Roshan Manasia POST CARDIAC ARREST SYNDROME AND POST



























- Slides: 59

Roshan Manasia POST CARDIAC ARREST SYNDROME AND POST ROSC CARE

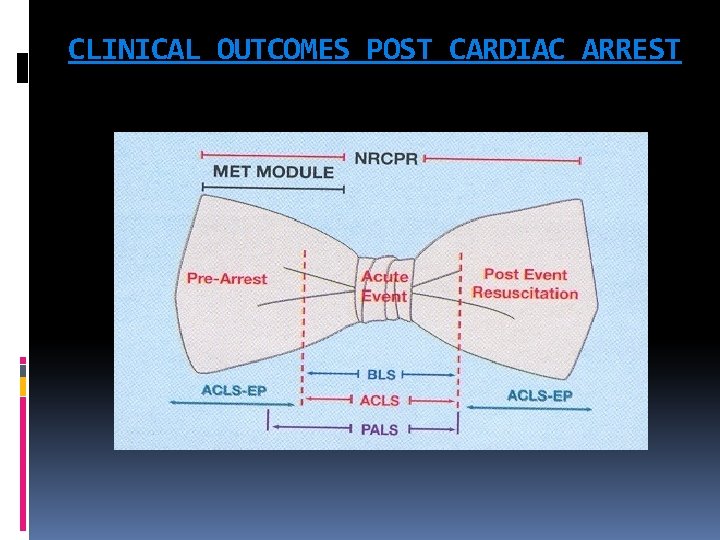
CLINICAL OUTCOMES POST CARDIAC ARREST

CLINICAL OUTCOMES POST ARREST The first large multicentre report on patients treated for cardiac arrest was published in 1953. The in-hospital mortality rate for the 672 adults and children whose ‘‘heart beat was restarted’’ was 50%. (Stephenson et al. , 1953) In Japan, one study reported that patients with ROSC after witnessed out-of-hospital cardiac arrest of presumed cardiac origin had an in-hospital mortality rate of 90% (Mashiko et al, 2002)

CLINICAL OUTCOMES POST ARREST In-hospital mortality rates for patients with out-ofhospital cardiac arrest who were taken to 4 different hospitals in Norway averaged 63% (range 54– 70%) for patients with ROSC, 57% (range 56– 70%) for patients arriving in the emergency department (ED) with a pulse, and 50% (range 41– 62%) for patients admitted to the hospital. (Langhelle et al. , 2003) al. , 2002). In a comprehensive review of nontraumatic out-of-hospital cardiac arrest in children, the overall rate of ROSC was 22. 8%, and the rate of survival to discharge was 6. 7%, resulting in a calculated post-ROSC mortality rate of 70% (Donoghue et al. , 2005).

CLINICAL OUTCOMES POST ARREST In Sweden the 1 -month mortality rate for 3853 patients admitted with a pulse to 21 hospitals after out-ofhospital cardiac arrest ranged from 58% to 86% (Herlitz et al. , 2006). The largest modern report of cardiac arrest epidemiology was published by the National Registry of CPR in 2006. Among the 19, 819 adults and 524 children who regained any spontaneous circulation, in-hospital mortality rates were 67% and 55%, respectively. (Nadkarni et al. , 2006).

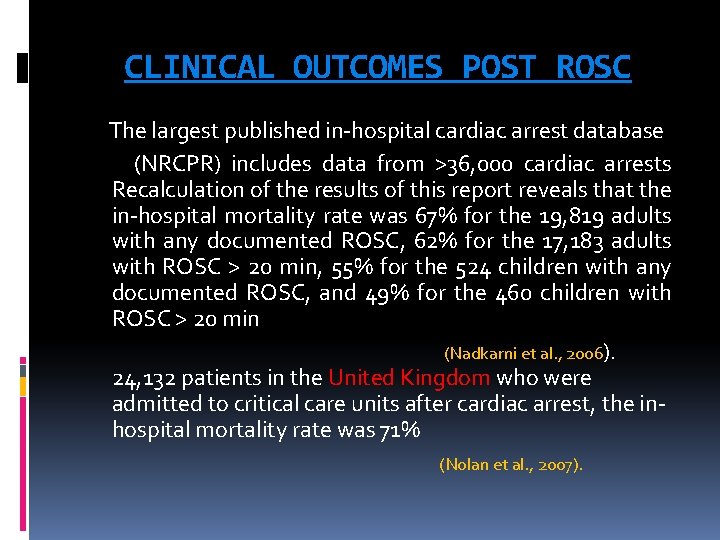
CLINICAL OUTCOMES POST ROSC The largest published in-hospital cardiac arrest database (NRCPR) includes data from >36, 000 cardiac arrests Recalculation of the results of this report reveals that the in-hospital mortality rate was 67% for the 19, 819 adults with any documented ROSC, 62% for the 17, 183 adults with ROSC > 20 min, 55% for the 524 children with any documented ROSC, and 49% for the 460 children with ROSC > 20 min (Nadkarni et al. , 2006). 24, 132 patients in the United Kingdom who were admitted to critical care units after cardiac arrest, the inhospital mortality rate was 71% (Nolan et al. , 2007).

CLINICAL OUTCOMES POST ROSC Data from the Canadian Critical Care Research Network indicates a 65% in-hospital mortality rate for 1483 patients admitted to the intensive care unit (ICU) after out-of-hospital arrest. (Keenan et al. , 2007) In the United Kingdom, 71. 4% of 8987 patients admitted to the ICU after out-of-hospital cardiac arrest died before being discharged from the hospital. (Nolan et al. , 2007).

RETURN OF SPONTANEOUS CIRCULATION (ROSC) ROSC is defined as a brief (approximately >30 s) return of pulse or spontaneous circulation sustained for >20 min

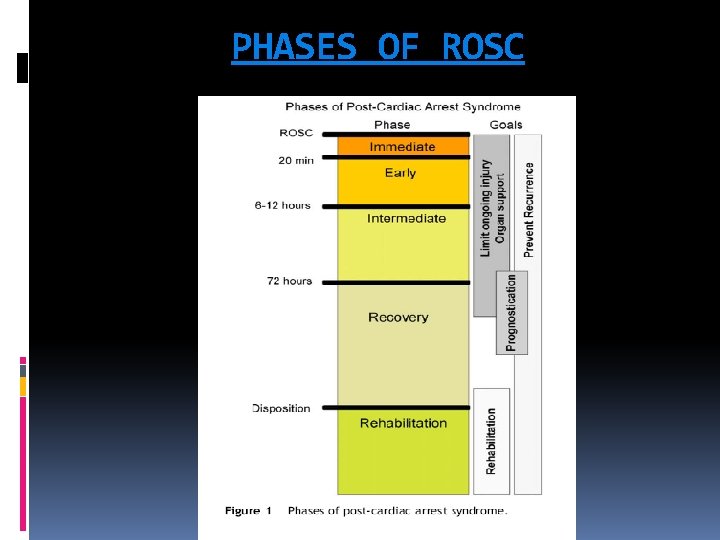
PHASES OF ROSC

POST CARDIAC ARREST SYNDROME Post-cardiac arrest syndrome is a unique and complex combination of pathophysiological processes including v Post-cardiac arrest brain injury v Post-cardiac arrest myocardial dysfunction v Systemic ischaemia/reperfusion response. v Persistent precipitating pathology If ROSC is rapidly achieved after onset of cardiac arrest, the post-cardiac arrest syndrome will not occur.

