Session 7 Reporting and Surveillance of MDROs Session



























- Slides: 48

Session 7: Reporting and Surveillance of MDROs Session 5

MDRO Reporting and Surveillance In this session, we are going to discuss • CMS and NHSN/CDC reporting • State of Texas reporting of CRE and MDR Acinetobacter – Resources and assistance: • DSHS laboratory services • Healthcare Associated Infection (HAI) team • Local Health Department Contact Info • Surveillance studies Session 5 2

MDRO Reporting and Surveillance The primary objectives of this session are as follows: 1. 2. Describe reporting requirements to NHSN Describe reporting requirements for CRE and MDRA to the state of Texas Session 5 3

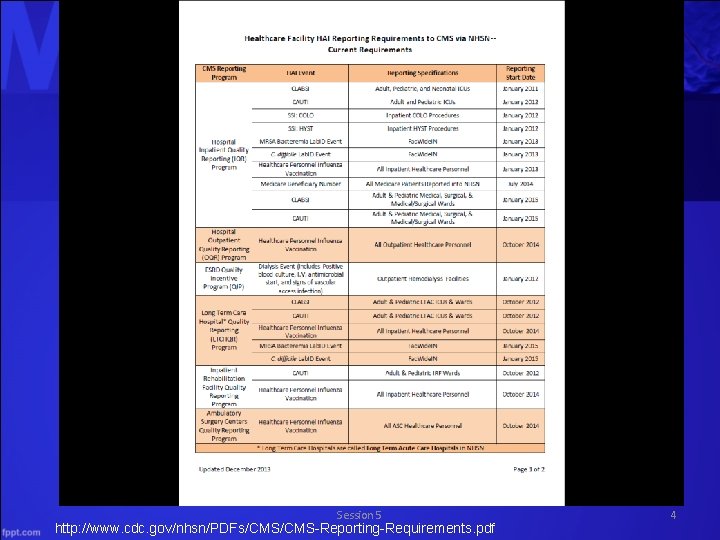
Session 5 http: //www. cdc. gov/nhsn/PDFs/CMS-Reporting-Requirements. pdf 4

http: //www. cdc. gov/nhsn/PDFs/CMS-Reporting-Requirements. pdf Session 5 5

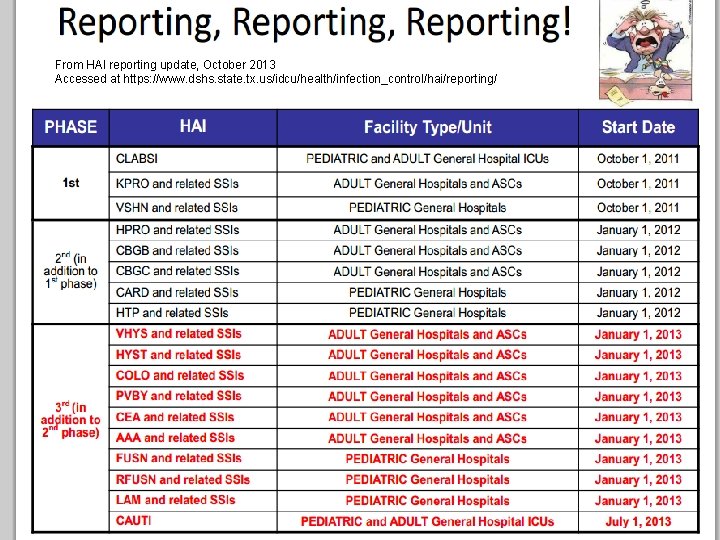
From HAI reporting update, October 2013 Accessed at https: //www. dshs. state. tx. us/idcu/health/infection_control/hai/reporting/ Session 5 6

NHSN MDRO Reporting • Why report (besides CMS requirements) • To help establish prevalence numbers – – National State Local Facility • Lets you know how you compare to other areas • Helps with your risk assessment From Texas Department of State Health Services Website: Texas Trends: Multi-drug resistant Acinetobacter has been found in Texas. The prevalence of MDR-A is currently unknown. Based on the 2012 Texas Annual Report on Health care-associated Infections Overall Antibiogram, antibiotic sensitivity for Acinetobacter baumannii ranged from 36% susceptible (Imipenem) to 70% Tetracycline. Session 5 7

NHSN MDRO Reporting Facilities must choose one of two Core reporting options for MDRO and C. difficile (or may choose both) 1. 2. Laboratory-identified (Lab. ID) events Infection Surveillance Facilities may also choose to report on Supplemental Measures 1. Prevention Process Measures • Hand Hygiene Adherence • Gown and Gloves Use Adherence • Active Surveillance Testing (AST) Adherence 2. Active Surveillance Testing Outcome Measures • Incident and Prevalent Cases using AST Multidrug Resistant Organism & Clostridium difficile Infection (MDRO/CDI) Module. January 2014. www. cdc. gov. Session 5 8 Accessed July 4, 2014

