Section 2 Hydrogen Ions and Acidity Introduction To

![6. Ion Product Constant for Water • When [H+] increases, [OH-] decreases, and vice 6. Ion Product Constant for Water • When [H+] increases, [OH-] decreases, and vice](https://slidetodoc.com/presentation_image_h2/dc5686a58eadfd9356b9cd4dd4cecf7f/image-4.jpg)


![The p. H Concept (cont. ) • A solution in which [H+] is greater The p. H Concept (cont. ) • A solution in which [H+] is greater](https://slidetodoc.com/presentation_image_h2/dc5686a58eadfd9356b9cd4dd4cecf7f/image-10.jpg)







- Slides: 24

Section 2 - Hydrogen Ions and Acidity Introduction • To test a diagnosis of diabetic coma, a doctor orders several tests, including the acidity of the patient’s blood. • Results from this test will be expressed in units of p. H. • You will learn how the p. H scale is used to indicate the acidity of a solution and why the p. H scale is used.

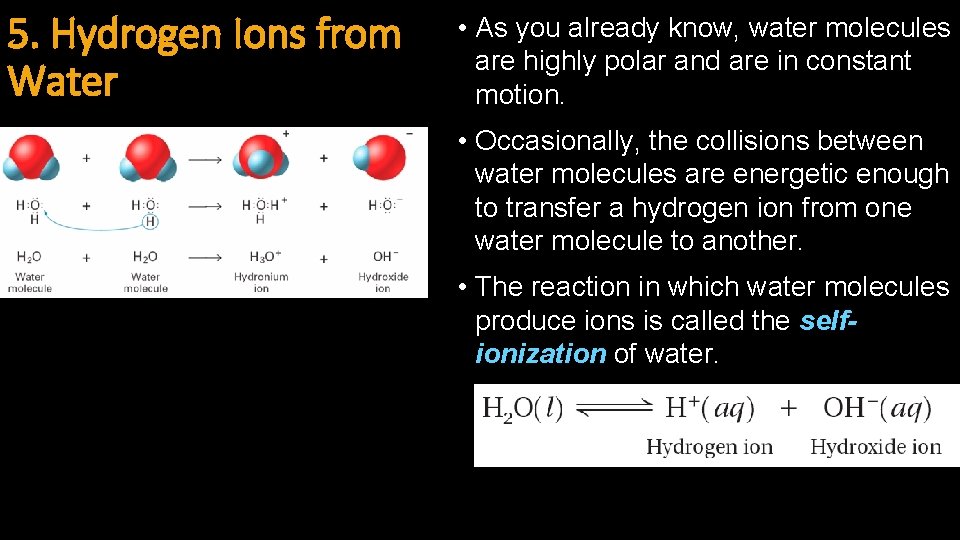
5. Hydrogen Ions from Water • As you already know, water molecules are highly polar and are in constant motion. • Occasionally, the collisions between water molecules are energetic enough to transfer a hydrogen ion from one water molecule to another. • The reaction in which water molecules produce ions is called the selfionization of water.

Hydrogen Ions from Water (cont. ) • Hydrogen ions (H+) are always joined to water molecules as hydronium ions (H 3 O+) • Sometimes called protons • The self-ionization of water occurs to a very small extent. • The concentrations of H+ and OH- are equal (1 x 10 -7 M) in pure water, which is a neutral solution.
![6 Ion Product Constant for Water When H increases OH decreases and vice 6. Ion Product Constant for Water • When [H+] increases, [OH-] decreases, and vice](https://slidetodoc.com/presentation_image_h2/dc5686a58eadfd9356b9cd4dd4cecf7f/image-4.jpg)
6. Ion Product Constant for Water • When [H+] increases, [OH-] decreases, and vice versa. • If additional ions are added to a solution, the equilibrium shifts. • The concentration of the other type of ion decreases. • More water molecules are formed in the process.