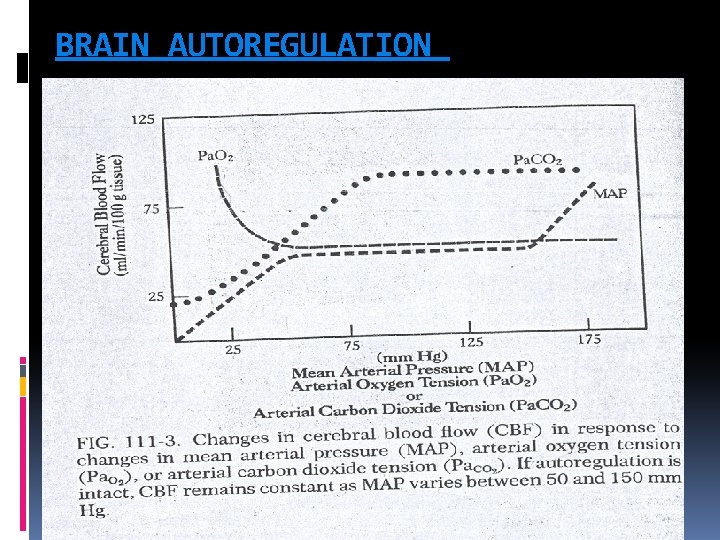
BRAIN AUTOREGULATION

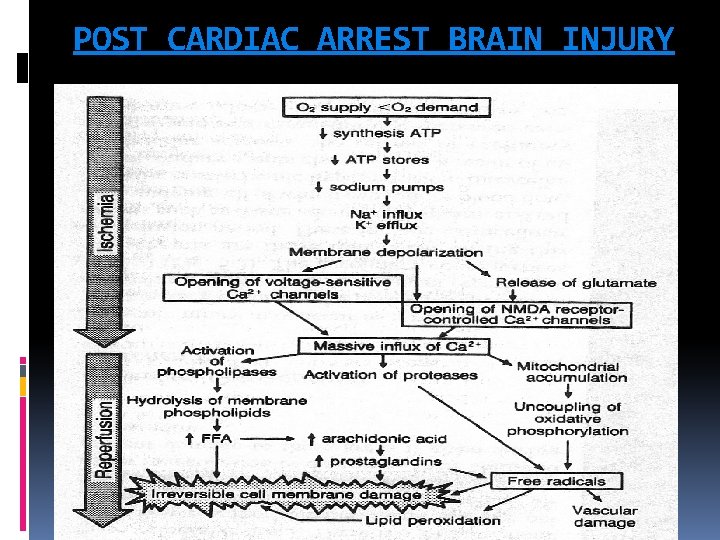
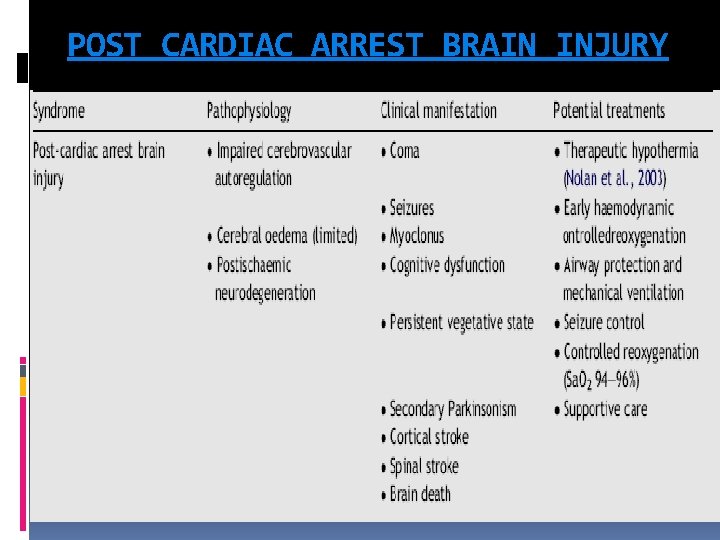
POST CARDIAC ARREST BRAIN INJURY

POST CARDIAC ARREST BRAIN INJURY The unique vulnerability of the brain is attributed to its limited tolerance of ischaemia as well as its unique response to reperfusion. The mechanisms of brain injury triggered by cardiac arrest and resuscitation are complex and include excitotoxicity, disrupted calcium homeostasis, free radical formation, pathological protease cascades, and activation of cell death signaling pathways. Both neuronal necrosis and apoptosis have been reported after cardiac arrest. Prolonged cardiac arrest can also be followed by fixed and/or dynamic failure of cerebral microcirculatory reperfusion despite adequate cerebral perfusion pressure (CPP). This impaired reflow can cause persistent ischaemia and small infarctions in some brain regions.

POST CARDIAC ARREST BRAIN INJURY Although resumption of oxygen and metabolic substrate delivery at the microcirculatory level is essential, a growing body of evidence suggests that too much oxygen during the initial stages of reperfusion can exacerbate neuronal injury through production of free radicals, nitric oxide, catecholamines, cytokines, and calcium shifts, which all lead to mitochondrial damage and cell death. This process may last as long as 24 to 48 hours. Despite cerebral microcirculatory failure, macroscopic reperfusion is often hyperaemic in the first few minutes after cardiac arrest because of elevated CPP and impaired cerebrovascular autoregulation These high initial perfusion pressures can theoretically minimize impaired reflow. Yet hyperaemic reperfusion can potentially exacerbate brain edema and reperfusion injury. The first 24— 48 h after resuscitation from cardiac arrest, there is increased cerebral vascular resistance, decreased CBF, decreased cerebral metabolic rate of oxygen consumption (CMRO 2), and decreased glucose consumption

POST CARDIAC ARREST BRAIN INJURY Beyond the initial reperfusion phase, several factors can potentially compromise cerebral oxygen delivery and possibly secondary injury in the hours to days after cardiac arrest. These include hypotension, hypoxaemia, impaired cerebrovascular autoregulation, brain oedema, pyrexia, hypergycemia, seizures There is limited evidence that brain oedema or elevated intracranial pressure (ICP) directly exacerbates post-cardiac arrest brain injury. Although transient brain oedema is observed early after ROSC, most commonly after asphyxial cardiac arrest, it is rarely associated with clinically relevant increases in ICP. In contrast, delayed brain oedema, occurring days to weeks after cardiac arrest, has been attributed to delayed hyperaemia; this is more likely the consequence rather than the cause of severe ischaemic neuro degeneration

