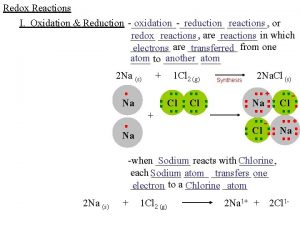
REDOX REDOX CONTENTS Definitions of oxidation and reduction









- Slides: 30

REDOX

REDOX CONTENTS • Definitions of oxidation and reduction • Calculating oxidation state • Use of H, O and F in calculating oxidation state • Naming compounds • Redox reactions • Balancing ionic half equations • Combining half equations to form a redox equation • Revision check list

REDOX Before you start it would be helpful to… • Recall the layout of the periodic table • Be able to balance simple equations

OXIDATION & REDUCTION - Definitions OXIDATION GAIN OF OXYGEN 2 Mg + O 2 ——> 2 Mg. O magnesium has been oxidised as it has gained oxygen REMOVAL (LOSS) OF HYDROGEN C 2 H 5 OH ——> CH 3 CHO + H 2 ethanol has been oxidised as it has ‘lost’ hydrogen

OXIDATION & REDUCTION - Definitions REDUCTION GAIN OF HYDROGEN C 2 H 4 + H 2 ——> C 2 H 6 ethene has been reduced as it has gained hydrogen REMOVAL (LOSS) OF OXYGEN Cu. O + H 2 ——> Cu + H 2 O copper(II) oxide has been reduced as it has ‘lost’ oxygen However as chemistry became more sophisticated, it was realised that another definition was required

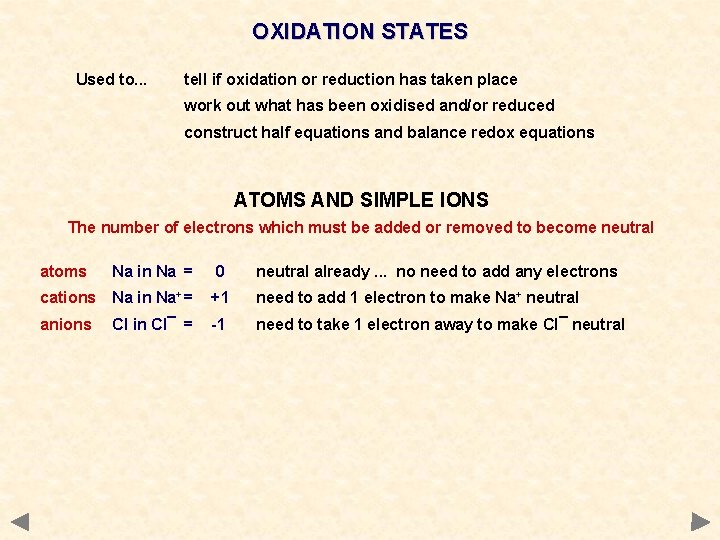
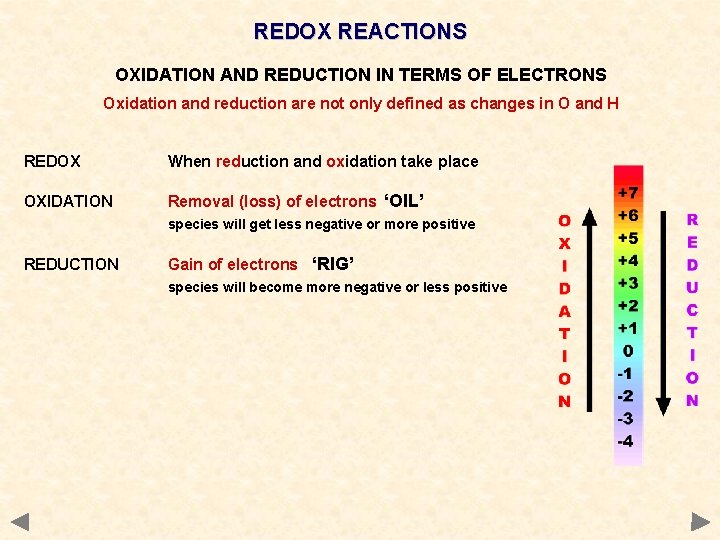
OXIDATION & REDUCTION - Definitions OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H. . . OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive REDOX When reduction and oxidation take place

OXIDATION & REDUCTION - Definitions OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H. . . OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive REDOX When reduction and oxidation take place OIL - Oxidation Is the Loss of electrons RIG - Reduction Is the Gain of electrons

