Acidity and Alkalinity Definitions Alkalinity Measurement Task Acidity




- Slides: 12

Acidity and Alkalinity Definitions Alkalinity Measurement ------ Task -----Acidity Measurement Importance

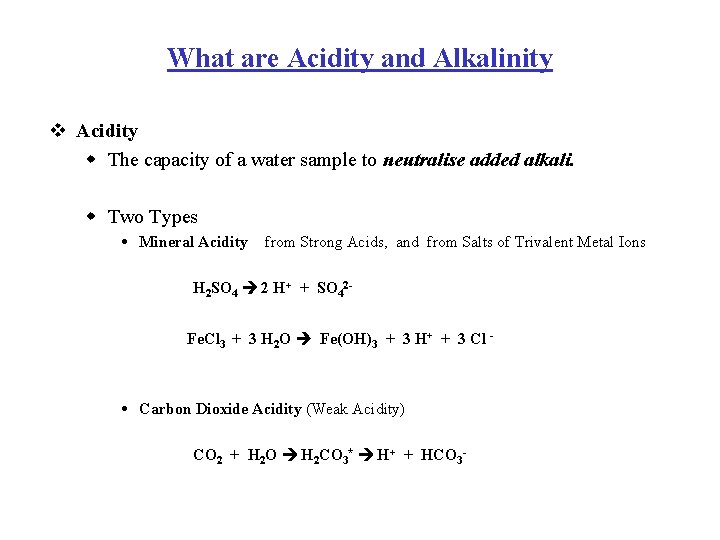
What are Acidity and Alkalinity v Acidity w The capacity of a water sample to neutralise added alkali. w Two Types Mineral Acidity from Strong Acids, and from Salts of Trivalent Metal Ions H 2 SO 4 2 H+ + SO 42 Fe. Cl 3 + 3 H 2 O Fe(OH)3 + 3 H+ + 3 Cl - Carbon Dioxide Acidity (Weak Acidity) CO 2 + H 2 O H 2 CO 3* H+ + HCO 3 -

What are Acidity and Alkalinity v Alkalinity w The capacity of a water sample to neutralise added acid. v Mainly from: 1. The Carbonate - Bicarbonate buffering system. 2. The salts of weak acids CO 2 + Ca. CO 3 + H 2 O Na Acetate, Na Propionate 3. Hydroxide Ammonia Ca 2+ + HCO 3 -

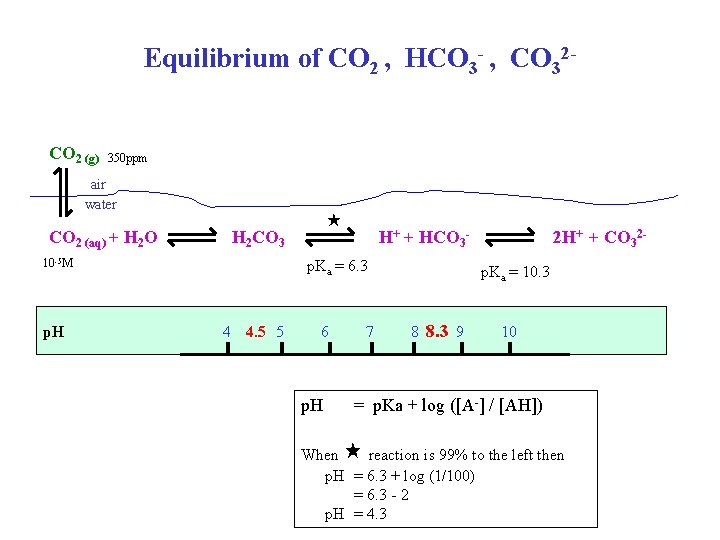
Equilibrium of CO 2 , HCO 3 - , CO 32 CO 2 (g) 350 ppm air water CO 2 (aq) + H 2 O H 2 CO 3 10 -5 M p. H H+ + HCO 3 - p. Ka = 6. 3 4 4. 5 5 6 p. H 7 2 H+ + CO 32 p. Ka = 10. 3 8 8. 3 9 10 = p. Ka + log ([A-] / [AH]) When reaction is 99% to the left then p. H = 6. 3 + log (1/100) = 6. 3 - 2 p. H = 4. 3