POST CARDIAC ARREST BRAIN INJURY Hyperglycaemia is common in post-cardiac arrest patients and is associated with poor neurological outcome. elevated post ischaemic blood glucose concentrations exacerbate ischaemic brain injury. Seizures in the post-cardiac arrest period are associated with worse prognosis and are likely to be caused by, as well as exacerbate, post-cardiac arrest brain injury. In a small case series, patients with temperatures >39 ◦C in the first 72 h after out-of-hospital cardiac arrest had a significantly increased risk of brain death. the risk of unfavorable outcome is increased for every degree Celsius that the peak temperature exceeded 37 ◦C. Maximal recorded temperature >37. 8 ◦C was associated with increased in-hospital mortality

POST CARDIAC ARREST BRAIN INJURY

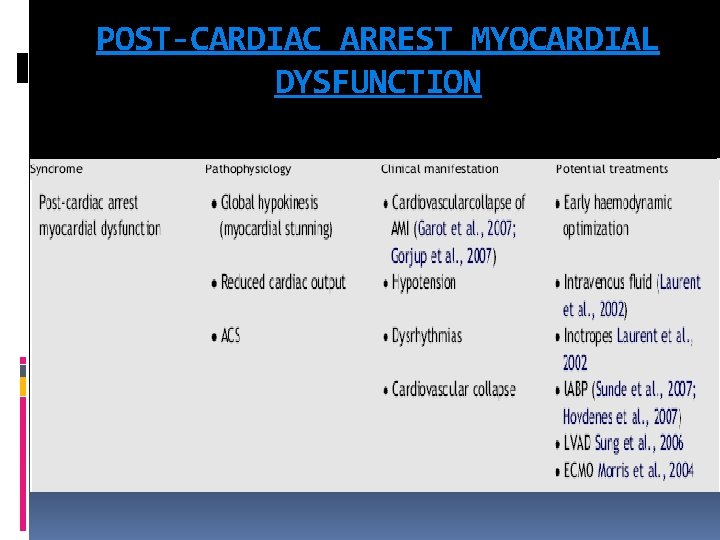
POST-CARDIAC ARREST MYOCARDIAL DYSFUNCTION

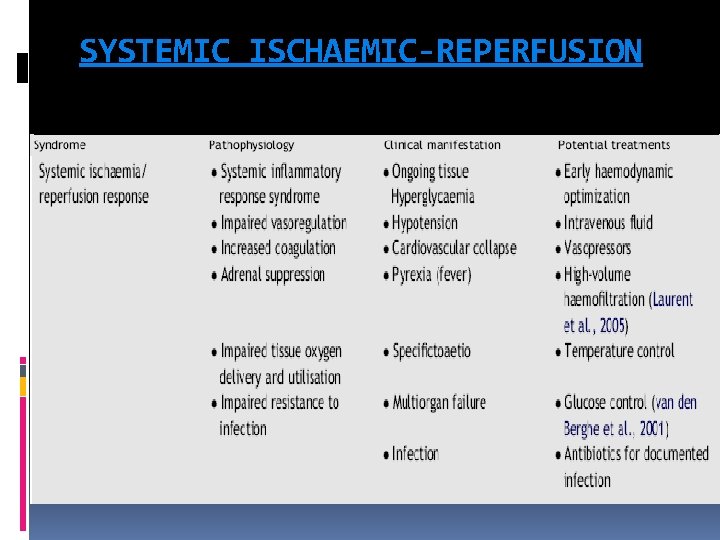
SYSTEMIC ISCHAEMIC-REPERFUSION

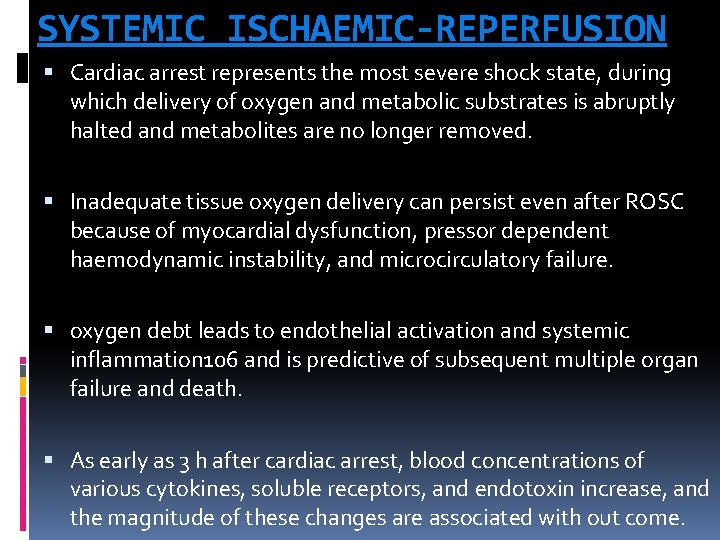
SYSTEMIC ISCHAEMIC-REPERFUSION Cardiac arrest represents the most severe shock state, during which delivery of oxygen and metabolic substrates is abruptly halted and metabolites are no longer removed. Inadequate tissue oxygen delivery can persist even after ROSC because of myocardial dysfunction, pressor dependent haemodynamic instability, and microcirculatory failure. oxygen debt leads to endothelial activation and systemic inflammation 106 and is predictive of subsequent multiple organ failure and death. As early as 3 h after cardiac arrest, blood concentrations of various cytokines, soluble receptors, and endotoxin increase, and the magnitude of these changes are associated with out come.

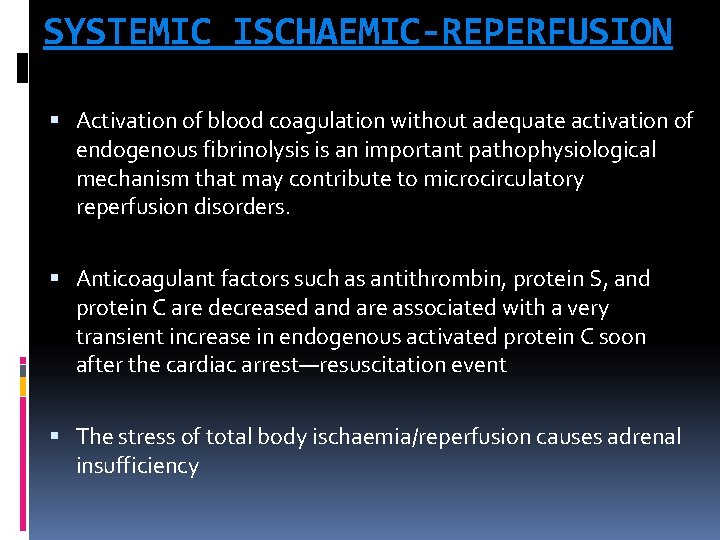
SYSTEMIC ISCHAEMIC-REPERFUSION Activation of blood coagulation without adequate activation of endogenous fibrinolysis is an important pathophysiological mechanism that may contribute to microcirculatory reperfusion disorders. Anticoagulant factors such as antithrombin, protein S, and protein C are decreased and are associated with a very transient increase in endogenous activated protein C soon after the cardiac arrest—resuscitation event The stress of total body ischaemia/reperfusion causes adrenal insufficiency