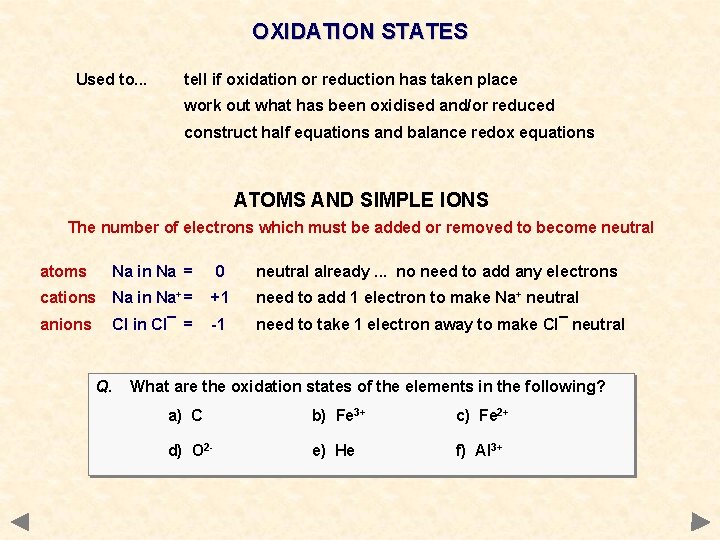
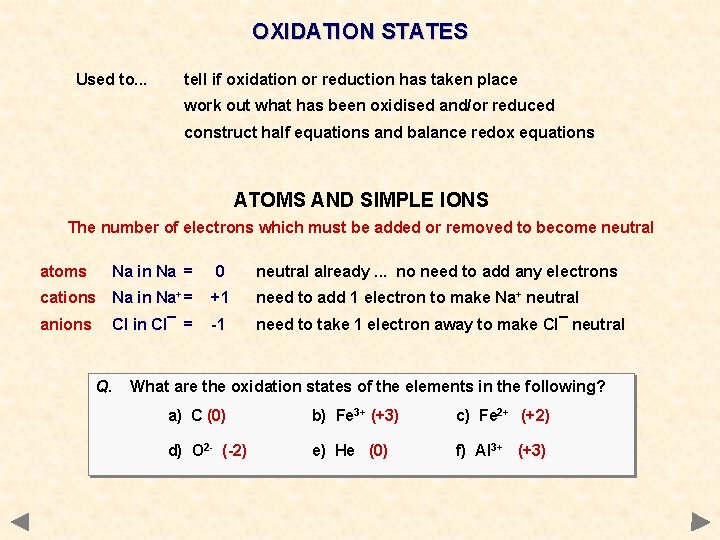
OXIDATION STATES Used to. . . tell if oxidation or reduction has taken place work out what has been oxidised and/or reduced construct half equations and balance redox equations ATOMS AND SIMPLE IONS The number of electrons which must be added or removed to become neutral atoms Na in Na = 0 neutral already. . . no need to add any electrons cations Na in Na+ = +1 need to add 1 electron to make Na+ neutral anions Cl in Cl¯ = -1 need to take 1 electron away to make Cl¯ neutral

OXIDATION STATES Used to. . . tell if oxidation or reduction has taken place work out what has been oxidised and/or reduced construct half equations and balance redox equations ATOMS AND SIMPLE IONS The number of electrons which must be added or removed to become neutral atoms Na in Na = 0 neutral already. . . no need to add any electrons cations Na in Na+ = +1 need to add 1 electron to make Na+ neutral anions Cl in Cl¯ = -1 need to take 1 electron away to make Cl¯ neutral Q. What are the oxidation states of the elements in the following? a) C b) Fe 3+ c) Fe 2+ d) O 2 - e) He f) Al 3+

OXIDATION STATES Used to. . . tell if oxidation or reduction has taken place work out what has been oxidised and/or reduced construct half equations and balance redox equations ATOMS AND SIMPLE IONS The number of electrons which must be added or removed to become neutral atoms Na in Na = 0 neutral already. . . no need to add any electrons cations Na in Na+ = +1 need to add 1 electron to make Na+ neutral anions Cl in Cl¯ = -1 need to take 1 electron away to make Cl¯ neutral Q. What are the oxidation states of the elements in the following? a) C (0) b) Fe 3+ (+3) c) Fe 2+ (+2) d) O 2 - (-2) e) He (0) f) Al 3+ (+3)

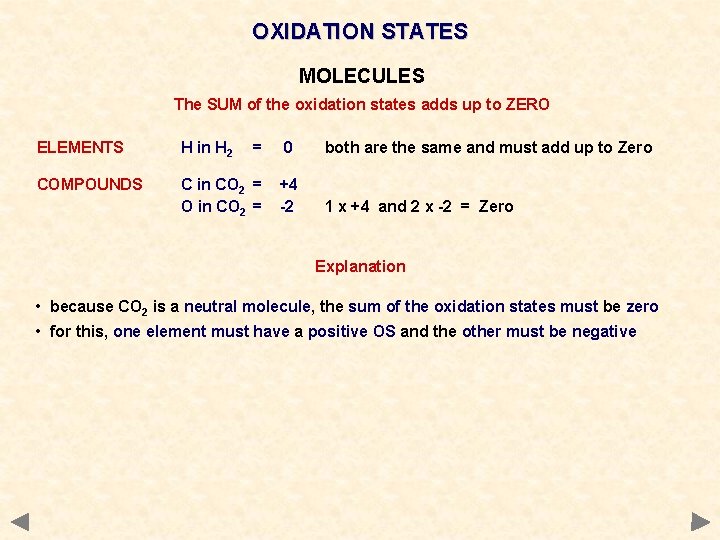
OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x -2 = Zero

OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x -2 = Zero Explanation • because CO 2 is a neutral molecule, the sum of the oxidation states must be zero • for this, one element must have a positive OS and the other must be negative

OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x -2 = Zero HOW DO YOU DETERMINE WHICH IS THE POSITIVE ONE? • the more electronegative species will have the negative value • electronegativity increases across a period and decreases down a group • O is further to the right than C in the periodic table so it has the negative value

OXIDATION STATES MOLECULES The SUM of the oxidation states adds up to ZERO ELEMENTS H in H 2 = COMPOUNDS C in CO 2 = O in CO 2 = 0 both are the same and must add up to Zero +4 -2 1 x +4 and 2 x -2 = Zero HOW DO YOU DETERMINE THE VALUE OF AN ELEMENT’S OXIDATION STATE? • from its position in the periodic table and/or • the other element(s) present in the formula

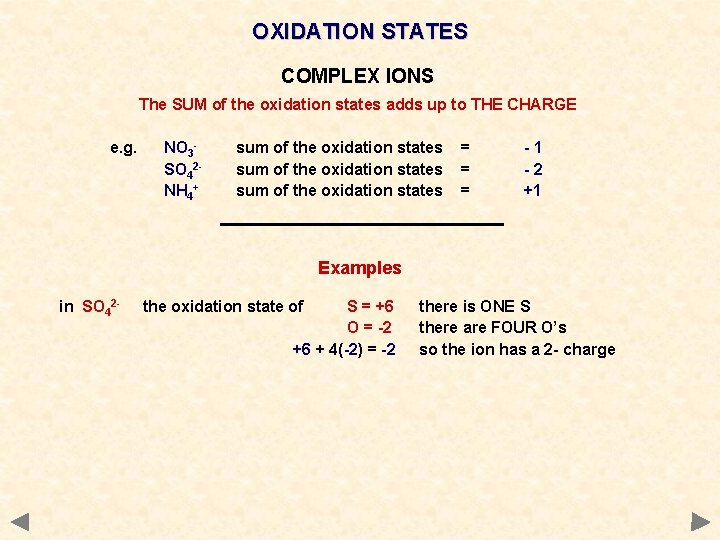
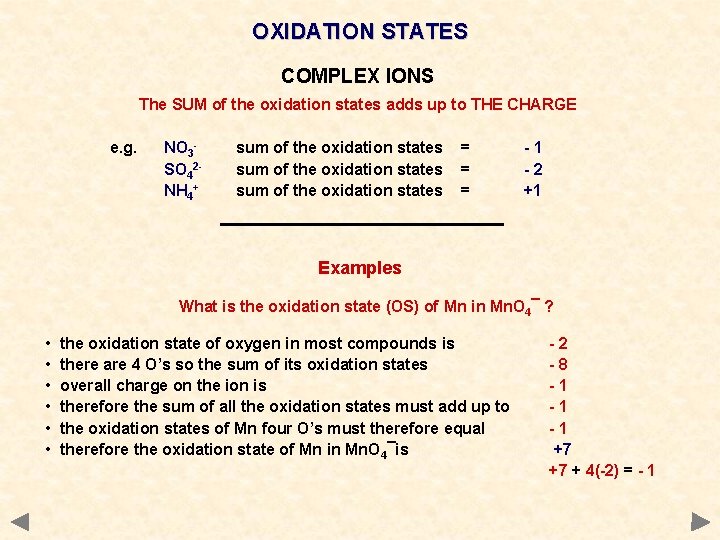
OXIDATION STATES COMPLEX IONS The SUM of the oxidation states adds up to THE CHARGE e. g. NO 3 SO 42 NH 4+ sum of the oxidation states = = = -1 -2 +1 Examples in SO 42 - the oxidation state of S = +6 O = -2 +6 + 4(-2) = -2 there is ONE S there are FOUR O’s so the ion has a 2 - charge