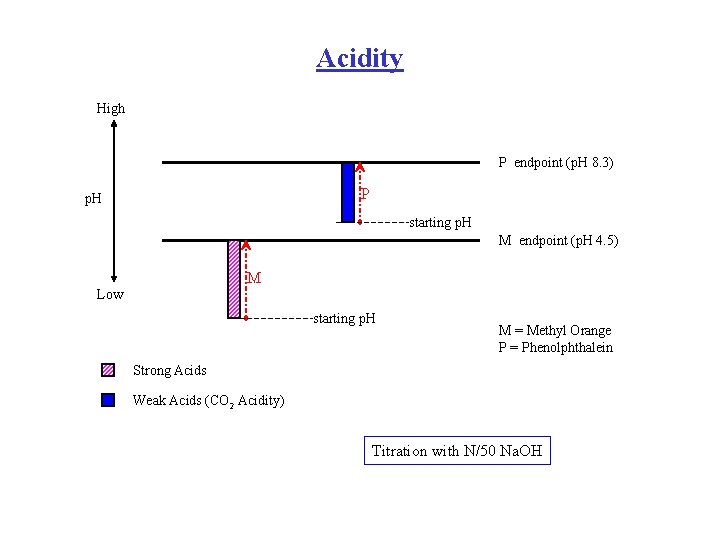
How is Acidity Determined v By titration with a Standard Alkali solution to a specific Endpoint. v Mineral Acidity w Indicator used is Methyl Orange (endpoint at p. H 4. 5 ) v Carbon Dioxide Acidity w Indicator used is Phenolphthalein (endpoint at p. H 8. 3 ) In practice CO 2 is the major weak acid. Others are possible, eg. Acetic

Acidity High P endpoint (p. H 8. 3) P p. H starting p. H M endpoint (p. H 4. 5) M Low starting p. H M = Methyl Orange P = Phenolphthalein Strong Acids Weak Acids (CO 2 Acidity) Titration with N/50 Na. OH

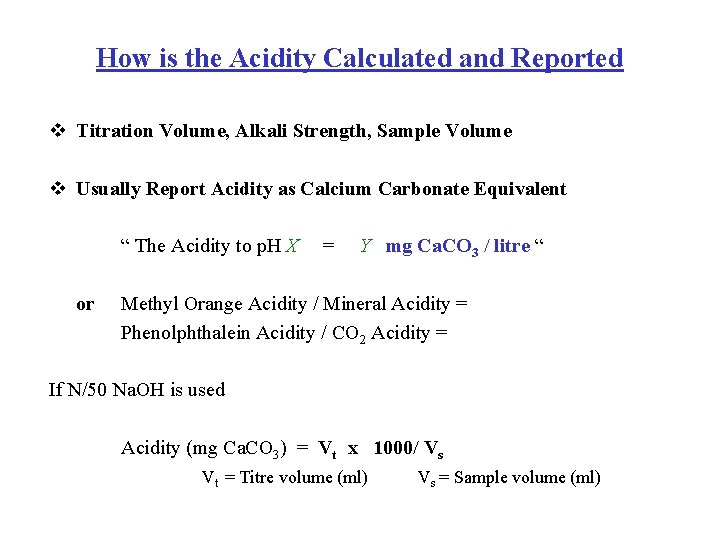
How is the Acidity Calculated and Reported v Titration Volume, Alkali Strength, Sample Volume v Usually Report Acidity as Calcium Carbonate Equivalent “ The Acidity to p. H X or = Y mg Ca. CO 3 / litre “ Methyl Orange Acidity / Mineral Acidity = Phenolphthalein Acidity / CO 2 Acidity = If N/50 Na. OH is used Acidity (mg Ca. CO 3) = Vt x 1000/ Vs Vt = Titre volume (ml) Vs = Sample volume (ml)