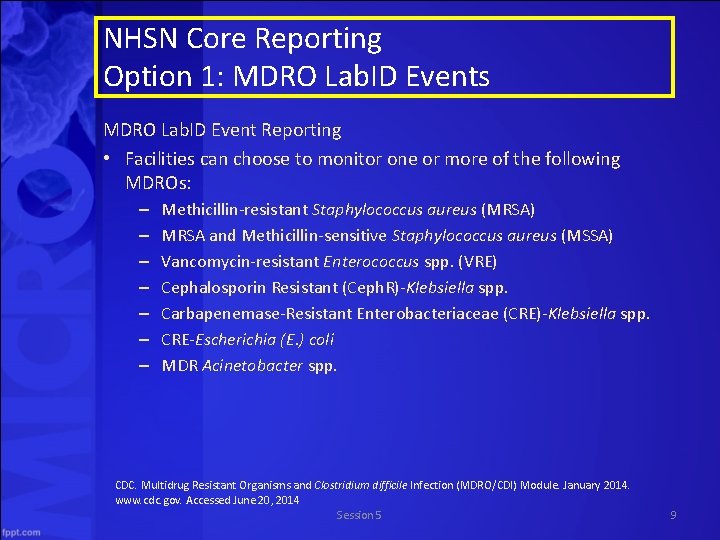
NHSN Core Reporting Option 1: MDRO Lab. ID Events MDRO Lab. ID Event Reporting • Facilities can choose to monitor one or more of the following MDROs: – – – – Methicillin-resistant Staphylococcus aureus (MRSA) MRSA and Methicillin-sensitive Staphylococcus aureus (MSSA) Vancomycin-resistant Enterococcus spp. (VRE) Cephalosporin Resistant (Ceph. R)-Klebsiella spp. Carbapenemase-Resistant Enterobacteriaceae (CRE)-Klebsiella spp. CRE-Escherichia (E. ) coli MDR Acinetobacter spp. CDC. Multidrug Resistant Organisms and Clostridium difficile Infection (MDRO/CDI) Module. January 2014. www. cdc. gov. Accessed June 20, 2014 Session 5 9

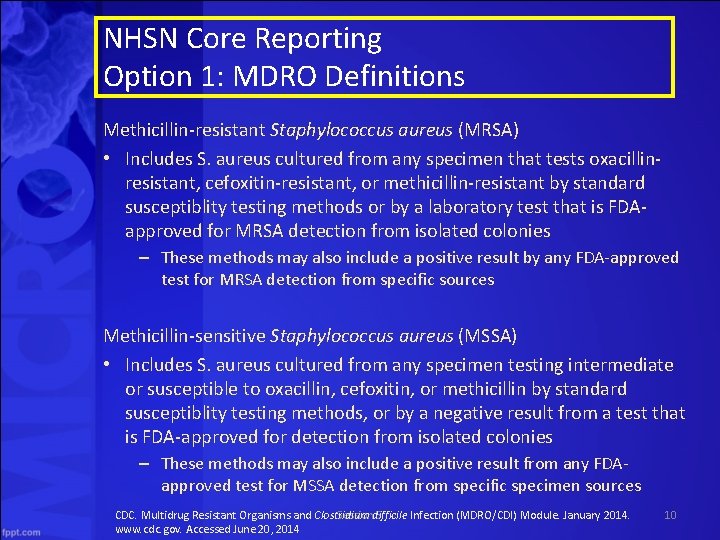
NHSN Core Reporting Option 1: MDRO Definitions Methicillin-resistant Staphylococcus aureus (MRSA) • Includes S. aureus cultured from any specimen that tests oxacillinresistant, cefoxitin-resistant, or methicillin-resistant by standard susceptiblity testing methods or by a laboratory test that is FDAapproved for MRSA detection from isolated colonies – These methods may also include a positive result by any FDA-approved test for MRSA detection from specific sources Methicillin-sensitive Staphylococcus aureus (MSSA) • Includes S. aureus cultured from any specimen testing intermediate or susceptible to oxacillin, cefoxitin, or methicillin by standard susceptiblity testing methods, or by a negative result from a test that is FDA-approved for detection from isolated colonies – These methods may also include a positive result from any FDAapproved test for MSSA detection from specific specimen sources CDC. Multidrug Resistant Organisms and Clostridium difficile Infection (MDRO/CDI) Module. January 2014. Session 5 www. cdc. gov. Accessed June 20, 2014 10

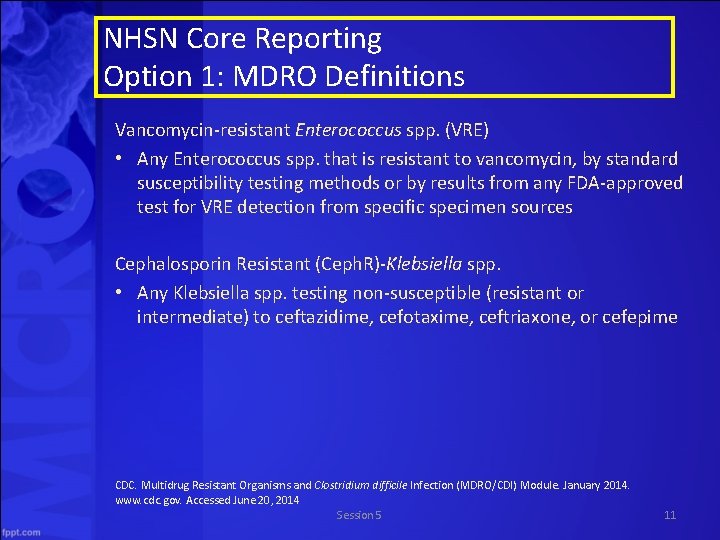
NHSN Core Reporting Option 1: MDRO Definitions Vancomycin-resistant Enterococcus spp. (VRE) • Any Enterococcus spp. that is resistant to vancomycin, by standard susceptibility testing methods or by results from any FDA-approved test for VRE detection from specific specimen sources Cephalosporin Resistant (Ceph. R)-Klebsiella spp. • Any Klebsiella spp. testing non-susceptible (resistant or intermediate) to ceftazidime, cefotaxime, ceftriaxone, or cefepime CDC. Multidrug Resistant Organisms and Clostridium difficile Infection (MDRO/CDI) Module. January 2014. www. cdc. gov. Accessed June 20, 2014 Session 5 11

