p H Hydrogen Ions and Acidity Water molecules

![�A can of Coke has [H+]= 2. 6*10 -3 M. What is the concentration �A can of Coke has [H+]= 2. 6*10 -3 M. What is the concentration](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-4.jpg)
![�Calculate the [H+] concentration in each of the following: �[OH-] = 5. 99*10 -8 �Calculate the [H+] concentration in each of the following: �[OH-] = 5. 99*10 -8](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-5.jpg)
![The p. H Concept p. H = -log[H+] �p. H is the negative logarithm The p. H Concept p. H = -log[H+] �p. H is the negative logarithm](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-6.jpg)
![p. H practice �Calculate the p. H of a solution with [H+] = 7. p. H practice �Calculate the p. H of a solution with [H+] = 7.](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-8.jpg)
![�Calculate the p. OH of a solution with [H+] = -5 M. 6. 32*10 �Calculate the p. OH of a solution with [H+] = -5 M. 6. 32*10](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-10.jpg)
- Slides: 17

p. H

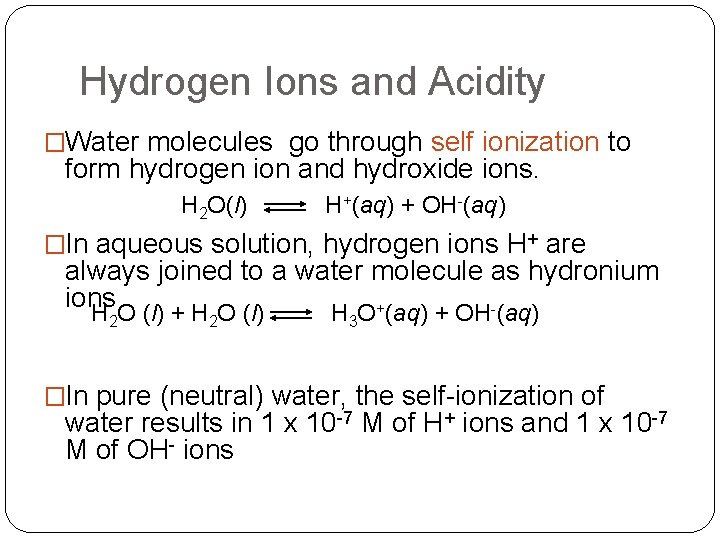
Hydrogen Ions and Acidity �Water molecules go through self ionization to form hydrogen ion and hydroxide ions. H 2 O(l) H+(aq) + OH-(aq) �In aqueous solution, hydrogen ions H+ are always joined to a water molecule as hydronium ions H O (l) + H O (l) H O+(aq) + OH-(aq) 2 2 3 �In pure (neutral) water, the self-ionization of water results in 1 x 10 -7 M of H+ ions and 1 x 10 -7 M of OH- ions

H 2 O (l) + H 2 O (l) H 3 O+(aq) + OH-(aq) �You can write an equation for an equilibrium constant for this!! Keq = [H 3 O+]*[OH-] You can use [H+] as an alternative here a �Called the Kw or the ion product constant �Kw ALWAYS equals 1 x 10 -14 at 25ºC, regardless of what else is in the solution, like an acid or base �In a basic/alkaline solution, the hydroxide ion (OH-) is greater than 1 x 10 -7 M and the hydrogen ion (H+) is less 1 x 10 -7 M �In a acidic solution, the hydrogen ion (H+) is greater than 1 x 10 -7 M and the hydroxide ion (OH-) is less -7
![A can of Coke has H 2 610 3 M What is the concentration �A can of Coke has [H+]= 2. 6*10 -3 M. What is the concentration](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-4.jpg)
�A can of Coke has [H+]= 2. 6*10 -3 M. What is the concentration of OH-? Kw = [H+]*[OH-] 1 x 10 -14 = (2. 6*10 -3 M)* [OH-] 3. 85* 10 -12 = [OH-]
![Calculate the H concentration in each of the following OH 5 9910 8 �Calculate the [H+] concentration in each of the following: �[OH-] = 5. 99*10 -8](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-5.jpg)
�Calculate the [H+] concentration in each of the following: �[OH-] = 5. 99*10 -8 M �[OH-] = 1. 43 * 10 -12 M 1 x 10 -14 = (5. 99*10 -8 M)* -7 = [H+] [H+1. 67* 10 ] 1 x 10 -14 = (1. 43 * 10 -12 M)* -3 = [H+] [H+6. 99* 10 ] KW = [H+]*[OH-]
![The p H Concept p H logH p H is the negative logarithm The p. H Concept p. H = -log[H+] �p. H is the negative logarithm](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-6.jpg)
The p. H Concept p. H = -log[H+] �p. H is the negative logarithm of the hydrogen ion concentration �A neutral solution H+ = 1 x 10 -7 has a p. H = -log[1 x 10 -7]= 7 �A solution in which the [H+] is greater than 1 x 10 -7 M and has a p. H less than 7. 0 is acidic �A solution with a p. H greater than 7 is basic and has a [H+] concentration of less than 1 x 10 -7 M �You can also calculate p. OH which is the negative logarithm of hydroxide ion concentration p. OH = -log[OH-] p. H + p. OH = 14