OXIDATION STATES COMPLEX IONS The SUM of the oxidation states adds up to THE CHARGE e. g. NO 3 SO 42 NH 4+ sum of the oxidation states = = = -1 -2 +1 Examples What is the oxidation state (OS) of Mn in Mn. O 4¯ ? • • • the oxidation state of oxygen in most compounds is there are 4 O’s so the sum of its oxidation states overall charge on the ion is therefore the sum of all the oxidation states must add up to the oxidation states of Mn four O’s must therefore equal therefore the oxidation state of Mn in Mn. O 4¯is -2 -8 -1 -1 -1 +7 +7 + 4(-2) = - 1

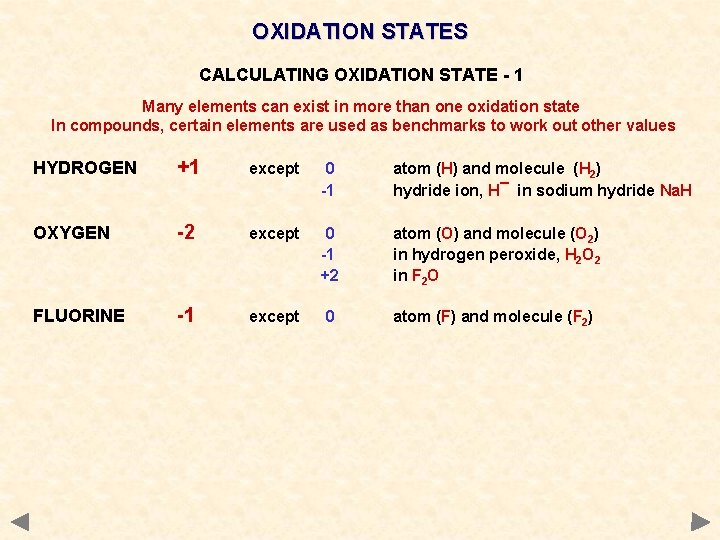
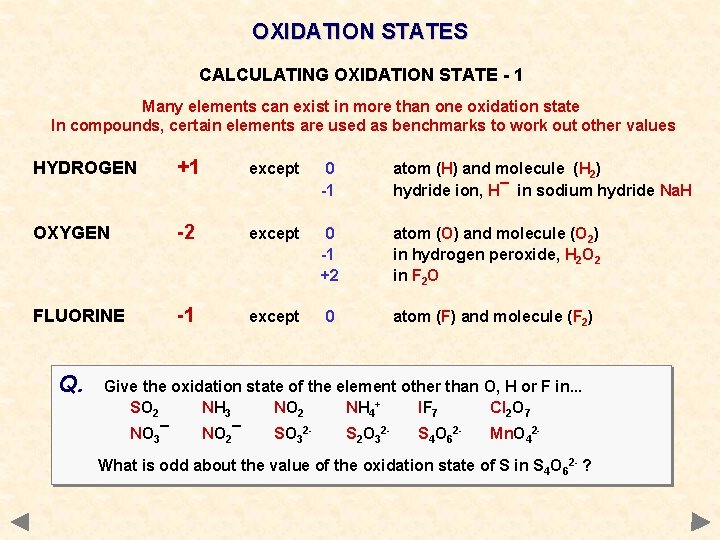
OXIDATION STATES CALCULATING OXIDATION STATE - 1 Many elements can exist in more than one oxidation state In compounds, certain elements are used as benchmarks to work out other values HYDROGEN +1 except 0 -1 atom (H) and molecule (H 2) hydride ion, H¯ in sodium hydride Na. H OXYGEN -2 except 0 -1 +2 atom (O) and molecule (O 2) in hydrogen peroxide, H 2 O 2 in F 2 O FLUORINE -1 except 0 atom (F) and molecule (F 2)

OXIDATION STATES CALCULATING OXIDATION STATE - 1 Many elements can exist in more than one oxidation state In compounds, certain elements are used as benchmarks to work out other values HYDROGEN +1 except 0 -1 atom (H) and molecule (H 2) hydride ion, H¯ in sodium hydride Na. H OXYGEN -2 except 0 -1 +2 atom (O) and molecule (O 2) in hydrogen peroxide, H 2 O 2 in F 2 O FLUORINE -1 except 0 atom (F) and molecule (F 2) Q. Give the oxidation state of the element other than O, H or F in. . . SO 2 NH 3 NO 2 NH 4+ IF 7 Cl 2 O 7 NO 3¯ NO 2¯ SO 32 - S 2 O 32 - S 4 O 62 - Mn. O 42 - What is odd about the value of the oxidation state of S in S 4 O 62 - ?