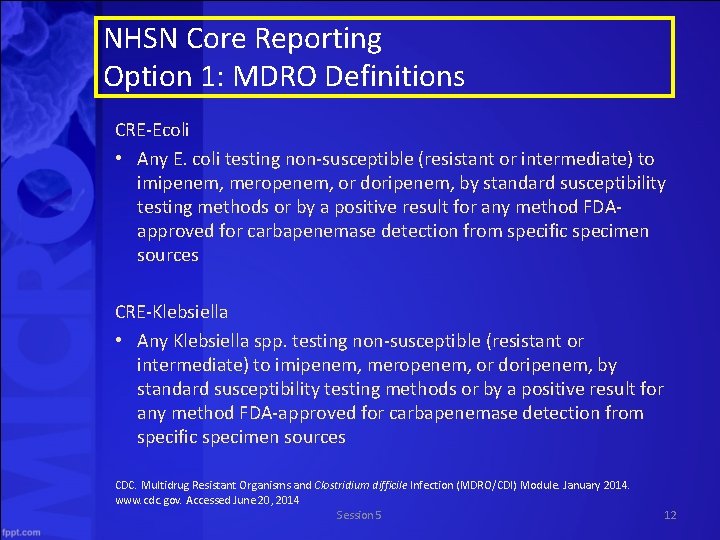
NHSN Core Reporting Option 1: MDRO Definitions CRE-Ecoli • Any E. coli testing non-susceptible (resistant or intermediate) to imipenem, meropenem, or doripenem, by standard susceptibility testing methods or by a positive result for any method FDAapproved for carbapenemase detection from specific specimen sources CRE-Klebsiella • Any Klebsiella spp. testing non-susceptible (resistant or intermediate) to imipenem, meropenem, or doripenem, by standard susceptibility testing methods or by a positive result for any method FDA-approved for carbapenemase detection from specific specimen sources CDC. Multidrug Resistant Organisms and Clostridium difficile Infection (MDRO/CDI) Module. January 2014. www. cdc. gov. Accessed June 20, 2014 Session 5 12

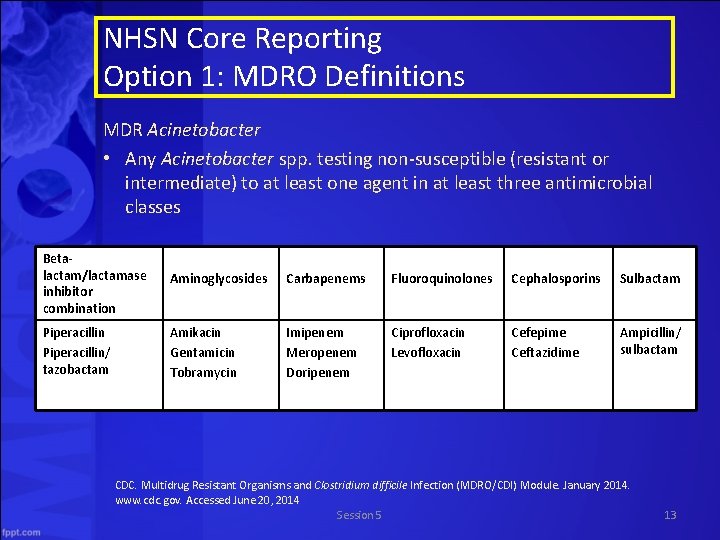
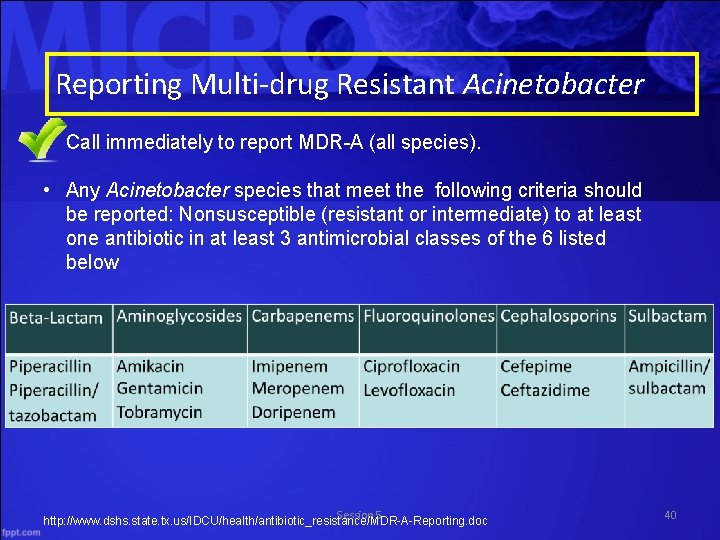
NHSN Core Reporting Option 1: MDRO Definitions MDR Acinetobacter • Any Acinetobacter spp. testing non-susceptible (resistant or intermediate) to at least one agent in at least three antimicrobial classes Betalactam/lactamase inhibitor combination Aminoglycosides Carbapenems Fluoroquinolones Cephalosporins Sulbactam Piperacillin/ tazobactam Amikacin Gentamicin Tobramycin Imipenem Meropenem Doripenem Ciprofloxacin Levofloxacin Cefepime Ceftazidime Ampicillin/ sulbactam CDC. Multidrug Resistant Organisms and Clostridium difficile Infection (MDRO/CDI) Module. January 2014. www. cdc. gov. Accessed June 20, 2014 Session 5 13