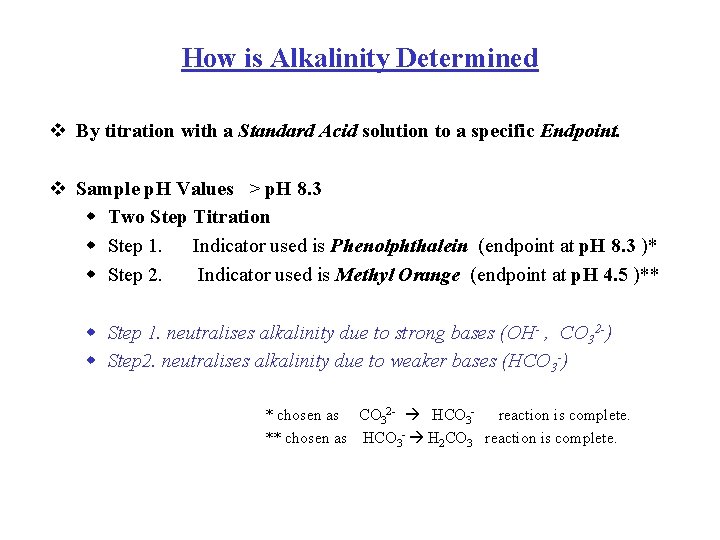
How is Alkalinity Determined v By titration with a Standard Acid solution to a specific Endpoint. v Sample p. H Values > p. H 8. 3 w Two Step Titration w Step 1. Indicator used is Phenolphthalein (endpoint at p. H 8. 3 )* w Step 2. Indicator used is Methyl Orange (endpoint at p. H 4. 5 )** w Step 1. neutralises alkalinity due to strong bases (OH- , CO 32 -) w Step 2. neutralises alkalinity due to weaker bases (HCO 3 -) * chosen as CO 32 - HCO 3 - reaction is complete. ** chosen as HCO 3 - H 2 CO 3 reaction is complete.

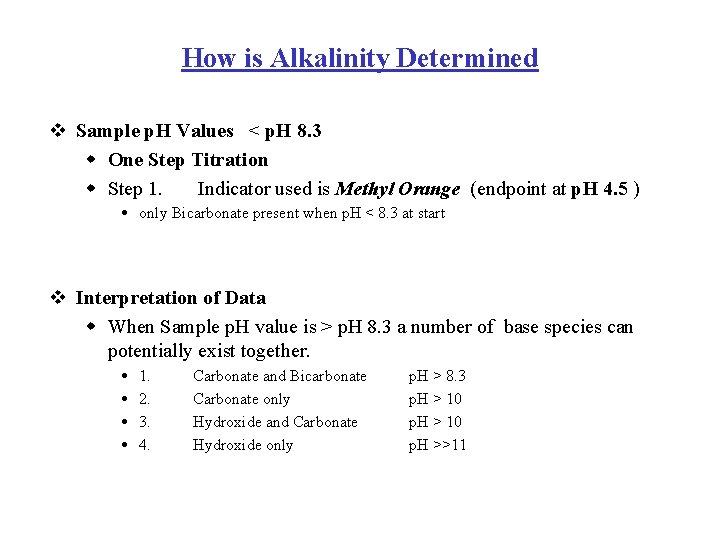
How is Alkalinity Determined v Sample p. H Values < p. H 8. 3 w One Step Titration w Step 1. Indicator used is Methyl Orange (endpoint at p. H 4. 5 ) only Bicarbonate present when p. H < 8. 3 at start v Interpretation of Data w When Sample p. H value is > p. H 8. 3 a number of base species can potentially exist together. 1. 2. 3. 4. Carbonate and Bicarbonate Carbonate only Hydroxide and Carbonate Hydroxide only p. H > 8. 3 p. H > 10 p. H >>11