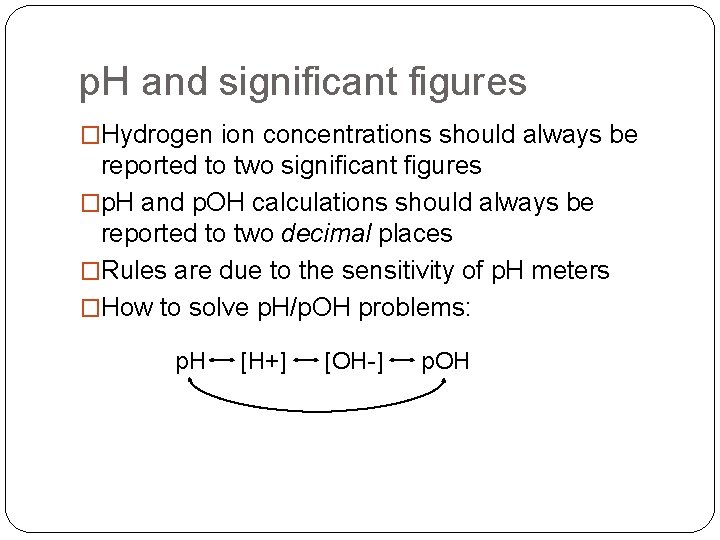
p. H and significant figures �Hydrogen ion concentrations should always be reported to two significant figures �p. H and p. OH calculations should always be reported to two decimal places �Rules are due to the sensitivity of p. H meters �How to solve p. H/p. OH problems: p. H [H+] [OH-] p. OH
![p H practice Calculate the p H of a solution with H 7 p. H practice �Calculate the p. H of a solution with [H+] = 7.](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-8.jpg)
p. H practice �Calculate the p. H of a solution with [H+] = 7. 42*105 M p. H = -log[H+] p. H = -log[7. 42*10 -5] p. H = 4. 13 �Calculate the p. OH of a solution with [OH-] = p. OH = -log[OH-] 4. 21*10 -9 M p. OH = -log[4. 21*10 -9] p. OH = 8. 38 p. H = ? p. H = 5. 62 p. H + p. OH = 14

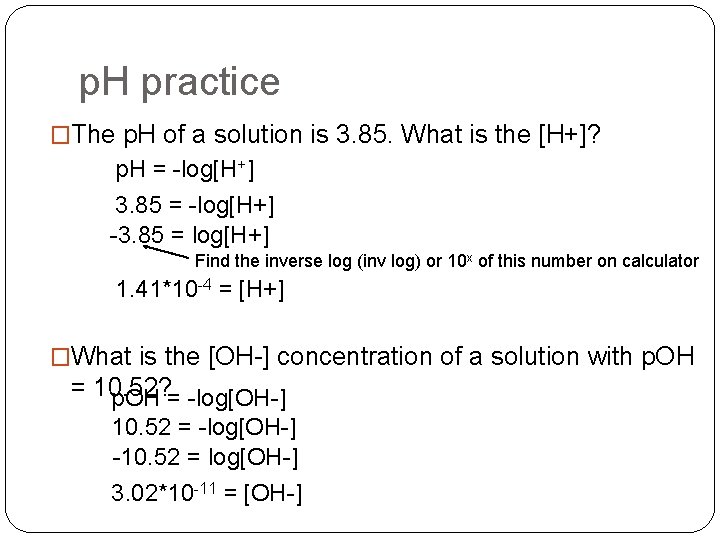
p. H practice �The p. H of a solution is 3. 85. What is the [H+]? p. H = -log[H+] 3. 85 = -log[H+] -3. 85 = log[H+] Find the inverse log (inv log) or 10 x of this number on calculator 1. 41*10 -4 = [H+] �What is the [OH-] concentration of a solution with p. OH = 10. 52? p. OH = -log[OH-] 10. 52 = -log[OH-] -10. 52 = log[OH-] 3. 02*10 -11 = [OH-]
![Calculate the p OH of a solution with H 5 M 6 3210 �Calculate the p. OH of a solution with [H+] = -5 M. 6. 32*10](https://slidetodoc.com/presentation_image/900893e2295f89ba39d1bb55929b7f70/image-10.jpg)
�Calculate the p. OH of a solution with [H+] = -5 M. 6. 32*10 + - Kw = [H ]*[OH ] 1 x 10 -14 = (6. 32*10 -5 M)* [OH-] p. H = -log[H+] p. H = -log[6. 32*10 -5] 1. 58* 10 -10 = [OH-] p. H = 4. 20 p. OH = -log[OH-] p. OH = -log[1. 58* 10 -10 ] p. H + p. OH = 14 p. OH = 9. 80 4. 20 + p. OH = 14