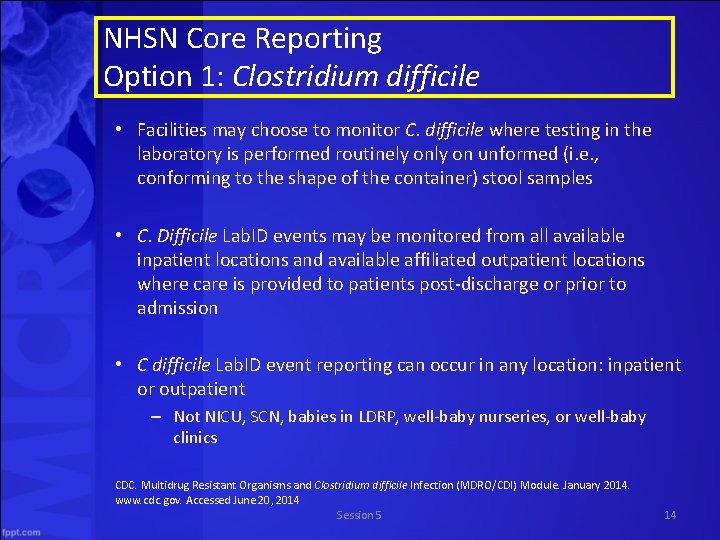
NHSN Core Reporting Option 1: Clostridium difficile • Facilities may choose to monitor C. difficile where testing in the laboratory is performed routinely on unformed (i. e. , conforming to the shape of the container) stool samples • C. Difficile Lab. ID events may be monitored from all available inpatient locations and available affiliated outpatient locations where care is provided to patients post-discharge or prior to admission • C difficile Lab. ID event reporting can occur in any location: inpatient or outpatient – Not NICU, SCN, babies in LDRP, well-baby nurseries, or well-baby clinics CDC. Multidrug Resistant Organisms and Clostridium difficile Infection (MDRO/CDI) Module. January 2014. www. cdc. gov. Accessed June 20, 2014 Session 5 14

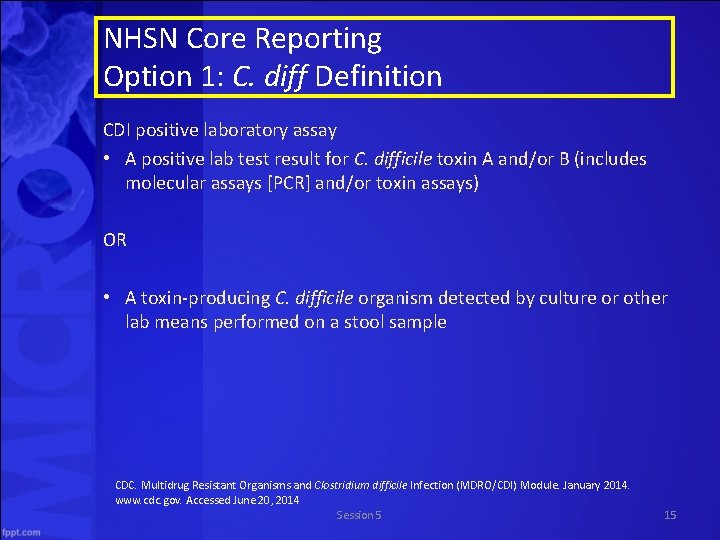
NHSN Core Reporting Option 1: C. diff Definition CDI positive laboratory assay • A positive lab test result for C. difficile toxin A and/or B (includes molecular assays [PCR] and/or toxin assays) OR • A toxin-producing C. difficile organism detected by culture or other lab means performed on a stool sample CDC. Multidrug Resistant Organisms and Clostridium difficile Infection (MDRO/CDI) Module. January 2014. www. cdc. gov. Accessed June 20, 2014 Session 5 15

NHSN Core Reporting – CMS rules • • To comply with NHSN and CMS reporting requirements, facilities must – map each of their inpatient locations, include MRSA bacteremia and C. difficile Lab. ID events each month – enter Lab. ID events when identified – Enter a summary data record each month – Indicate when they have zero lab. ID events to report If these requirements are not met, the facility’s data are not sent to CMS http: //www. cdc. gov/nhsn/PDFs/psc. Manual/12 psc. MDRO_CDADcurrent. pdf. Accessed June 25, 2014 Session 5 16