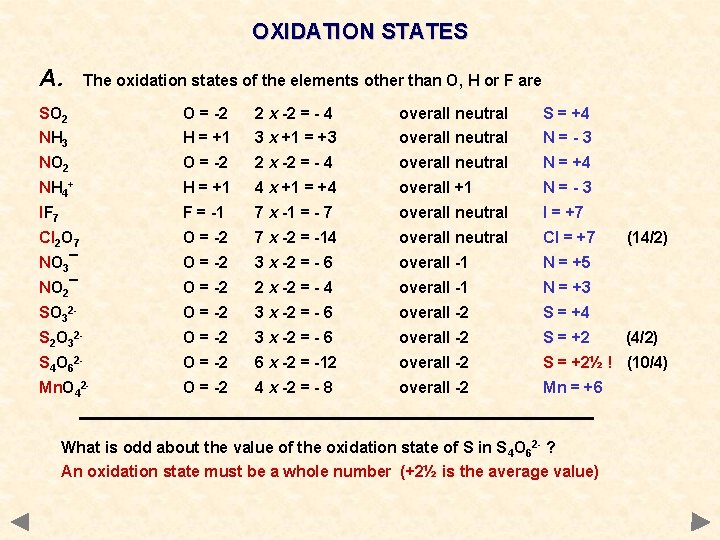
OXIDATION STATES A. The oxidation states of the elements other than O, H or F are SO 2 NH 3 O = -2 H = +1 2 x -2 = - 4 3 x +1 = +3 overall neutral S = +4 N=-3 NO 2 O = -2 2 x -2 = - 4 overall neutral N = +4 NH 4+ H = +1 4 x +1 = +4 overall +1 N=-3 IF 7 F = -1 7 x -1 = - 7 overall neutral I = +7 Cl 2 O 7 O = -2 7 x -2 = -14 overall neutral Cl = +7 NO 3¯ O = -2 3 x -2 = - 6 overall -1 N = +5 NO 2¯ O = -2 2 x -2 = - 4 overall -1 N = +3 SO 32 - O = -2 3 x -2 = - 6 overall -2 S = +4 S 2 O 32 - O = -2 3 x -2 = - 6 overall -2 S = +2 S 4 O 62 - O = -2 6 x -2 = -12 overall -2 S = +2½ ! (10/4) Mn. O 42 - O = -2 4 x -2 = - 8 overall -2 Mn = +6 What is odd about the value of the oxidation state of S in S 4 O 62 - ? An oxidation state must be a whole number (+2½ is the average value) (14/2) (4/2)

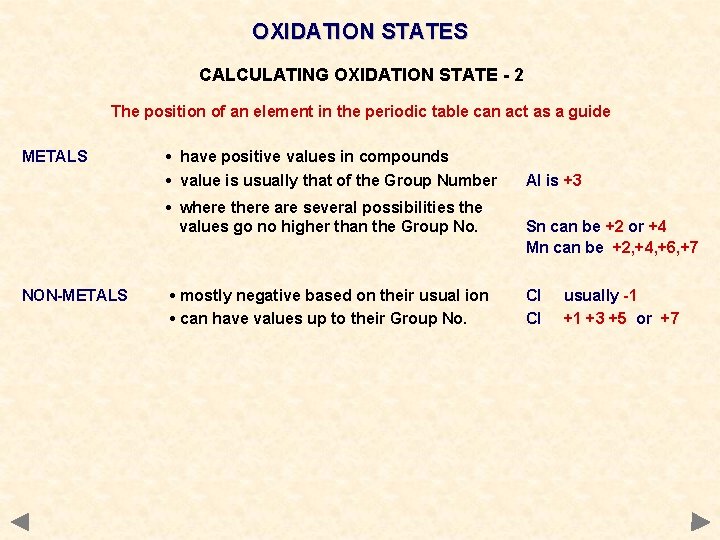
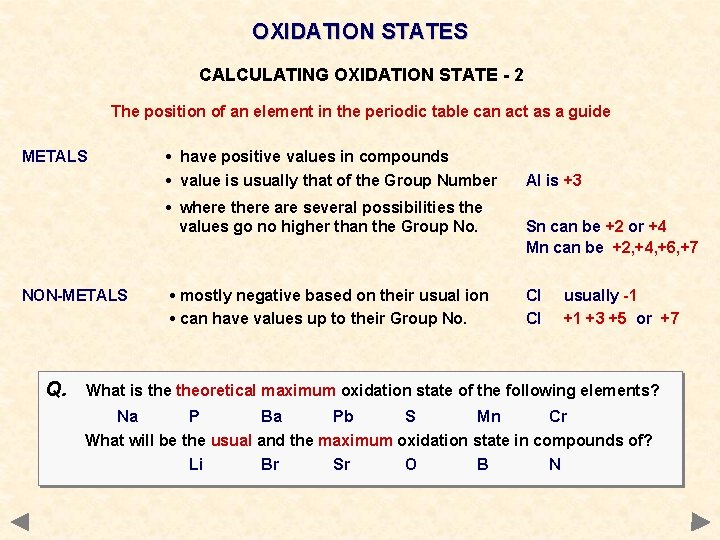
OXIDATION STATES CALCULATING OXIDATION STATE - 2 The position of an element in the periodic table can act as a guide METALS • have positive values in compounds • value is usually that of the Group Number • where there are several possibilities the values go no higher than the Group No. NON-METALS • mostly negative based on their usual ion • can have values up to their Group No. Al is +3 Sn can be +2 or +4 Mn can be +2, +4, +6, +7 Cl Cl usually -1 +1 +3 +5 or +7

