Hydrogen Ions and Acidity PrenticeHall Chapter 19 2

![Objectives v Describe how [H+] and [OH-] are related in an aqueous solution v Objectives v Describe how [H+] and [OH-] are related in an aqueous solution v](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-2.jpg)



![Acidic Solution An acidic solution is one in which [H+] is greater than [OH-]. Acidic Solution An acidic solution is one in which [H+] is greater than [OH-].](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-9.jpg)

![Basic Solution A basic solution is one in which [H+] is less than [OH Basic Solution A basic solution is one in which [H+] is less than [OH](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-11.jpg)






![Calculating Neutral p. H A solution in which [H+] is greater than 1 10 Calculating Neutral p. H A solution in which [H+] is greater than 1 10](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-19.jpg)
![p. H and [H+] p. H and [H+]](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-20.jpg)












![1. If the [OH-] in a solution is 7. 65 10 -3 M, what 1. If the [OH-] in a solution is 7. 65 10 -3 M, what](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-37.jpg)
![1. If the [OH-] in a solution is 7. 65 10 -3 M, what 1. If the [OH-] in a solution is 7. 65 10 -3 M, what](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-38.jpg)
![2. The [OH-] for four solutions is given below. Which one of the solutions 2. The [OH-] for four solutions is given below. Which one of the solutions](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-39.jpg)
![2. The [OH-] for four solutions is given below. Which one of the solutions 2. The [OH-] for four solutions is given below. Which one of the solutions](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-40.jpg)


- Slides: 42

Hydrogen Ions and Acidity Prentice-Hall Chapter 19. 2 Dr. Yager
![Objectives v Describe how H and OH are related in an aqueous solution v Objectives v Describe how [H+] and [OH-] are related in an aqueous solution v](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-2.jpg)
Objectives v Describe how [H+] and [OH-] are related in an aqueous solution v Classify a solution as neutral, acidic, or basic given the hydrogen-ion or hydroxide-ion concentration v Convert hydrogen-ion concentration into p. H values and hydroxide-ion concentrations into p. OH values v Describe the purpose of an acid-base indicator

To test a diagnosis of diabetic coma, a doctor tests for the acidity of the patient’s blood. Results from this test will be expressed in units of p. H. You will learn how the p. H scale is used to indicate the acidity of a solution.

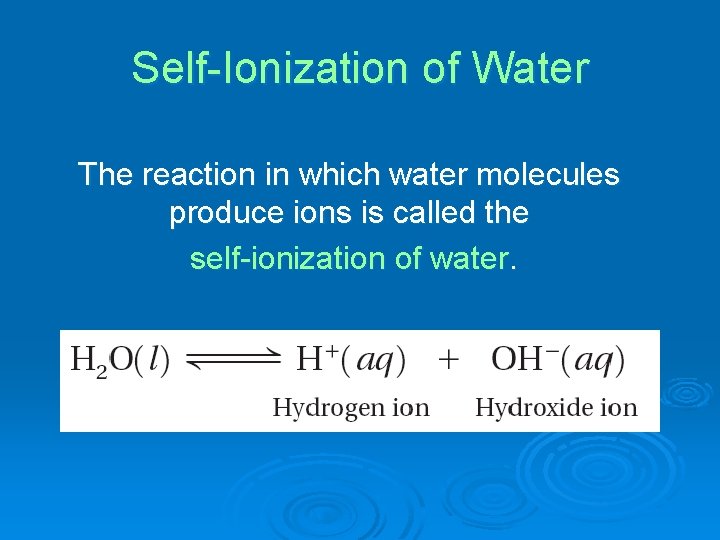
Self-Ionization of Water The reaction in which water molecules produce ions is called the self-ionization of water.

In the self-ionization of water, a proton (hydrogen ion) transfers from one water molecule to another water molecule.