NHSN Core Reporting Option 2: Infection Surveillance Reporting • Surveillance must occur from at least one patient care area and requires active patient-based prospective surveillance of the chosen MDRO(s) and/or C. difficile infections (CDIs) by a trained Infection Preventionist • The IP must seek to confirm and classify infections caused by the chosen organism(s) for monitoring during a patient’s stay in at least one patient care location during the surveillance period • Infection Surveillance can occur in any inpatient location where infections may be identified http: //www. cdc. gov/nhsn/PDFs/psc. Manual/12 psc. MDRO_CDADcurrent. pdf. Accessed June 25, 2014 Session 5 17

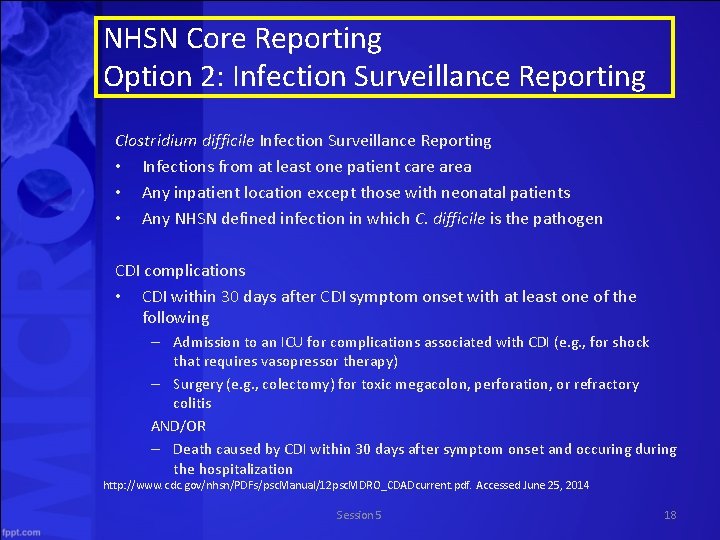
NHSN Core Reporting Option 2: Infection Surveillance Reporting Clostridium difficile Infection Surveillance Reporting • Infections from at least one patient care area • Any inpatient location except those with neonatal patients • Any NHSN defined infection in which C. difficile is the pathogen CDI complications • CDI within 30 days after CDI symptom onset with at least one of the following – Admission to an ICU for complications associated with CDI (e. g. , for shock that requires vasopressor therapy) – Surgery (e. g. , colectomy) for toxic megacolon, perforation, or refractory colitis AND/OR – Death caused by CDI within 30 days after symptom onset and occuring during the hospitalization http: //www. cdc. gov/nhsn/PDFs/psc. Manual/12 psc. MDRO_CDADcurrent. pdf. Accessed June 25, 2014 Session 5 18

NHSN Supplemental Reporting 1. 2. Prevention Process Measures Surveillance a. Monitoring adherence to hand hygiene b. Monitoring adherence to gown and glove use as part of contact precautions c. Monitoring adherence to Active Surveillance Testing Outcome Measures http: //www. cdc. gov/nhsn/PDFs/psc. Manual/12 psc. MDRO_CDADcurrent. pdf. Accessed June 25, 2014 Session 5 19

NHSN Supplemental Reporting Active Surveillance Testing Outcome Measures Definitions • MRSA colonization – Carriage of MRSA without evidence of infection Session 5 http: //www. cdc. gov/nhsn/PDFs/psc. Manual/12 psc. MDRO_CDADcurrent. pdf. Accessed June 25, 2014 20

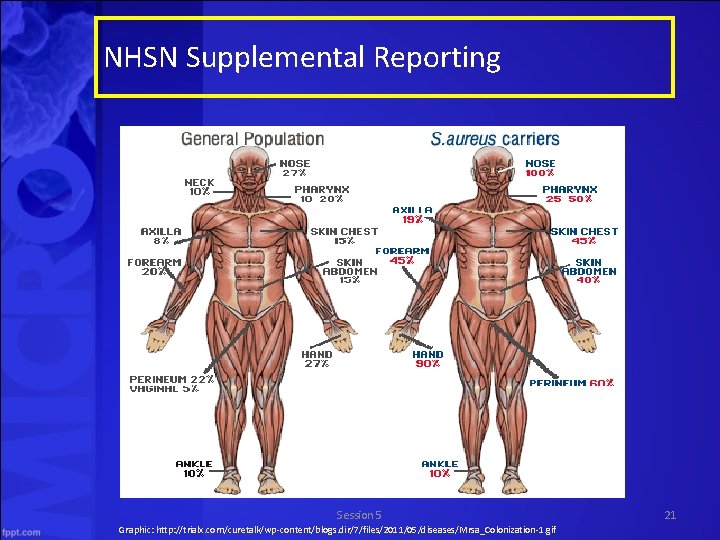
NHSN Supplemental Reporting Session 5 Graphic: http: //trialx. com/curetalk/wp-content/blogs. dir/7/files/2011/05/diseases/Mrsa_Colonization-1. gif 21