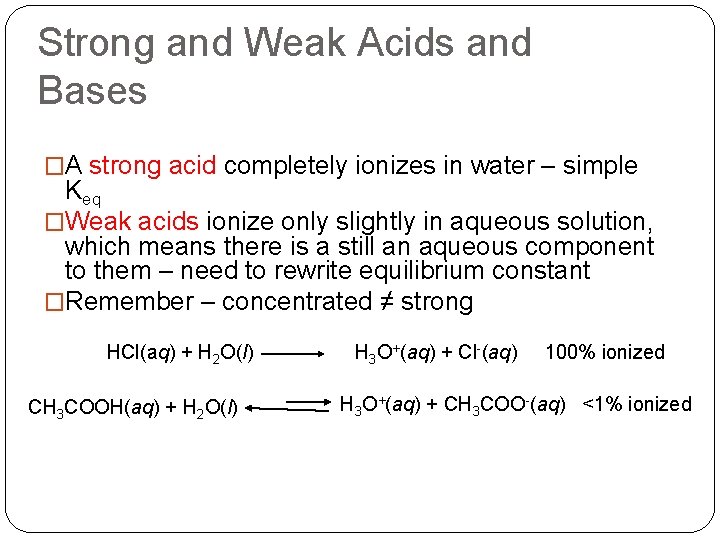
Strong and Weak Acids and Bases �A strong acid completely ionizes in water – simple Keq �Weak acids ionize only slightly in aqueous solution, which means there is a still an aqueous component to them – need to rewrite equilibrium constant �Remember – concentrated ≠ strong HCl(aq) + H 2 O(l) CH 3 COOH(aq) + H 2 O(l) H 3 O+(aq) + Cl-(aq) 100% ionized H 3 O+(aq) + CH 3 COO-(aq) <1% ionized

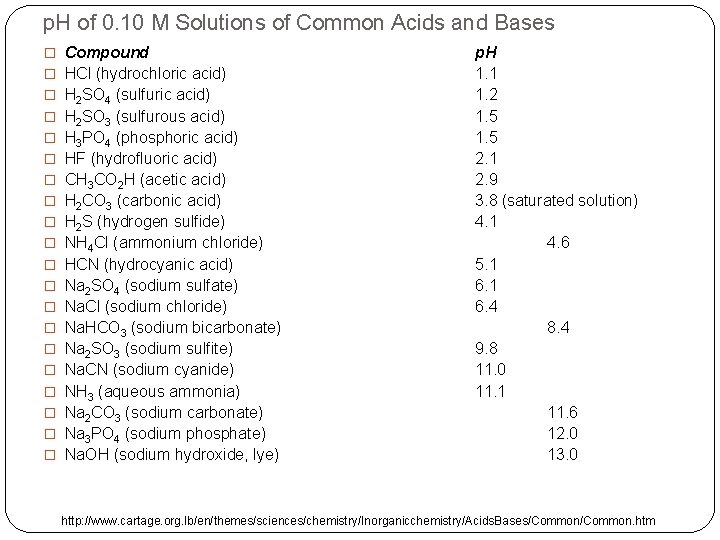
p. H of 0. 10 M Solutions of Common Acids and Bases � Compound � HCl (hydrochloric acid) � H 2 SO 4 (sulfuric acid) � H 2 SO 3 (sulfurous acid) � H 3 PO 4 (phosphoric acid) � HF (hydrofluoric acid) � CH 3 CO 2 H (acetic acid) � H 2 CO 3 (carbonic acid) � H 2 S (hydrogen sulfide) � NH 4 Cl (ammonium chloride) � HCN (hydrocyanic acid) � Na 2 SO 4 (sodium sulfate) � Na. Cl (sodium chloride) � Na. HCO 3 (sodium bicarbonate) � Na 2 SO 3 (sodium sulfite) � Na. CN (sodium cyanide) � NH 3 (aqueous ammonia) � Na 2 CO 3 (sodium carbonate) � Na 3 PO 4 (sodium phosphate) � Na. OH (sodium hydroxide, lye) p. H 1. 1 1. 2 1. 5 2. 1 2. 9 3. 8 (saturated solution) 4. 1 4. 6 5. 1 6. 4 8. 4 9. 8 11. 0 11. 1 11. 6 12. 0 13. 0 http: //www. cartage. org. lb/en/themes/sciences/chemistry/Inorganicchemistry/Acids. Bases/Common. htm