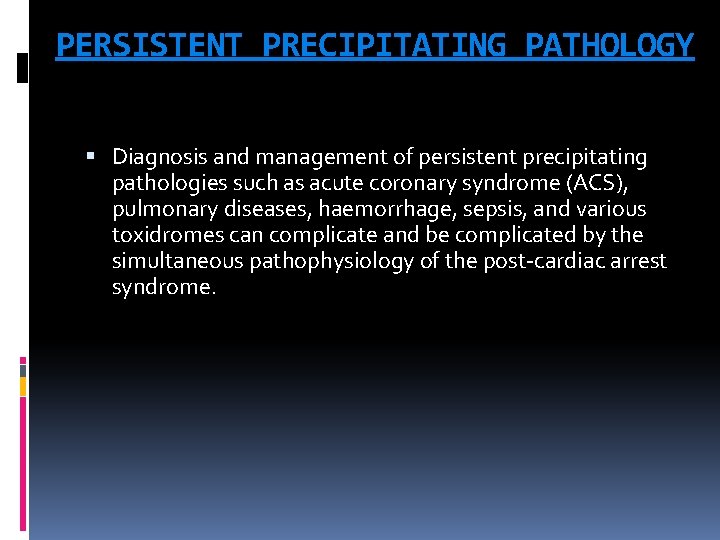
PERSISTENT PRECIPITATING PATHOLOGY Diagnosis and management of persistent precipitating pathologies such as acute coronary syndrome (ACS), pulmonary diseases, haemorrhage, sepsis, and various toxidromes can complicate and be complicated by the simultaneous pathophysiology of the post-cardiac arrest syndrome.

POST ROSC CARE Post ROSC protocol Post arrest therapeutic hypothermia

COOL BODY SAVES BRAIN

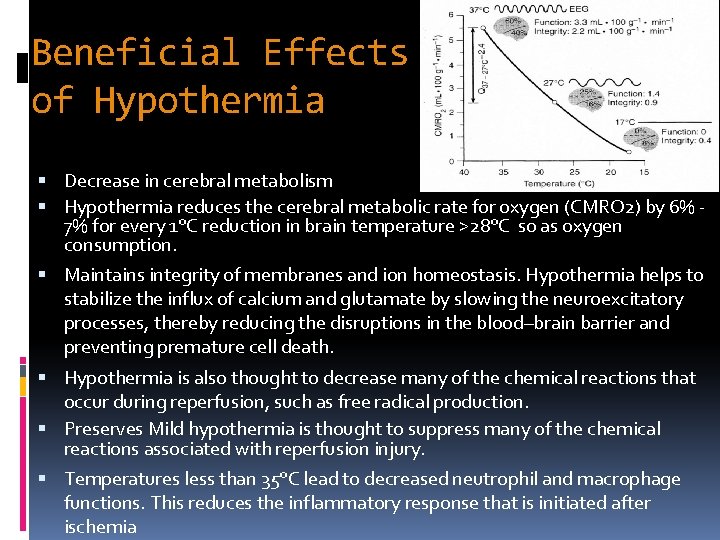
Beneficial Effects of Hypothermia Decrease in cerebral metabolism Hypothermia reduces the cerebral metabolic rate for oxygen (CMRO 2) by 6% 7% for every 1°C reduction in brain temperature >28°C so as oxygen consumption. Maintains integrity of membranes and ion homeostasis. Hypothermia helps to stabilize the influx of calcium and glutamate by slowing the neuroexcitatory processes, thereby reducing the disruptions in the blood–brain barrier and preventing premature cell death. Hypothermia is also thought to decrease many of the chemical reactions that occur during reperfusion, such as free radical production. Preserves Mild hypothermia is thought to suppress many of the chemical reactions associated with reperfusion injury. Temperatures less than 35°C lead to decreased neutrophil and macrophage functions. This reduces the inflammatory response that is initiated after ischemia

Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia Bernard SA, et al. 2002 – Melbourne, Australia N Engl J Med 2002; 346: 557 -63.

Aussie Arrest – Continued… Multicenter r. CT 77 Pts who remained unconscious s/p out of hospital cardiac arrest (V-fib @ scene). Randomized (by day) to hypothermia group (33 o. C 2 hrs after return of spont circulation, maintained for 12 hours) or normothermia. Outcome: survival to hospital discharge with sufficiently good neurologic function to be discharged to home or to a rehabilitation facility.

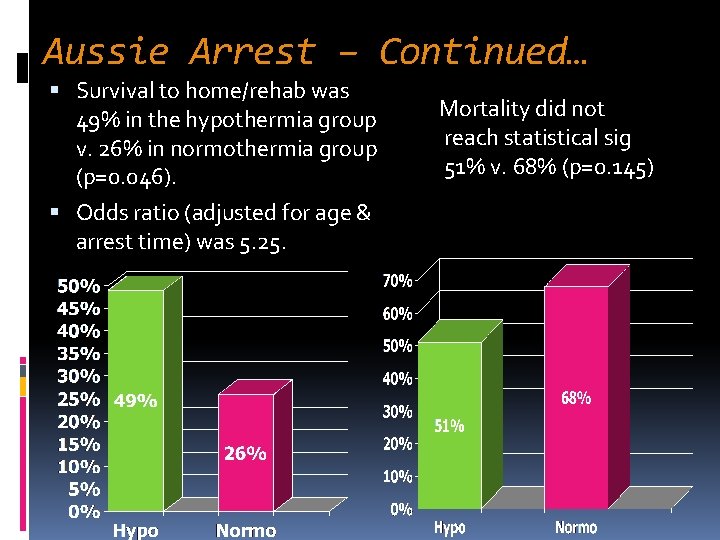
Aussie Arrest – Continued… Survival to home/rehab was 49% in the hypothermia group v. 26% in normothermia group (p=0. 046). Odds ratio (adjusted for age & arrest time) was 5. 25. Mortality did not reach statistical sig 51% v. 68% (p=0. 145)

Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest Michael Holzer, The Hypothermia after Cardiac Arrest Study Group 2002 – Vienna, Austria N Engl J Med 2002; 346: 549 -56.