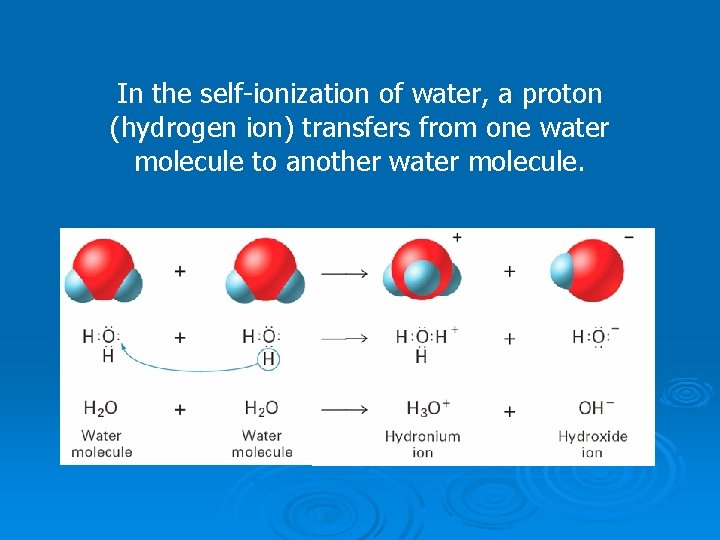
The self-ionization of water is a reversible reaction. The concentrations of hydronium and hydroxide ions are equal and known, that is: [H 3 O+] = [OH-] = 1 x 10 -7 M In any aqueous solution when [H 3 O+] and [OH-] are equal the solution is neutral.

Ion Constant Product for Water Calculate the equilibrium constant for the reaction of self-ionization of water: H 2 O (l) + H 2 O (l) H 3 O+(aq) + OH-(aq) [H 3 O+] [OH-] = (1. 0 x 10 -7) = 1. 0 10 -14 or Kw = 1. 0 10 -14.

The product of the concentrations of hydrogen ions and hydroxide ions in water is called the ion-product constant for water (Kw).
![Acidic Solution An acidic solution is one in which H is greater than OH Acidic Solution An acidic solution is one in which [H+] is greater than [OH-].](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-9.jpg)
Acidic Solution An acidic solution is one in which [H+] is greater than [OH-].

Unrefined hydrochloric acid, commonly called muriatic acid, is used to clean stone buildings and swimming pools.
![Basic Solution A basic solution is one in which H is less than OH Basic Solution A basic solution is one in which [H+] is less than [OH](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-11.jpg)
Basic Solution A basic solution is one in which [H+] is less than [OH ]. Basic solutions are also known as alkaline solutions.

Sodium hydroxide, or lye, is commonly used as a drain cleaner.





The p. H Concept What does p. H stand for? p. OWER of Hydrogen

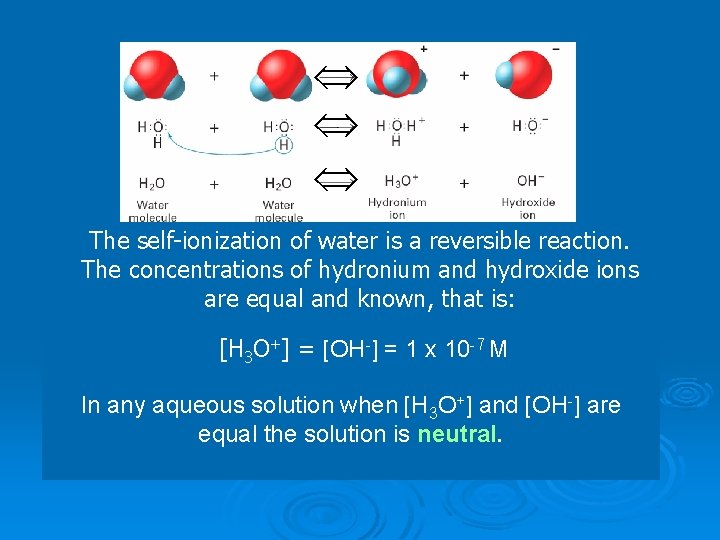
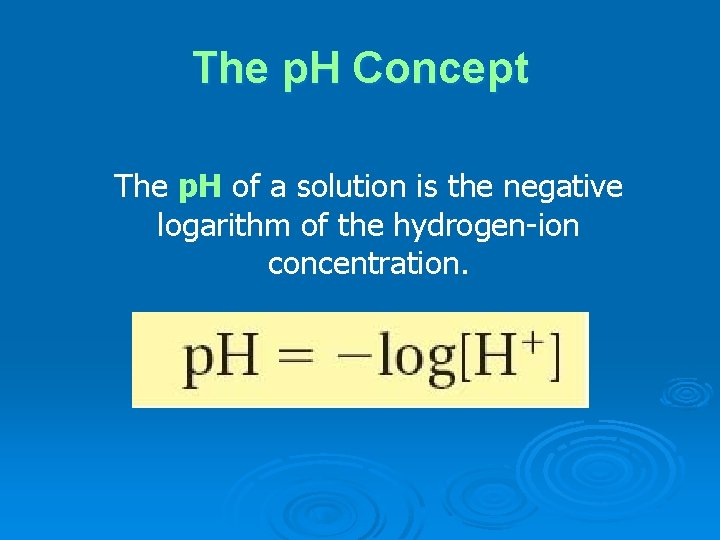
The p. H Concept The p. H of a solution is the negative logarithm of the hydrogen-ion concentration.
![Calculating Neutral p H A solution in which H is greater than 1 10 Calculating Neutral p. H A solution in which [H+] is greater than 1 10](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-19.jpg)
Calculating Neutral p. H A solution in which [H+] is greater than 1 10 -7 M has a p. H less than 7. 0 and is acidic. The p. H of pure water or a neutral aqueous solution is 7. 0. A solution with a p. H greater than 7 is basic and has a [H+] of less than 1 10 -7 M.
![p H and H p. H and [H+]](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-20.jpg)
p. H and [H+]