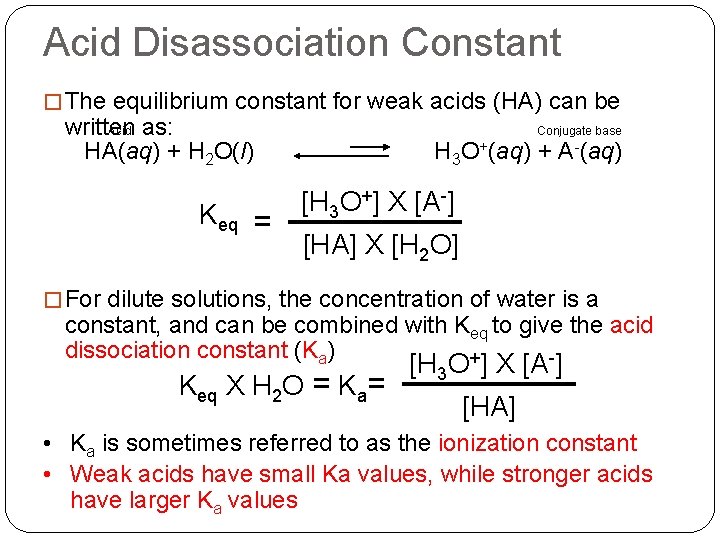
Acid Disassociation Constant � The equilibrium constant for weak acids (HA) can be written as: Acid HA(aq) + H 2 O(l) Keq = Conjugate base H 3 O+(aq) + A-(aq) [H 3 O+] X [A-] [HA] X [H 2 O] � For dilute solutions, the concentration of water is a constant, and can be combined with Keq to give the acid dissociation constant (Ka) + - Keq X H 2 O = Ka= [H 3 O ] X [A ] [HA] • Ka is sometimes referred to as the ionization constant • Weak acids have small Ka values, while stronger acids have larger Ka values

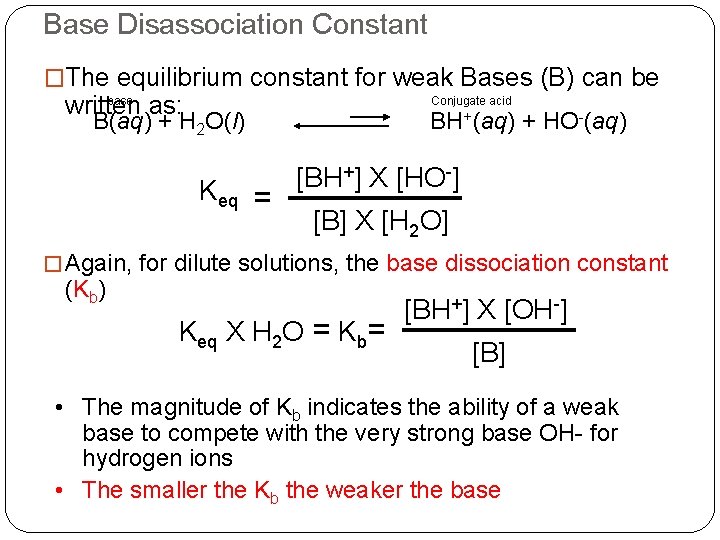
Base Disassociation Constant �The equilibrium constant for weak Bases (B) can be base written as: Conjugate acid BH+(aq) + HO-(aq) B(aq) + H 2 O(l) Keq = [BH+] X [HO-] [B] X [H 2 O] � Again, for dilute solutions, the base dissociation constant (Kb) Keq X H 2 O = Kb= [BH+] X [OH-] [B] • The magnitude of Kb indicates the ability of a weak base to compete with the very strong base OH- for hydrogen ions • The smaller the Kb the weaker the base