• Movie Quiz Session 5

State of Texas In this section, we will discuss • State and local health department roles, activities, and resources • State reporting requirements • Contacting your local Health Department Session 5 23

State of Texas • One priority of the State is to prevent and reduce the number of CDIs and MDRO infections along the continuum of patients’ and residents’ healthcare services • This effort requires a multi-disciplinary approach – Community health assessment process, facilities, residents Session 5 24

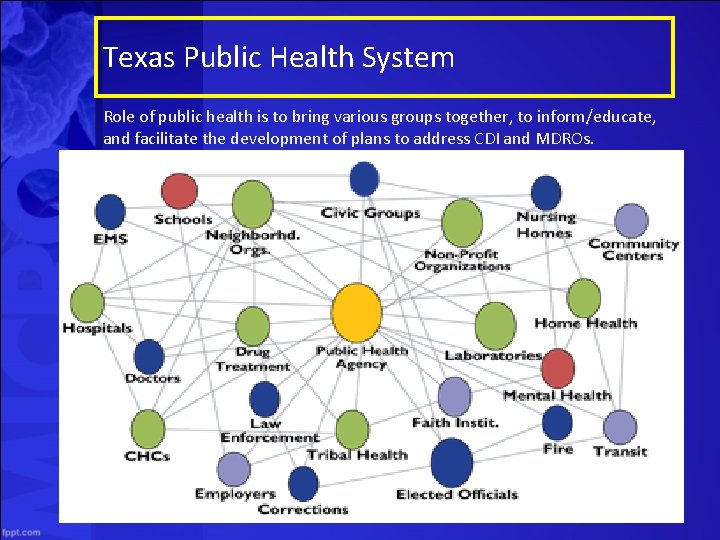
Texas Public Health System Role of public health is to bring various groups together, to inform/educate, and facilitate the development of plans to address CDI and MDROs. Session 5 25

Public Health CDI Surveillance • Surveillance systems are vital to monitor trends and inform action • Setting up a system in Texas would require: – Rules update – Additional resources at local and state levels – Standardized definitions for cases and outbreaks – Education and training of public health system work force – Campaign to roll out new requirements to providers Session 5 26

What does the Texas Administrative Code (TAC) say? • Reporting of CR-E. coli, CR-Klebsiella species, MDRAcinetobacter as defined in the NHSN Manual, Protocol for MDRO/CDI

Exceptional Item #9 2014 – 2015 Biennium • Offer free Infection Control Training • Foster a “CDI-prevention” culture – Prevention/response activities evaluated – CDI rates tracked by facility – Share best practices • Leverage expertise of academic centers – University of Houston and the UT Health Science Center at Houston – Texas A & M School of Public Health Session 5 28

Public Health Roles & Responsibilities • Support projects that integrate evidence-based best practices to reduce and eliminate the occurrence of CDI among Texas residents • Oversee implementation of facility-specific action plans to improve infection prevention and control • Encourage organizations to share best practices and engage in discussion on conference calls or at conferences • Support ongoing training programs • Provide technical assistance in epidemiology, analysis, presentation of data, and program evaluation Session 5 29

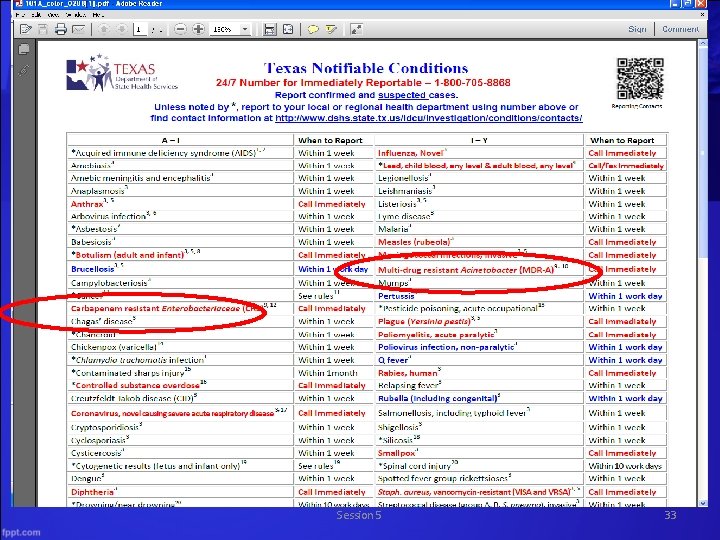
Reporting Nuts and Bolts WHAT to Report? • Notifiable conditions • Any outbreaks, exotic diseases, and unusual group expressions of disease • Reported by name, age, sex, race/ethnicity, Date of Birth, address, telephone number, disease, date of onset, method of diagnosis, and name, address, and telephone number of physician Session 5 30

Mandate/Authority • Communicable disease reporting is exempt from HIPAA - Health Insurance Portability and Accountability Act of 1996 • Texas Health & Safety Code – Chapter 81: Communicable Diseases – Chapter 98: Reporting of Healthcare-Associated Infections • Texas Administrative Code – Title 25, part 1, Chapter 97 Communicable Diseases – Title 25, part 1, Chapter 96 Bloodborne Pathogen Control Session 5 31

Mandate/Authority • 80 th Legislature enacted Chapter 98 of the Health and Safety Code to require reporting of select HAIs by certain facilities • Clarification provided during the 81 st and 82 nd legislature on requirements and procedures Session 5 32