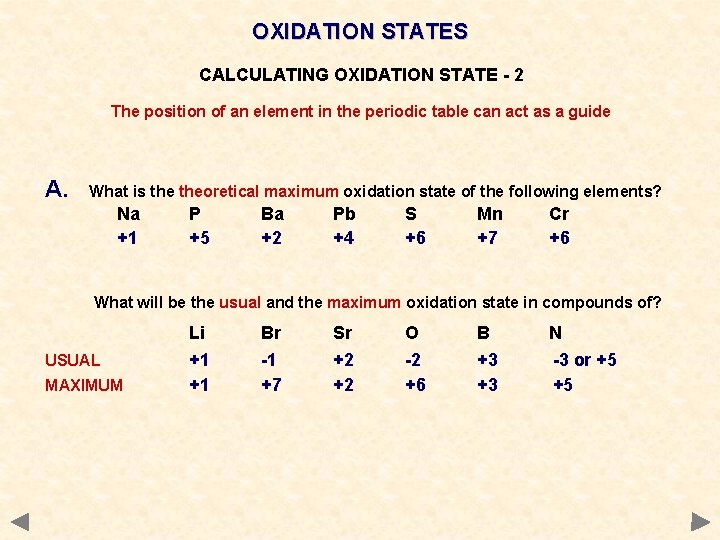
OXIDATION STATES CALCULATING OXIDATION STATE - 2 The position of an element in the periodic table can act as a guide METALS • have positive values in compounds • value is usually that of the Group Number • where there are several possibilities the values go no higher than the Group No. NON-METALS Q. • mostly negative based on their usual ion • can have values up to their Group No. Al is +3 Sn can be +2 or +4 Mn can be +2, +4, +6, +7 Cl Cl usually -1 +1 +3 +5 or +7 What is theoretical maximum oxidation state of the following elements? Na P Ba Pb S Mn Cr What will be the usual and the maximum oxidation state in compounds of? Li Br Sr O B N +1

OXIDATION STATES CALCULATING OXIDATION STATE - 2 The position of an element in the periodic table can act as a guide A. What is theoretical maximum oxidation state of the following elements? Na +1 P +5 Ba +2 Pb +4 S +6 Mn +7 Cr +6 What will be the usual and the maximum oxidation state in compounds of? USUAL MAXIMUM Li +1 +1 Br -1 +7 Sr +2 +2 O -2 +6 B +3 +3 N -3 or +5 +5

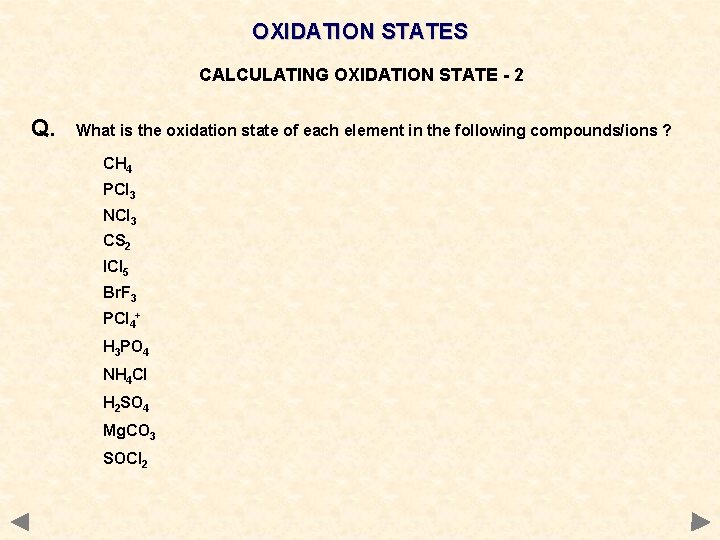
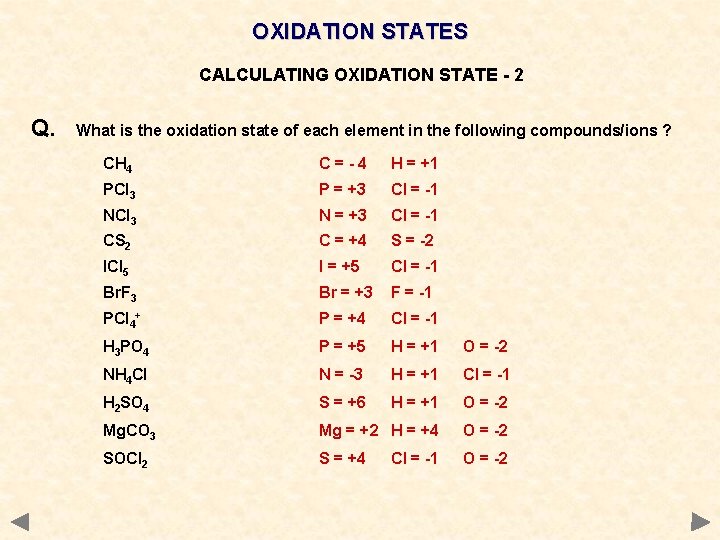
OXIDATION STATES CALCULATING OXIDATION STATE - 2 Q. What is the oxidation state of each element in the following compounds/ions ? CH 4 PCl 3 NCl 3 CS 2 ICl 5 Br. F 3 PCl 4+ H 3 PO 4 NH 4 Cl H 2 SO 4 Mg. CO 3 SOCl 2