Calculating Dissociation Constants �Problems involving Ka of weak acid or the Kb of a weak base: 1. Write the expression for Ka or Kb using the actual compounds in the chemical equation. Rearrange for the unknown variable. 2. Substitute the known numbers of all the variables at equilibrium into the expression for Ka or Kb. 3. Solve for the unknown.

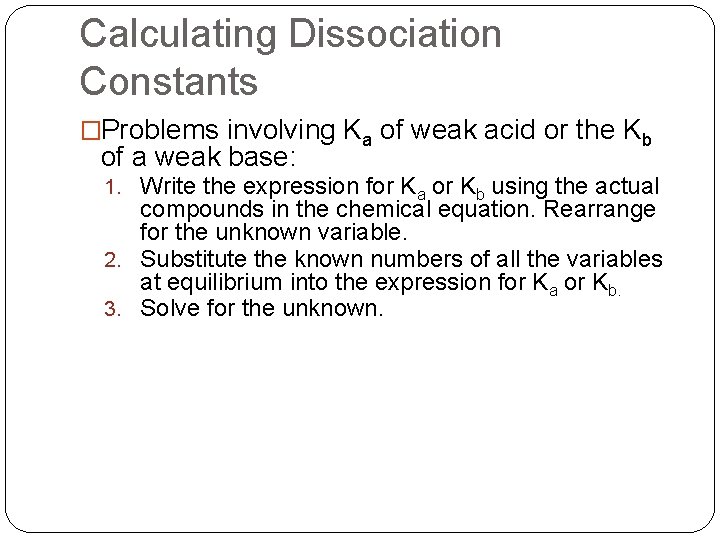
� A 0. 1000 M solution of acetic acid is only partially ionized and has a p. H of 2. 87. What is the acid dissociation constant (Ka) or ethanoic acid? HC 2 H 3 O 2(aq) + H 2 O(l) Ka = 1. 82*10 -5 H 3 O+(aq) + C 2 H 3 O 2 -(aq) p. H = -log[H+] 2. 87 = -log[H+] -2. 87 = log[H+] 1. 35*10 -3 = [H+]= [C 2 H 3 O 2 -]

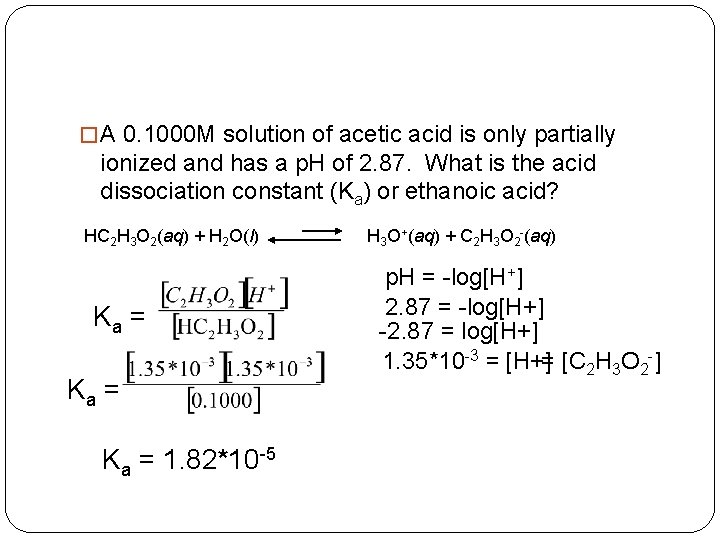
�The Ka of nitrous acid is 4. 0 *10 -4. What is the concentration of [H+] in a 0. 85 M solution of nitrous acid? What is the p. H? HNO 2(aq) + H 2 O(l) H 3 O+(aq) + NO 2 -(aq) Ka = Ka * [HNO 2] = [NO 2 -]*[H+] Ka * [HNO 2] = x*x = x 2 1. 84*10 -2= x = [H+] p. H = -log[1. 84*10 -2] p. H = 1. 73