Session 5 33

Reporting Nuts and Bolts WHEN to Report? • Immediately - cases or suspected cases of illness considered to be public health emergencies, outbreaks, exotic diseases, and unusual group expressions of disease • Within one working day - diseases for which there must be a quick public health response • Within one week - all other conditions HOW to Report? • Most should be reported directly to the Local Health Department or DSHS Health Services Region • Last resort or in case of emergency call the central DSHS office in Austin at 888 -963 -7111. Session 5 34

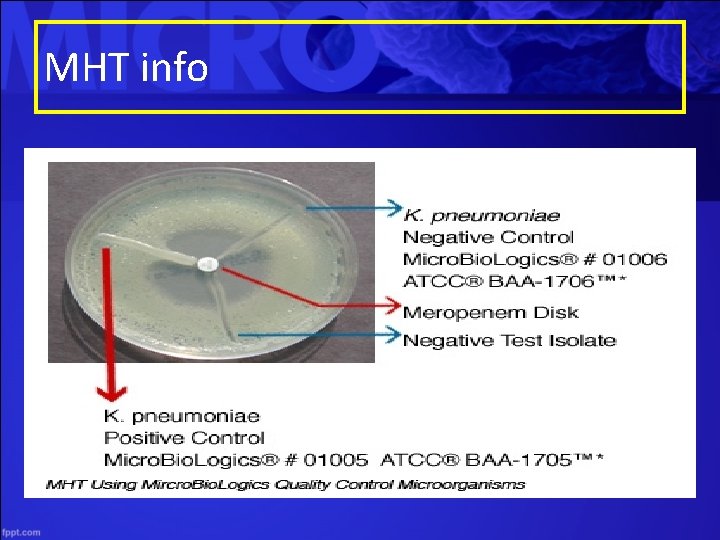
Lab Detection for CRE • Clinical and Laboratory Standards Institute (CLSI) breakpoints for determining carbapenem susceptibility – Breakpoints were lowered to improve detection • Modified Hodge Test (MHT) – Tests for carbapenemase – Not necessary with the recommended lowered breakpoints • Other methods – PCR- only for KPC or NDM

MHT info

Reporting Carbapenem-Resistant Enterobacteriaceae • Report a suspected or confirmed case immediately by phone to your local health department. CDC – NHSN MDRO Protocol E. coli or any Klebsiella spp. testing non-susceptible to imipenem, meropenem or doripenem by standard susceptibility testing methods or by a positive result for any method FDA-approved for carbapenemase detection from specific specimen sources Session 5 http: //www. dshs. state. tx. us/IDCU/health/antibiotic_resistance/Reporting-CRE. doc 37


Reporting Carbapenem-Resistant Enterobacteriaceae • When reporting CRE, please include (if available): – a copy of the lab report – carbapenems tested – susceptibility of antibiotics – minimum inhibitory concentration (MIC) – positive phenotypic or PCR test, if applicable. • *A DSHS or local health department epidemiologist may contact you for follow-up after receiving your report. http: //www. dshs. state. tx. us/IDCU/health/antibiotic_resistance/Reporting-CRE. doc Session 5 39

Reporting Multi-drug Resistant Acinetobacter • Call immediately to report MDR-A (all species). • Any Acinetobacter species that meet the following criteria should be reported: Nonsusceptible (resistant or intermediate) to at least one antibiotic in at least 3 antimicrobial classes of the 6 listed below Session 5 http: //www. dshs. state. tx. us/IDCU/health/antibiotic_resistance/MDR-A-Reporting. doc 40

Reporting Multi-drug Resistant Acinetobacter • Please include the following additional information when reporting (if available) – Attach a copy of the lab report – Antibiotics tested – Susceptibility of Antibiotics – Minimum inhibitory concentration (MIC) • *You may be contacted by either a DSHS or local health department epidemiologist after reporting for additional follow-up. http: //www. dshs. state. tx. us/IDCU/health/antibiotic_resistance/MDR-A-Reporting. doc Session 5 41

Molecular Subtyping • PFGE (Pulsed Field Gel Electrophoresis) • Isolates may be submitted for PFGE with approval of the DSHS MDRO epi. **DSHS Lab is in process of validation of PCR testing for drug resistant mechanisms (KPC and NDM)

How to Submit? • Contact DSHS MDRO epidemiologist Jessica Ross in the Central Office prior to submission (Phone: 512 -776 -6356) • Make sure to include: – Antimicrobial susceptibility Test (AST) data – MHT data if done – Patient information • Fill out G 2 B form • Information on DSHS lab website on how to request submission forms • http: //www. dshs. state. tx. us/lab/

Contacting your local health department • A list of local health departments is available at the following link: • http: //www. dshs. state. tx. us/regions/lhds. shtm Session 5 44