OXIDATION STATES CALCULATING OXIDATION STATE - 2 Q. What is the oxidation state of each element in the following compounds/ions ? CH 4 C=-4 H = +1 PCl 3 P = +3 Cl = -1 NCl 3 N = +3 Cl = -1 CS 2 C = +4 S = -2 ICl 5 I = +5 Cl = -1 Br. F 3 Br = +3 F = -1 PCl 4+ P = +4 Cl = -1 H 3 PO 4 P = +5 H = +1 O = -2 NH 4 Cl N = -3 H = +1 Cl = -1 H 2 SO 4 S = +6 H = +1 O = -2 Mg. CO 3 Mg = +2 H = +4 O = -2 SOCl 2 S = +4 O = -2 Cl = -1

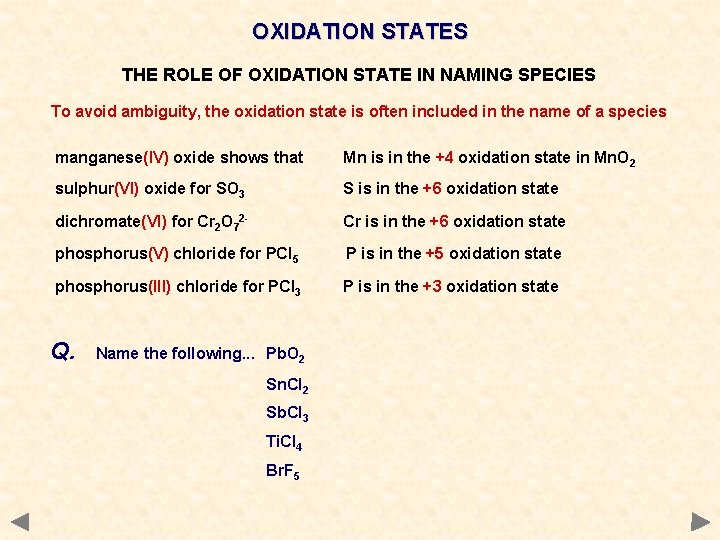
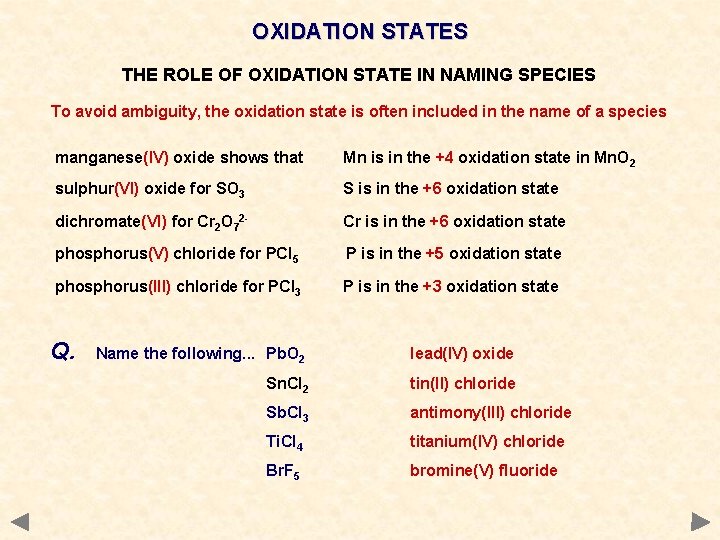
OXIDATION STATES THE ROLE OF OXIDATION STATE IN NAMING SPECIES To avoid ambiguity, the oxidation state is often included in the name of a species manganese(IV) oxide shows that Mn is in the +4 oxidation state in Mn. O 2 sulphur(VI) oxide for SO 3 S is in the +6 oxidation state dichromate(VI) for Cr 2 O 72 - Cr is in the +6 oxidation state phosphorus(V) chloride for PCl 5 P is in the +5 oxidation state phosphorus(III) chloride for PCl 3 P is in the +3 oxidation state Q. Name the following. . . Pb. O 2 Sn. Cl 2 Sb. Cl 3 Ti. Cl 4 Br. F 5