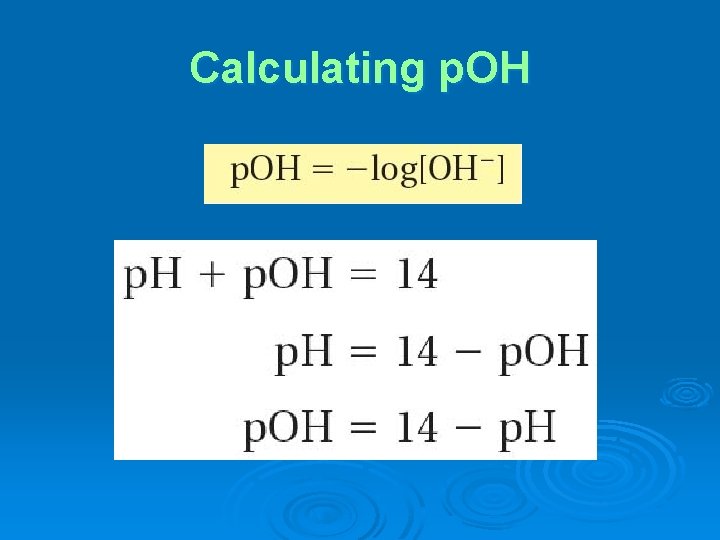
Calculating p. OH










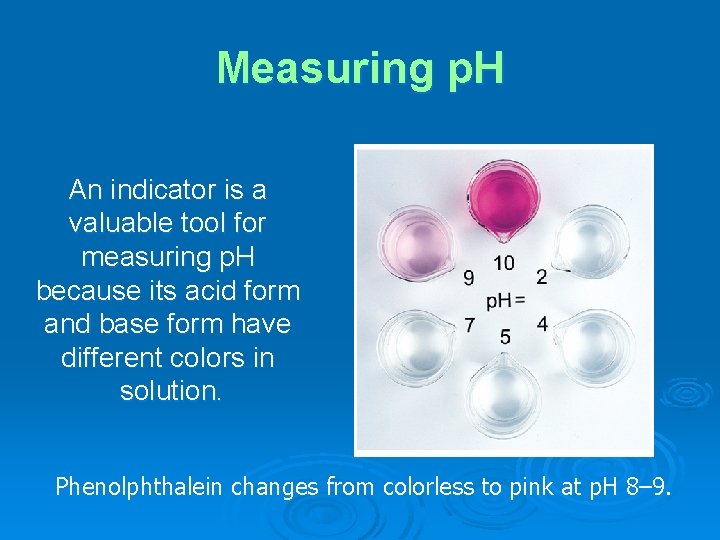
Measuring p. H An indicator is a valuable tool for measuring p. H because its acid form and base form have different colors in solution. Phenolphthalein changes from colorless to pink at p. H 8– 9.