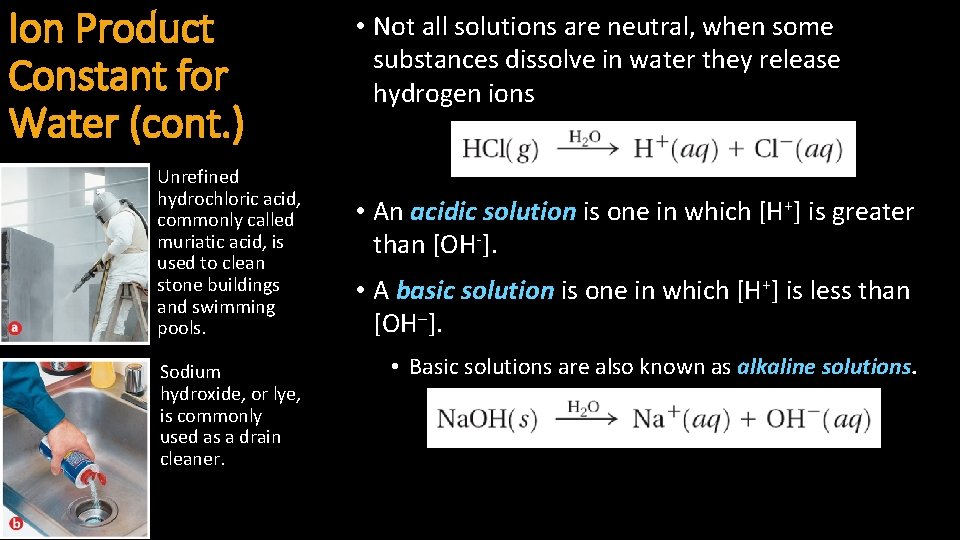
• For aqueous solutions, the product of the Ion Product hydrogen ion concentration and the hydroxide Constant for Water ion concentration equals 1. 0 10 -14. (cont. ) • The product of the concentrations of the hydrogen ions and hydroxide ions in water is called the ion-product constant for water (Kw).

Ion Product Constant for Water (cont. ) Unrefined hydrochloric acid, commonly called muriatic acid, is used to clean stone buildings and swimming pools. Sodium hydroxide, or lye, is commonly used as a drain cleaner. • Not all solutions are neutral, when some substances dissolve in water they release hydrogen ions • An acidic solution is one in which [H+] is greater than [OH-]. • A basic solution is one in which [H+] is less than [OH ]. • Basic solutions are also known as alkaline solutions.

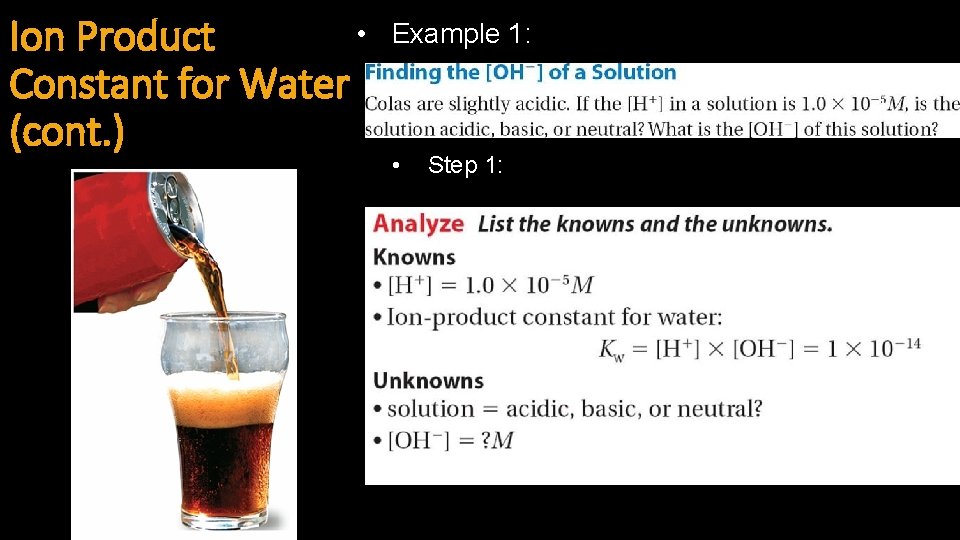
• Ion Product Constant for Water (cont. ) Example 1: • Step 1:

Ion Product Constant for Water (cont. ) • Step 2: • Step 3:

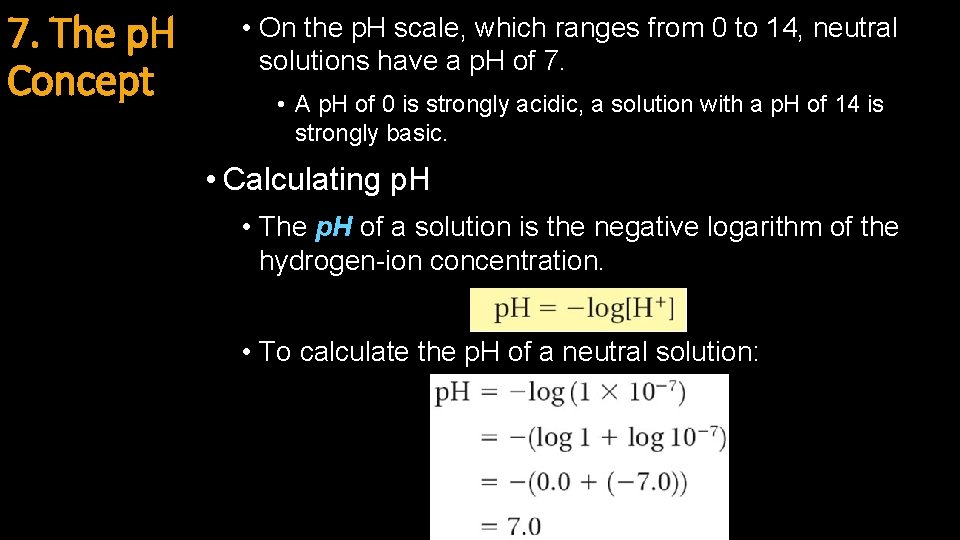
7. The p. H Concept • On the p. H scale, which ranges from 0 to 14, neutral solutions have a p. H of 7. • A p. H of 0 is strongly acidic, a solution with a p. H of 14 is strongly basic. • Calculating p. H • The p. H of a solution is the negative logarithm of the hydrogen-ion concentration. • To calculate the p. H of a neutral solution:
![The p H Concept cont A solution in which H is greater The p. H Concept (cont. ) • A solution in which [H+] is greater](https://slidetodoc.com/presentation_image_h2/dc5686a58eadfd9356b9cd4dd4cecf7f/image-10.jpg)
The p. H Concept (cont. ) • A solution in which [H+] is greater than 1 10– 7 M has a p. H less than 7. 0 and is acidic. • The p. H of pure water or a neutral aqueous solution is 7. 0. • A solution with a p. H greater than 7 is basic and has a [H+] of less than 1 10– 7 M.

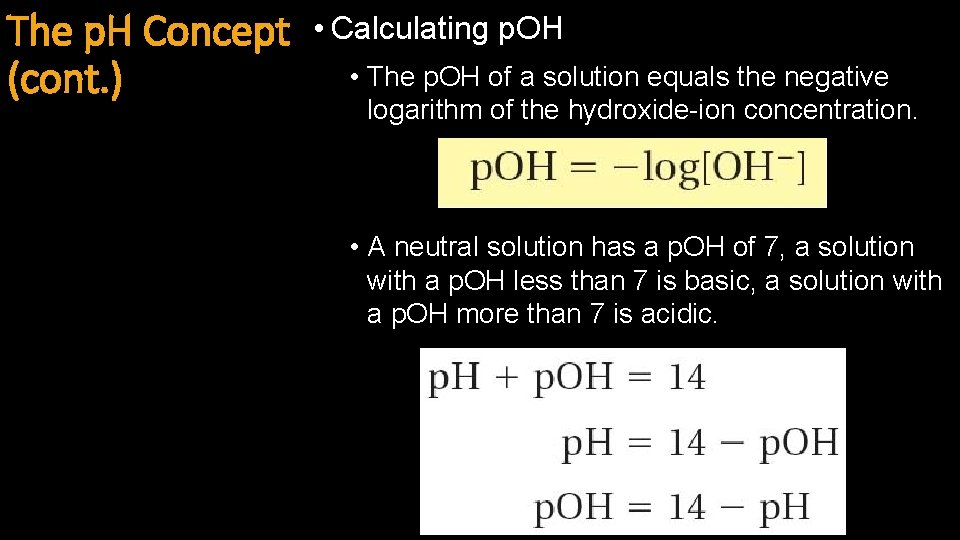
The p. H Concept (cont. ) • Calculating p. OH • The p. OH of a solution equals the negative logarithm of the hydroxide-ion concentration. • A neutral solution has a p. OH of 7, a solution with a p. OH less than 7 is basic, a solution with a p. OH more than 7 is acidic.