Euro Arrest – Continued… Multictr RCT, Blinded assessment outcome. 275 Pts s/p witnessed V-fib arrest randomized to hypothermia (32 -34 o. C) x 24 hrs v. normothermia (37 -38 o. C). Primary endpoint: favorable neuro outcome* w/in 6 mo; secondary: 6 mo mortality & 7 day complication rate. * Pittsburgh cerebral-performance category, 1 [good recovery] or 2 [moderate disability]

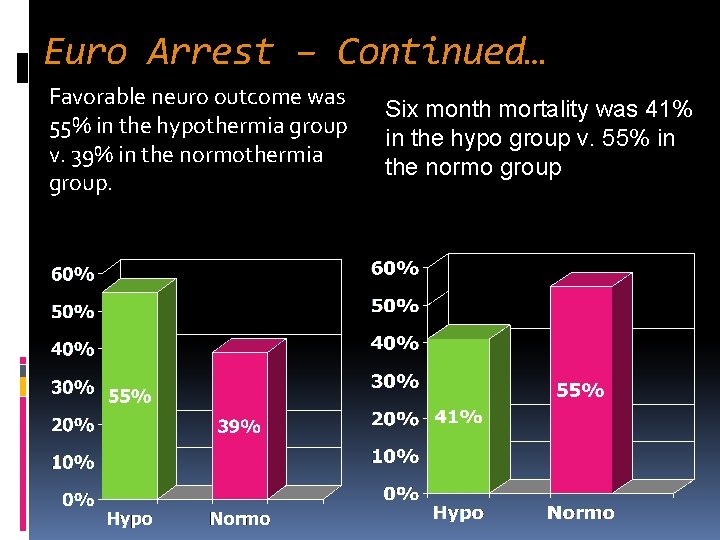
Euro Arrest – Continued… Favorable neuro outcome was 55% in the hypothermia group v. 39% in the normothermia group. Six month mortality was 41% in the hypo group v. 55% in the normo group

Inclusion Criteria Adult patients (over age of 18 years) whose initial cardiac arrest rhythm was Ventricular Fibrillation (VF) or Pulseless Ventricular Tachycardia (VT). Patients who had Pulseless Electrical Activity (PEA) and Asystolic arrest may also benefit from therapeutic hypothermia and should be considered for therapy. ROSC following CPR within 60 minutes of collapse. Persistent coma following ROSC. It is defined as inability to follow commands which is not attributed to pre-cardiac arrest medical condition or GCS of < 8.

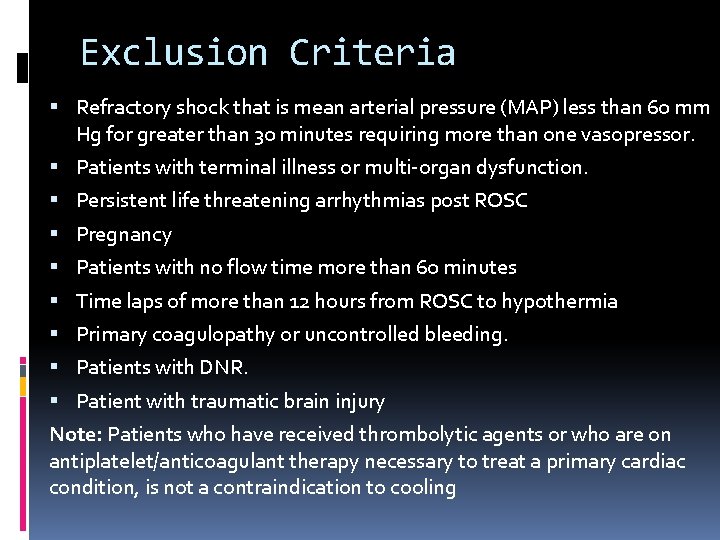
Exclusion Criteria Refractory shock that is mean arterial pressure (MAP) less than 60 mm Hg for greater than 30 minutes requiring more than one vasopressor. Patients with terminal illness or multi-organ dysfunction. Persistent life threatening arrhythmias post ROSC Pregnancy Patients with no flow time more than 60 minutes Time laps of more than 12 hours from ROSC to hypothermia Primary coagulopathy or uncontrolled bleeding. Patients with DNR. Patient with traumatic brain injury Note: Patients who have received thrombolytic agents or who are on antiplatelet/anticoagulant therapy necessary to treat a primary cardiac condition, is not a contraindication to cooling

General guidelines The patient must be on mechanical ventilation and on continuous cardiac monitoring. Cooling should be initiated as soon as possible, preferably within 6 hours of ROSC. Patient should be cooled as soon as possible to achieve the target temperature of 32 C – 34 C ( 33 C) within 4 hours of initiation of initiating cooling measures. Target temperature should be maintained for 24 hours, with time beginning once patient reaches the goal temperature. To optimize the positive outcomes of the hypothermia take measures to maintain MAP 65 -100 mm. Hg; urine output > 0. 5 ml/kg/hr; CVP 8 -12 mm. Hg; O 2 saturation 94%-96%; control hyperglycemia following target blood sugar less than 150 mm. Hg.

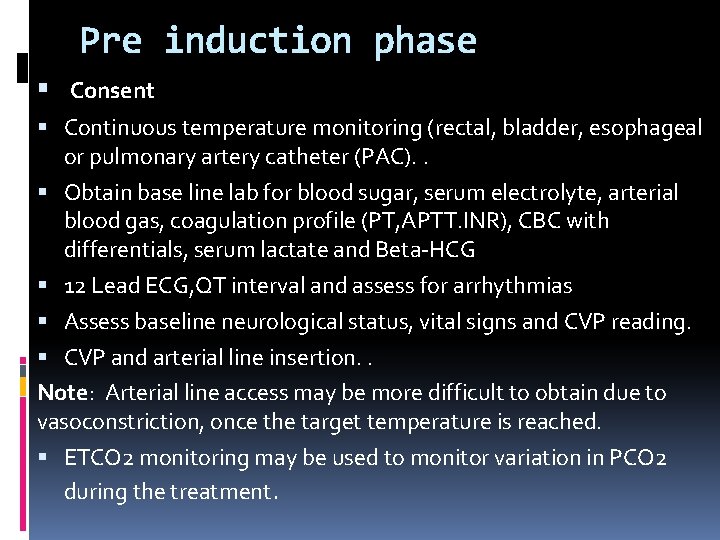
Pre induction phase Consent Continuous temperature monitoring (rectal, bladder, esophageal or pulmonary artery catheter (PAC). . Obtain base line lab for blood sugar, serum electrolyte, arterial blood gas, coagulation profile (PT, APTT. INR), CBC with differentials, serum lactate and Beta-HCG 12 Lead ECG, QT interval and assess for arrhythmias Assess baseline neurological status, vital signs and CVP reading. CVP and arterial line insertion. . Note: Arterial line access may be more difficult to obtain due to vasoconstriction, once the target temperature is reached. ETCO 2 monitoring may be used to monitor variation in PCO 2 during the treatment.