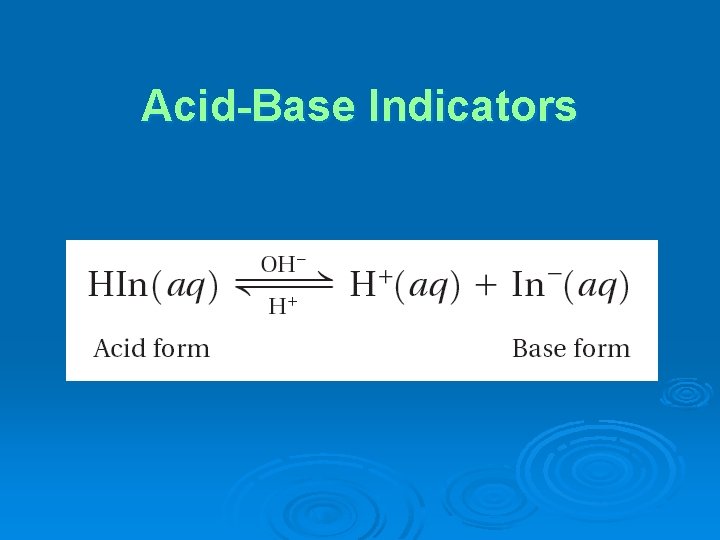
Acid-Base Indicators


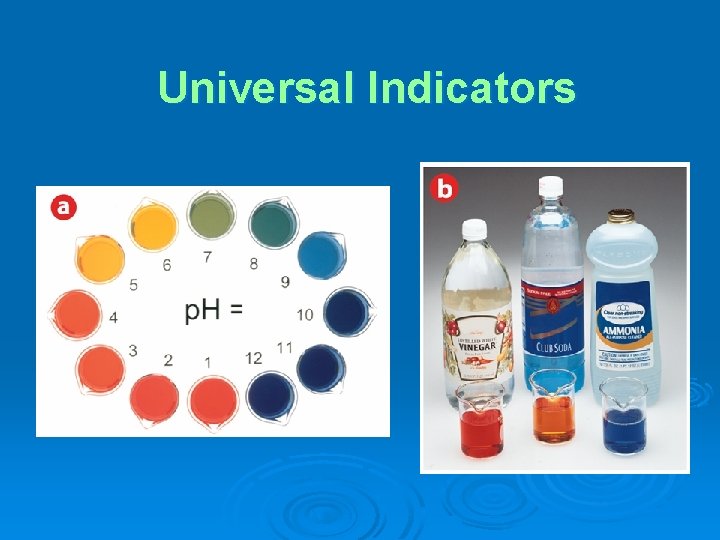
Universal Indicators
![1 If the OH in a solution is 7 65 10 3 M what 1. If the [OH-] in a solution is 7. 65 10 -3 M, what](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-37.jpg)
1. If the [OH-] in a solution is 7. 65 10 -3 M, what is the [H+] of this solution? a) 7. 65 10 -17 M b) 1. 31 10 -12 M c) 2. 12 M d) 11. 88 M
![1 If the OH in a solution is 7 65 10 3 M what 1. If the [OH-] in a solution is 7. 65 10 -3 M, what](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-38.jpg)
1. If the [OH-] in a solution is 7. 65 10 -3 M, what is the [H+] of this solution? a) 7. 65 10 -17 M b) 1. 31 10 -12 M c) 2. 12 M d) 11. 88 M
![2 The OH for four solutions is given below Which one of the solutions 2. The [OH-] for four solutions is given below. Which one of the solutions](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-39.jpg)
2. The [OH-] for four solutions is given below. Which one of the solutions is basic? a) 1. 0 x 10 -6 M b) 1. 0 x 10 -8 M c) 1. 0 x 10 -7 M d) 1. 0 x 10 -14 M
![2 The OH for four solutions is given below Which one of the solutions 2. The [OH-] for four solutions is given below. Which one of the solutions](https://slidetodoc.com/presentation_image_h2/39a4187a5b18eccdb1c35045c760f287/image-40.jpg)
2. The [OH-] for four solutions is given below. Which one of the solutions is basic? a) 1. 0 x 10 -6 M b) 1. 0 x 10 -8 M c) 1. 0 x 10 -7 M d) 1. 0 x 10 -14 M

3. What is the p. H of a solution with a hydrogen-ion concentration of 8. 5 x 10 -2 M? a) 12. 93 b) 8. 50 c) 5. 50 d) 1. 07

3. What is the p. H of a solution with a hydrogen-ion concentration of 8. 5 x 10 -2 M? a) 12. 93 b) 8. 50 c) 5. 50 d) 1. 07