Surveillance Literature Session 5

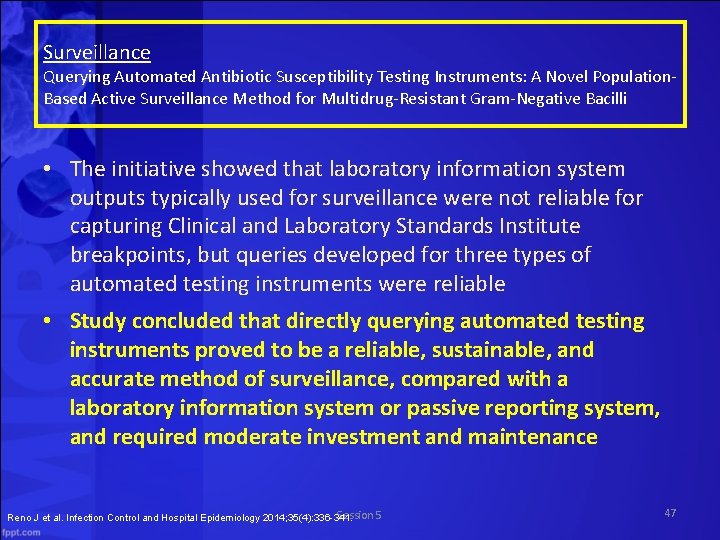
Surveillance Querying Automated Antibiotic Susceptibility Testing Instruments: A Novel Population-Based Active Surveillance Method for Multidrug-Resistant Gram. Negative Bacilli • In 2010, the CDC’s Emerging Infections Program (EIP) piloted the Multi-Site Resistant Gram-Negative Bacilli Surveillance Initiative (Mu. GSI) in Georgia and Minnesota • The program was implemented by Georgia EIP staff in three phases – Phase I: Determine local labs’ antibiotic susceptibility testing practices and capabilities for detecting MDR-GNB – Phase II: Pilot surveillance to determine feasibility over the long term and to approximate the proportion of GNB that were MDR – Phase III: Initiate ongoing active surveillance for MDR-GNB in metro Atlanta Reno J et al. Infection Control and Hospital Epidemiology 2014; 35(4): 336 -341. Session 5 46

Surveillance Querying Automated Antibiotic Susceptibility Testing Instruments: A Novel Population. Based Active Surveillance Method for Multidrug-Resistant Gram-Negative Bacilli • The initiative showed that laboratory information system outputs typically used for surveillance were not reliable for capturing Clinical and Laboratory Standards Institute breakpoints, but queries developed for three types of automated testing instruments were reliable • Study concluded that directly querying automated testing instruments proved to be a reliable, sustainable, and accurate method of surveillance, compared with a laboratory information system or passive reporting system, and required moderate investment and maintenance Session 5 Reno J et al. Infection Control and Hospital Epidemiology 2014; 35(4): 336 -341. 47

Questions and Discussion Session 5
 Continuous analysis and surveillance system
Continuous analysis and surveillance system Reconnaissance and surveillance leaders course
Reconnaissance and surveillance leaders course Informants, surveillance and undercover operations
Informants, surveillance and undercover operations Regulatory agencies
Regulatory agencies Who rsv surveillance
Who rsv surveillance Texas brfss
Texas brfss Surveillance post opératoire
Surveillance post opératoire Types of surveillance
Types of surveillance Surveillance fixateur externe
Surveillance fixateur externe Nav canada cfps
Nav canada cfps Sentinel surveillance is for
Sentinel surveillance is for Pss pro surveillance system
Pss pro surveillance system Drainage biliaire externe definition
Drainage biliaire externe definition Surveillance photos
Surveillance photos Passive surveillance
Passive surveillance Manovac drainage
Manovac drainage Reservoir surveillance definition
Reservoir surveillance definition Ephi ethiopia
Ephi ethiopia Sentinel surveillance definition
Sentinel surveillance definition Raven surveillance drone
Raven surveillance drone Developmental screening vs surveillance
Developmental screening vs surveillance Natural surveillance
Natural surveillance What does rid stand for in lifeguarding
What does rid stand for in lifeguarding Hostile surveillance techniques
Hostile surveillance techniques Airborne surveillance applications
Airborne surveillance applications Perform surveillance without the aid of electronic device
Perform surveillance without the aid of electronic device Georgia law on video surveillance
Georgia law on video surveillance Parental surveillance
Parental surveillance Drain transcystique surveillance
Drain transcystique surveillance Les différents types de drainage en chirurgie
Les différents types de drainage en chirurgie Cafe racer shooting surveillance video
Cafe racer shooting surveillance video Drain thoracique infirmier
Drain thoracique infirmier Automated video evidence
Automated video evidence Texas syndromic surveillance
Texas syndromic surveillance Pneumothorax soins infirmiers
Pneumothorax soins infirmiers Element de surveillance
Element de surveillance Syndrome malin surveillance ide
Syndrome malin surveillance ide Redon non aspiratif
Redon non aspiratif Define health supervision
Define health supervision Activity diagram for access camera surveillance
Activity diagram for access camera surveillance Automatic dependant surveillance
Automatic dependant surveillance Primary vs secondary surveillance radar
Primary vs secondary surveillance radar Aefi surveillance officer
Aefi surveillance officer Disadvantages of rhino poaching
Disadvantages of rhino poaching Syndromic surveillance
Syndromic surveillance Oxygenotherapie materiel
Oxygenotherapie materiel Panniculite mésentérique et ganglions
Panniculite mésentérique et ganglions Surveillance of
Surveillance of Principles of surveillance
Principles of surveillance