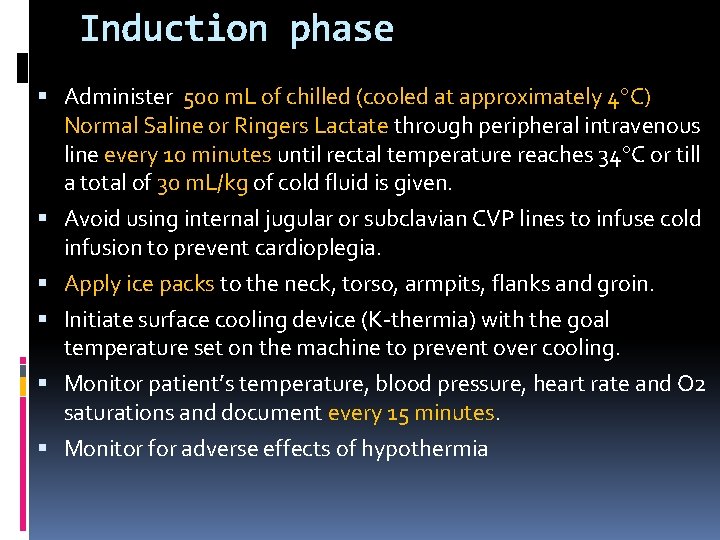
Induction phase Administer 500 m. L of chilled (cooled at approximately 4 C) Normal Saline or Ringers Lactate through peripheral intravenous line every 10 minutes until rectal temperature reaches 34 C or till a total of 30 m. L/kg of cold fluid is given. Avoid using internal jugular or subclavian CVP lines to infuse cold infusion to prevent cardioplegia. Apply ice packs to the neck, torso, armpits, flanks and groin. Initiate surface cooling device (K-thermia) with the goal temperature set on the machine to prevent over cooling. Monitor patient’s temperature, blood pressure, heart rate and O 2 saturations and document every 15 minutes. Monitor for adverse effects of hypothermia

Assess patient for shivering every hour using following Bed Side Shivering Assessment Scale (BSAS). Notify physician if score is more than 0. Patient should be administered opioids analgesia (fentanyl, morphine sulphate, pethedine) and hypnotics (propofol) or benzodiazepine (midazolam) to prevent shivering. If shivering occurs despite optimal sedation, neuromuscular blocking agent (atracurium, pancuronium, vecuronium) as a bolus or infusion should be considered SCORE 0 None DEFINITION No shivering noted on palpation of the masseter, neck or chest wall. 1 Shivering localized to neck and/or thorax only. May only be Mild noticed on palpation. 2 Shivering involves gross muscle movement of upper Moderate extremities in addition to neck and thorax. 3 Shivering involves gross movement of the trunk and upper and Severe

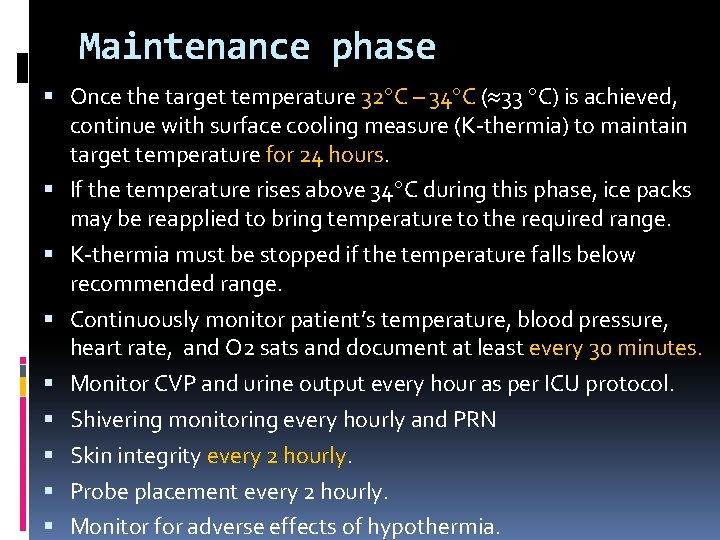
Maintenance phase Once the target temperature 32 C – 34 C ( 33 C) is achieved, continue with surface cooling measure (K-thermia) to maintain target temperature for 24 hours. If the temperature rises above 34 C during this phase, ice packs may be reapplied to bring temperature to the required range. K-thermia must be stopped if the temperature falls below recommended range. Continuously monitor patient’s temperature, blood pressure, heart rate, and O 2 sats and document at least every 30 minutes. Monitor CVP and urine output every hour as per ICU protocol. Shivering monitoring every hourly and PRN Skin integrity every 2 hourly. Probe placement every 2 hourly. Monitor for adverse effects of hypothermia.

Reflo Monitor blood glucose at least every 2 hourly as per patient’s baseline or ICU protocol. If hyperglycemia develops, monitor blood glucose every hou PT/APTT/INR/Platelet Every 12 hourly CBC Every day Amylase and Lipase Once per day Electrolytes Check electrolyte every 6 hour QTc interval Every 4 hourly

Rewarming phase Begins upon completion of 24 hours of maintenance phase. Allow patient to rewarm passively to a temperature of 36 C, it should not be faster than 0. 25 - 0. 5 C per hour. Monitor patient’s temperature, blood pressure, heart rate, and O 2 sats and document every 30 minutes. Use of K-thermia or bair hugger should only be reserved if temperature does not rise in initial 6 hours of rewarming phase. Kthermia/Bair Hugger and all other measures must be stopped once patient’s temperature reaches 36 C. Monitor for adverse effects that is hypotension, cerebral edema, ICP, arrhythmias, and shift of electrolytes back into the plasma Efforts must be made to prevent pyrexia (38 C) during initial 72 hours from the time of cardiac arrest.

Discontinuation of hypothermia Hemodynamically unstable arrhythmia that is refractory to the treatment. Severe bradycardia with hemodynamic compromise (less than 40 beats per minute). Severe bleeding If hypothermia is aborted rewarming should not be faster than 0. 25 - 0. 5 C per hour.

ADVERSE EFFECTS OF THERAPEUTIC HYPOTHERMIA

Shivering It increases metabolic rate and oxygen consumption and makes it difficult to achieve target temperature. Shivering is less likely to occur during the maintenance and warming phases. Signs and symptoms of shivering include a drop in mixed venous oxygen saturation, increase in RR, facial tensing, artifacts on ECG and palpation of muscle fasciculations on the face or chest. Bedside shivering assessment scale (Target is 0)

Management of Shivering Analgesia and sedation. Sedation and analgesic drugs, such as midazolam, fentanyl, and propofol, have a 30% to 50% decrease in systemic clearance during hypothermia. Vecuronium and atracurium, clearance is decreased 10% for every 1 C below 37 C. Thus, their duration of action is also increased. During hypothermia, the amount of drug required may be less than what is typically used. Patient may take longer to wake up after drugs are hold. (Doses) train of 4 should be done in case of continuous paralytics and goal is 1 out 4. (Add in the policy, order and monitoring sheet) The paralytic and sedation medications should be stopped as soon as possible after the completion of induced hypothermia treatment unless indicated otherwise. The paralytic may be stopped during the warming phase. Magnesium sulphate