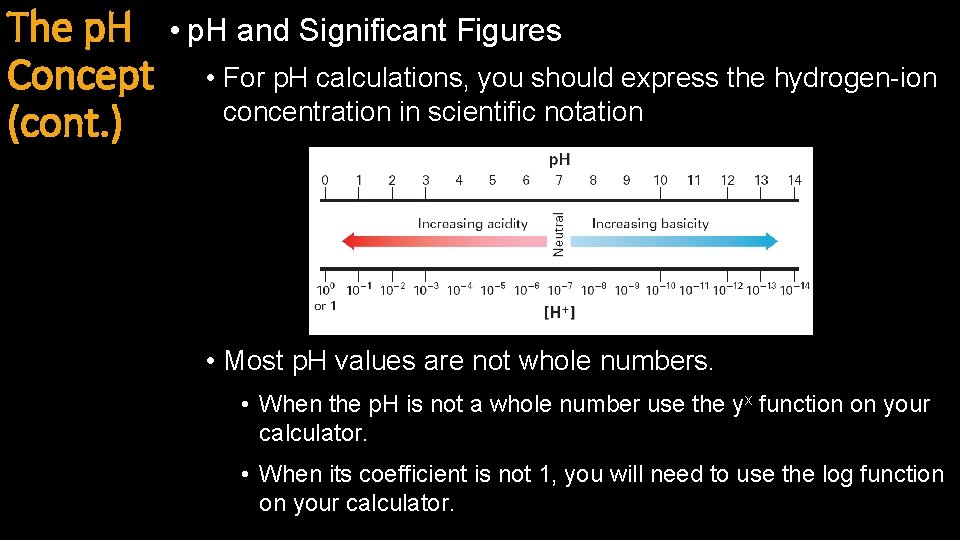
The p. H Concept (cont. ) • p. H and Significant Figures • For p. H calculations, you should express the hydrogen-ion concentration in scientific notation • Most p. H values are not whole numbers. • When the p. H is not a whole number use the yx function on your calculator. • When its coefficient is not 1, you will need to use the log function on your calculator.

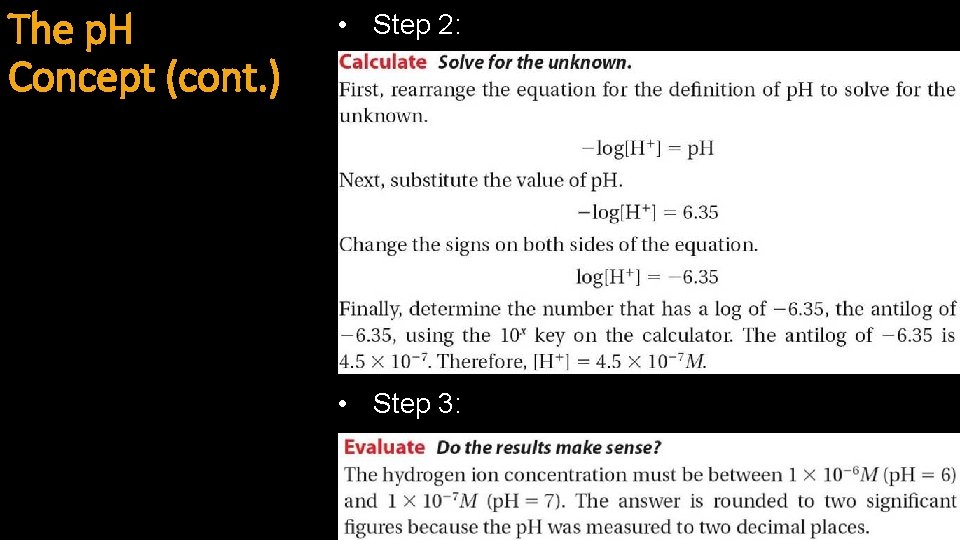
The p. H Concept (cont. ) • Example 2: • Step 1:

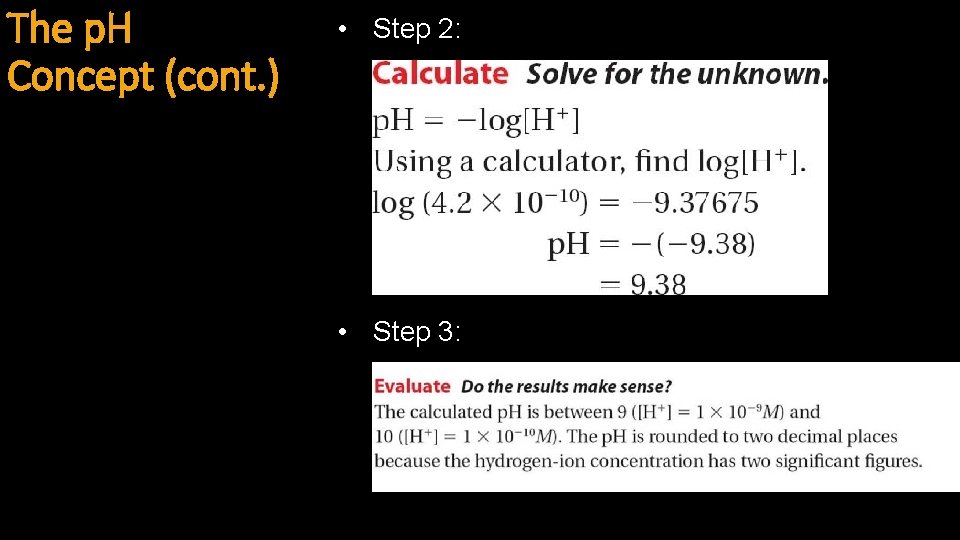
The p. H Concept (cont. ) • Step 2: • Step 3:

The p. H Concept (cont. ) • Example 3: • Step 1:

The p. H Concept (cont. ) • Step 2: • Step 3:

8. Measuring p. H • For small-volume samples, an indicator is used. • If you know the [OH-] of a solution, you can find its p. H. • The ion-product for water defines the relationship between [H+] and [OH-]. • Therefore, you can determine [H+] by dividing Kw by the known [OH-]. Phenolphthalein changes from colorless to pink at p. H 7– 9.

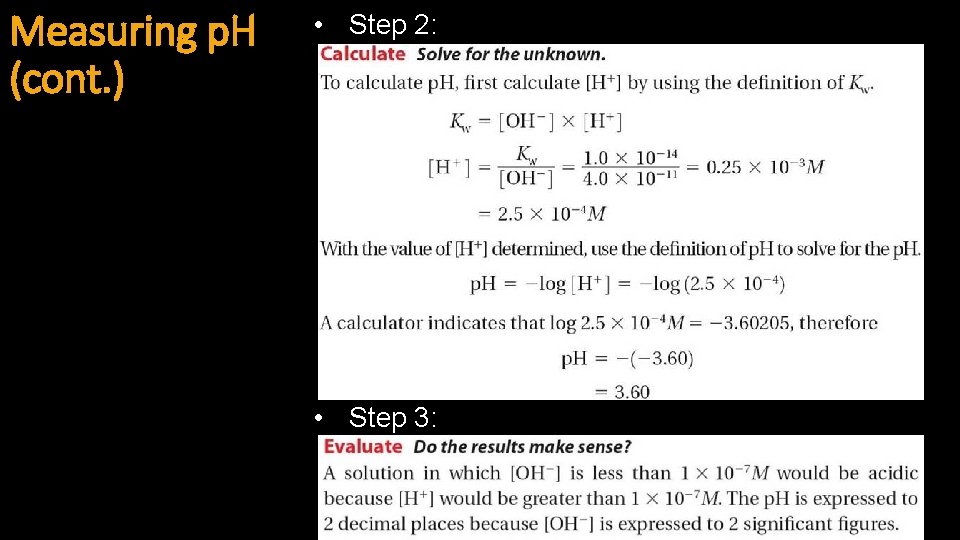
Measuring p. H (cont. ) • Example 4: • Step 1:

Measuring p. H (cont. ) • Step 2: • Step 3:

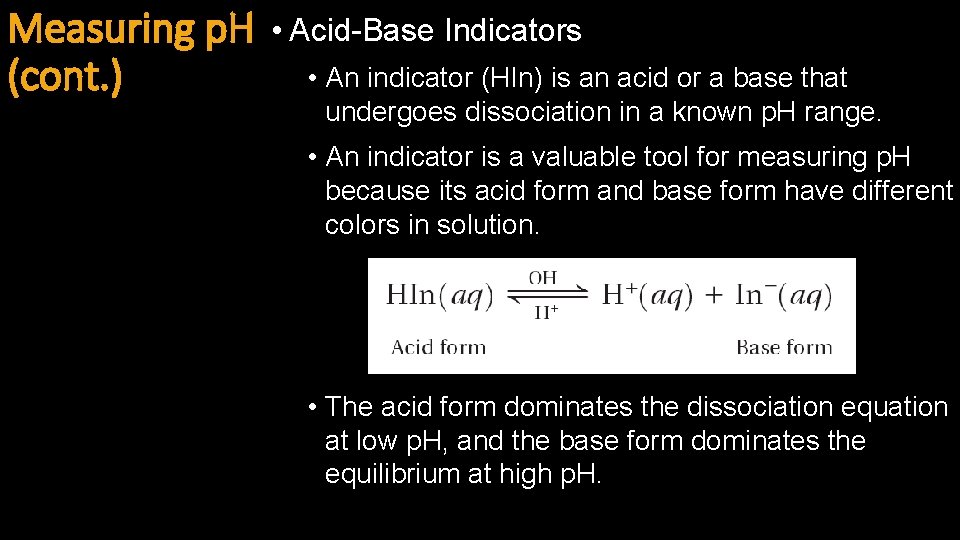
Measuring p. H (cont. ) • Acid-Base Indicators • An indicator (HIn) is an acid or a base that undergoes dissociation in a known p. H range. • An indicator is a valuable tool for measuring p. H because its acid form and base form have different colors in solution. • The acid form dominates the dissociation equation at low p. H, and the base form dominates the equilibrium at high p. H.

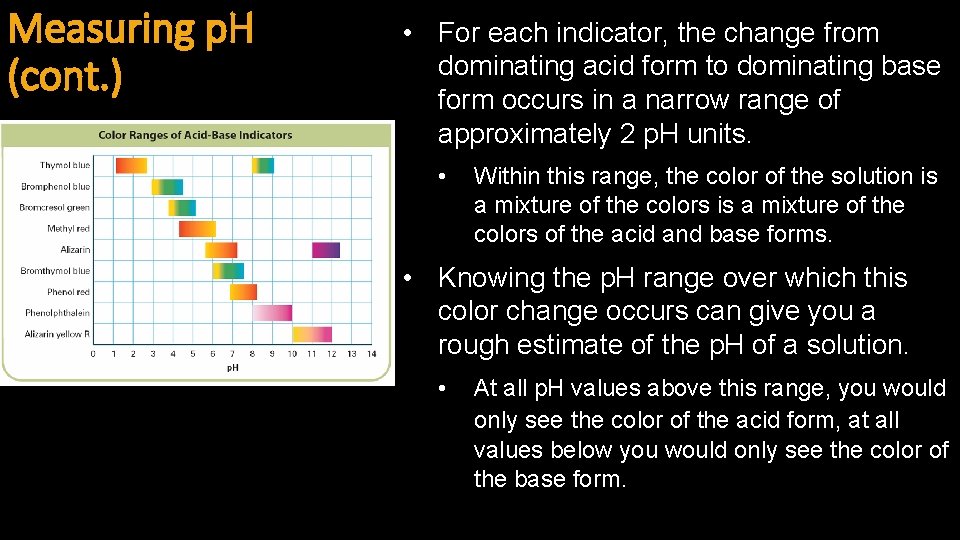
Measuring p. H (cont. ) • For each indicator, the change from dominating acid form to dominating base form occurs in a narrow range of approximately 2 p. H units. • Within this range, the color of the solution is a mixture of the colors of the acid and base forms. • Knowing the p. H range over which this color change occurs can give you a rough estimate of the p. H of a solution. • At all p. H values above this range, you would only see the color of the acid form, at all values below you would only see the color of the base form.

Measuring p. H (cont. ) • Indicators have certain characteristics that limit their usefulness. • At temperatures other than 25⁰ C an indicator may change color at a different p. H. • If the solution being tested is not colorless, the color of the indicator may be distorted Universal Indicators • Dissolved salts in a solution may also affect the indicator’s dissociation. • Indicator strips can help overcome these problems.

Measuring p. H (cont. ) • p. H Meters • Make rapid, accurate p. H measurements. • Easier to use than liquid indicators or indicator strips. • Measurements are typically more accurate to within 0. 01 p. H unit of the true p. H. • The color and cloudiness of the unknown solution do not affect the accuracy of the p. H value obtained.

END OF SECTION 2