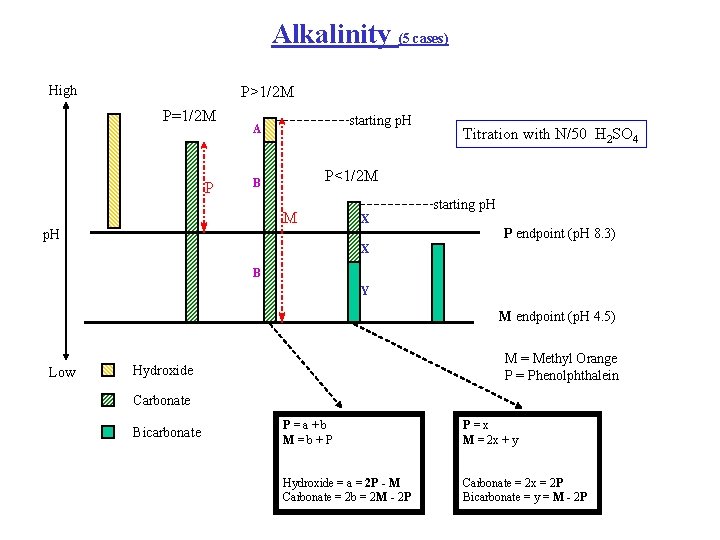
Alkalinity (5 cases) High P>1/2 M P=1/2 M P starting p. H A Titration with N/50 H 2 SO 4 P<1/2 M B M starting p. H X p. H P endpoint (p. H 8. 3) X B Y M endpoint (p. H 4. 5) Low M = Methyl Orange P = Phenolphthalein Hydroxide Carbonate Bicarbonate P=a+b M=b+P P=x M = 2 x + y Hydroxide = a = 2 P - M Carbonate = 2 b = 2 M - 2 P Carbonate = 2 x = 2 P Bicarbonate = y = M - 2 P

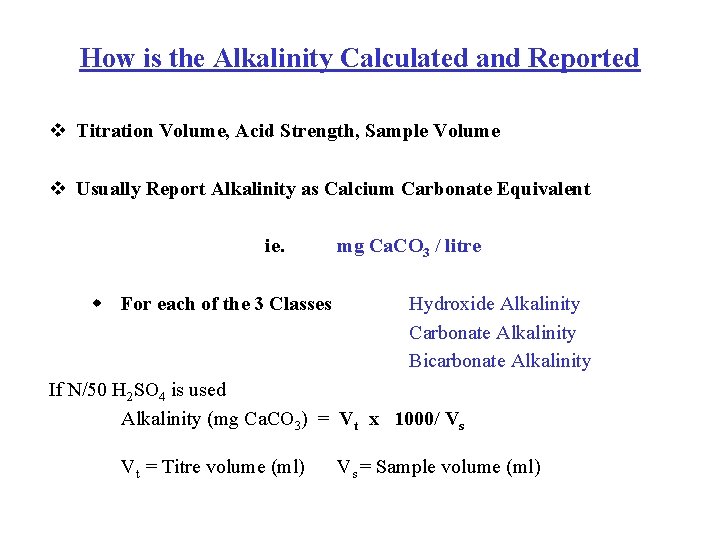
How is the Alkalinity Calculated and Reported v Titration Volume, Acid Strength, Sample Volume v Usually Report Alkalinity as Calcium Carbonate Equivalent ie. w For each of the 3 Classes mg Ca. CO 3 / litre Hydroxide Alkalinity Carbonate Alkalinity Bicarbonate Alkalinity If N/50 H 2 SO 4 is used Alkalinity (mg Ca. CO 3) = Vt x 1000/ Vs Vt = Titre volume (ml) Vs = Sample volume (ml)

Application of Acidity and Alkalinity Data v Acidity w Selection of new Water Supplies minimise treatment costs (Lime, Na. OH) w Industrial Wastewater Metal Pickling Liquor (phosphoric acid) Consent to Discharge v Alkalinity w Calculate safe levels of Ferric and Alum w Biological WWT Plants - gives buffering capacity w Potable - range 50 - 300 mg/l w Industrial - consent to discharge