Bradycardia and vasoconstriction patient’s normal response to hypothermia is to increase the heart rate and vasoconstriction to conserve heat. Use of sedation and paralytic agents prevents this normal response to hypothermia. Bradycardia and increased systemic vascular resistance will be seen in the absence of shivering with a continued decrease in temperature. The patient may be pale and peripheral pulses may be difficult to obtain because of the vasoconstriction

Hypotension Cooling phase Bradycardia is usually not hemodynamically significant and usually refractory to atropine. It is not always necessary to terminate treatment for patients who are bradycardic. Blood pressure is usually maintained without the use of vasopressors secondary to the increased systemic vascular resistance. If needed vasopressors may be used to maintain mean arterial pressure > 65 mm. Hg. Rewarming phase The greatest risk for hypotension is during the warming phase secondary to vasodilation. kept well hydrated during cooling to help prevent hypotension during warming when vasodilation occurs

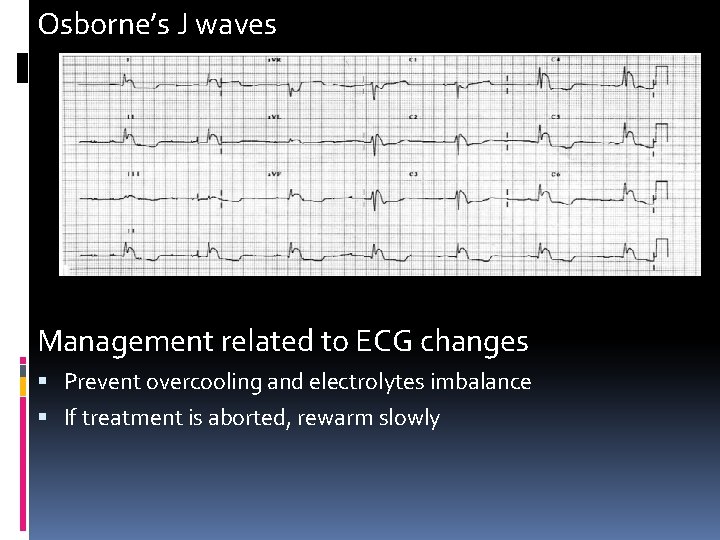
ECG changes Dysrhythmias are rare in mild hypothermia. Tachyarrhythmias beginning by atrial fibrillation. Prolong PR, QRS and QT interval in case of temperature < 33 C. QTc evaluation…notify if more than 0. 5 sec to prevent torsades de pointes. The patient is more at risk when the temperature drops below 32 C. Temperatures below 30 C may cause VF and may be refractory to defibrillation. Osborne’s J waves. Osborne’s waves are camel-hump waves, or hypothermic waves, are best seen in the inferior and lateral precordial leads. A small extra wave is seen immediately after the QRS complex. hey become more prominent as the body temperature decreases, and they resolve gradually with rewarming

Osborne’s J waves Management related to ECG changes Prevent overcooling and electrolytes imbalance If treatment is aborted, rewarm slowly

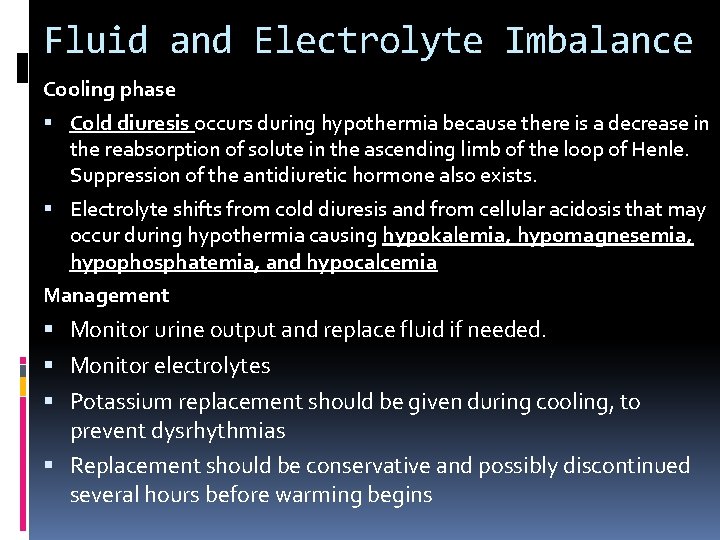
Fluid and Electrolyte Imbalance Cooling phase Cold diuresis occurs during hypothermia because there is a decrease in the reabsorption of solute in the ascending limb of the loop of Henle. Suppression of the antidiuretic hormone also exists. Electrolyte shifts from cold diuresis and from cellular acidosis that may occur during hypothermia causing hypokalemia, hypomagnesemia, hypophosphatemia, and hypocalcemia Management Monitor urine output and replace fluid if needed. Monitor electrolytes Potassium replacement should be given during cooling, to prevent dysrhythmias Replacement should be conservative and possibly discontinued several hours before warming begins

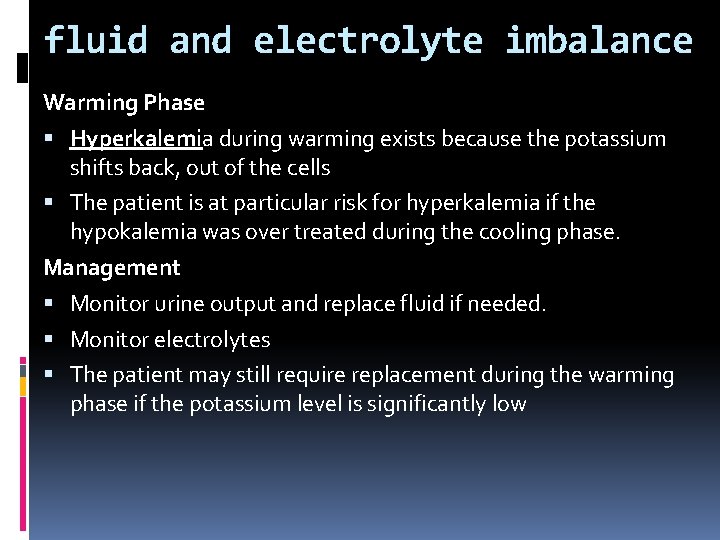
fluid and electrolyte imbalance Warming Phase Hyperkalemia during warming exists because the potassium shifts back, out of the cells The patient is at particular risk for hyperkalemia if the hypokalemia was over treated during the cooling phase. Management Monitor urine output and replace fluid if needed. Monitor electrolytes The patient may still require replacement during the warming phase if the potassium level is significantly low

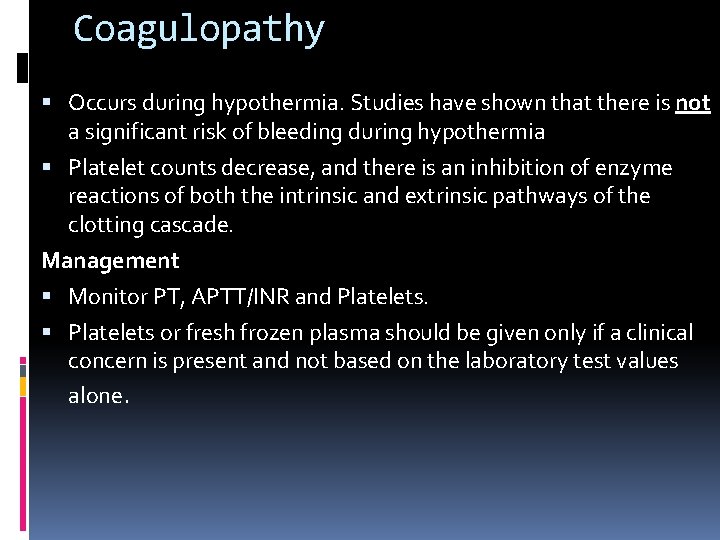
Coagulopathy Occurs during hypothermia. Studies have shown that there is not a significant risk of bleeding during hypothermia Platelet counts decrease, and there is an inhibition of enzyme reactions of both the intrinsic and extrinsic pathways of the clotting cascade. Management Monitor PT, APTT/INR and Platelets or fresh frozen plasma should be given only if a clinical concern is present and not based on the laboratory test values alone.

Increase risk of infection Hypothermia decreases the number of circulating white blood cells. Management Implement VAP bundle and other measures to prevent infections. Beware that elevated temperatures related to infection will be masked by hypothermia

Hyperglycemia It is caused by decreased release of insulin from the pancreas and causes insulin resistance at the cellular level during cooling phase. Management Hyperglycemia should be controlled using insulin treatment with frequent monitoring Glucose should be controlled at levels less than 150 mg/d. L. Tight glycemic control less than 110 mg/d. L is not recommended and carries risk of hypoglycemia.

Reduced Metabolism Decreased metabolic demand Decreased CO 2 production Decreased oxygen consumption. Management Frequent blood gases and adjustment of minute ventilation.

Skin breakdown Peripheral vasoconstriction places the patient at a particularly high risk for skin breakdown. Extra attention to skin assessment, skin care, and frequent turning is necessary.

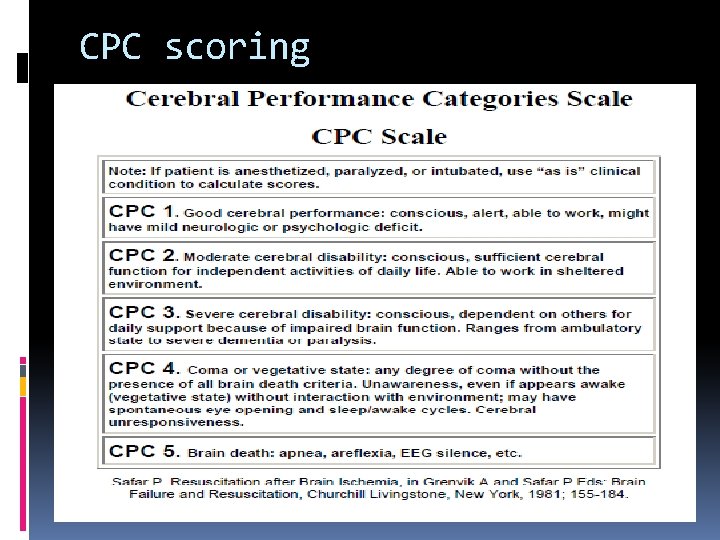
CPC scoring


Arrhythmias interpretation

References attached into TH policy
 Pediatric assessment triangle
Pediatric assessment triangle Lidocaine in cardiac arrest
Lidocaine in cardiac arrest Code red hospital
Code red hospital Adult chain of survival
Adult chain of survival Dissected heart
Dissected heart Cardiac arrest reason
Cardiac arrest reason Rohana roshan
Rohana roshan Roshan chitrakar
Roshan chitrakar Bnfo
Bnfo Usman roshan njit
Usman roshan njit Luxborg
Luxborg Usman roshan
Usman roshan Usman roshan
Usman roshan Usman roshan
Usman roshan Roshan dalvi
Roshan dalvi Usman roshan
Usman roshan Usman roshan
Usman roshan Roshan dalvi
Roshan dalvi Personal fall arrest snap-hooks must be locking and be
Personal fall arrest snap-hooks must be locking and be Advantage arrest
Advantage arrest Respiratory arrest definition
Respiratory arrest definition Ecg rhythm strip interpretation, basic lesson 7
Ecg rhythm strip interpretation, basic lesson 7 Tachy brady syndrome
Tachy brady syndrome Chicago arrest search
Chicago arrest search Total sirkulatuar arrest
Total sirkulatuar arrest Bridget riley arrest 1
Bridget riley arrest 1 Kaushik arrest
Kaushik arrest Gary goulin arrest
Gary goulin arrest Gary goulin arrest
Gary goulin arrest Gary goulin arrest
Gary goulin arrest Gary goulin arrest
Gary goulin arrest The crucible act 2 internal conflict
The crucible act 2 internal conflict How quickly did things get out of hand in salem
How quickly did things get out of hand in salem Khadija diallo arrest
Khadija diallo arrest Cipd arrest report
Cipd arrest report Sinuzal blok
Sinuzal blok Dr douglas vaughan
Dr douglas vaughan Asistoli ekg
Asistoli ekg Hastane dışı kardiyak arrest
Hastane dışı kardiyak arrest A positioning device system is a fall arrest system
A positioning device system is a fall arrest system Amsta arrest
Amsta arrest What is post polio syndrome
What is post polio syndrome Neuroendocrine syndrome in gynecology
Neuroendocrine syndrome in gynecology Mirizzi syndrome types
Mirizzi syndrome types Post tubal ligation syndrome
Post tubal ligation syndrome Sarcoplasmic
Sarcoplasmic Characteristics of skeletal smooth and cardiac muscle
Characteristics of skeletal smooth and cardiac muscle What is b in the figure
What is b in the figure Lesson 6: cardiac emergencies and using an aed
Lesson 6: cardiac emergencies and using an aed Ekg rhythms and interventions
Ekg rhythms and interventions Slide
Slide Comparison of skeletal cardiac and smooth muscle
Comparison of skeletal cardiac and smooth muscle Refractory period cardiac
Refractory period cardiac Pressure and volume changes during cardiac cycle ppt
Pressure and volume changes during cardiac cycle ppt Characteristics of skeletal smooth and cardiac muscle
Characteristics of skeletal smooth and cardiac muscle Characteristics of skeletal smooth and cardiac muscle
Characteristics of skeletal smooth and cardiac muscle Cardiac stimulants and depressants
Cardiac stimulants and depressants Factors affecting cardiac output
Factors affecting cardiac output Cardiac output and stroke volume
Cardiac output and stroke volume Heart sounds and murmurs
Heart sounds and murmurs