OXIDATION STATES THE ROLE OF OXIDATION STATE IN NAMING SPECIES To avoid ambiguity, the oxidation state is often included in the name of a species manganese(IV) oxide shows that Mn is in the +4 oxidation state in Mn. O 2 sulphur(VI) oxide for SO 3 S is in the +6 oxidation state dichromate(VI) for Cr 2 O 72 - Cr is in the +6 oxidation state phosphorus(V) chloride for PCl 5 P is in the +5 oxidation state phosphorus(III) chloride for PCl 3 P is in the +3 oxidation state Q. Name the following. . . Pb. O 2 lead(IV) oxide Sn. Cl 2 tin(II) chloride Sb. Cl 3 antimony(III) chloride Ti. Cl 4 titanium(IV) chloride Br. F 5 bromine(V) fluoride

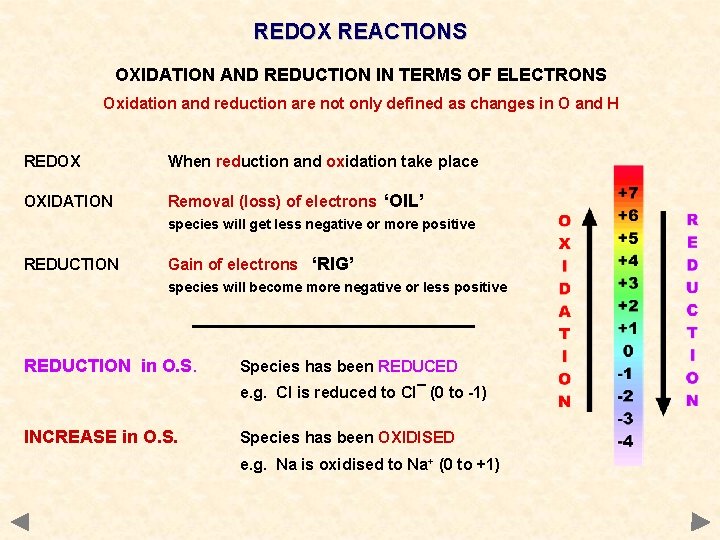
REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H REDOX When reduction and oxidation take place OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive

REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS Oxidation and reduction are not only defined as changes in O and H REDOX When reduction and oxidation take place OXIDATION Removal (loss) of electrons ‘OIL’ species will get less negative or more positive REDUCTION Gain of electrons ‘RIG’ species will become more negative or less positive REDUCTION in O. S. Species has been REDUCED e. g. Cl is reduced to Cl¯ (0 to -1) INCREASE in O. S. Species has been OXIDISED e. g. Na is oxidised to Na+ (0 to +1)

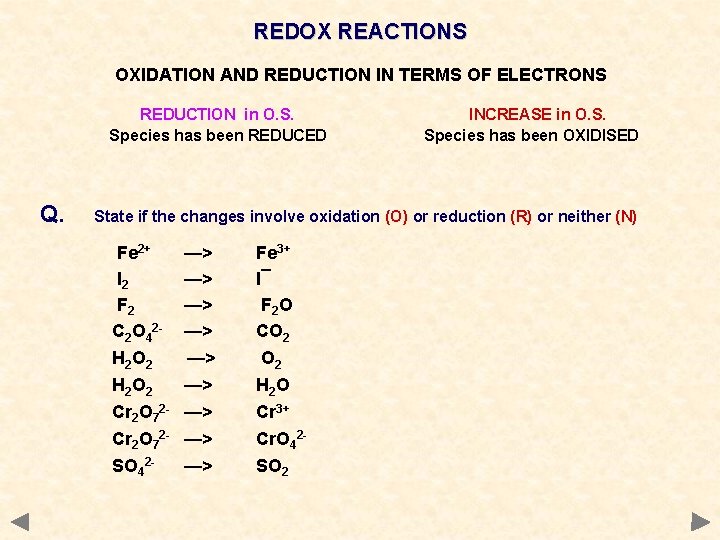
REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS REDUCTION in O. S. Species has been REDUCED Q. INCREASE in O. S. Species has been OXIDISED State if the changes involve oxidation (O) or reduction (R) or neither (N) Fe 2+ I 2 F 2 C 2 O 42 H 2 O 2 Cr 2 O 72 SO 42 - —> —> —> Fe 3+ I¯ F 2 O CO 2 H 2 O Cr 3+ Cr. O 42 SO 2

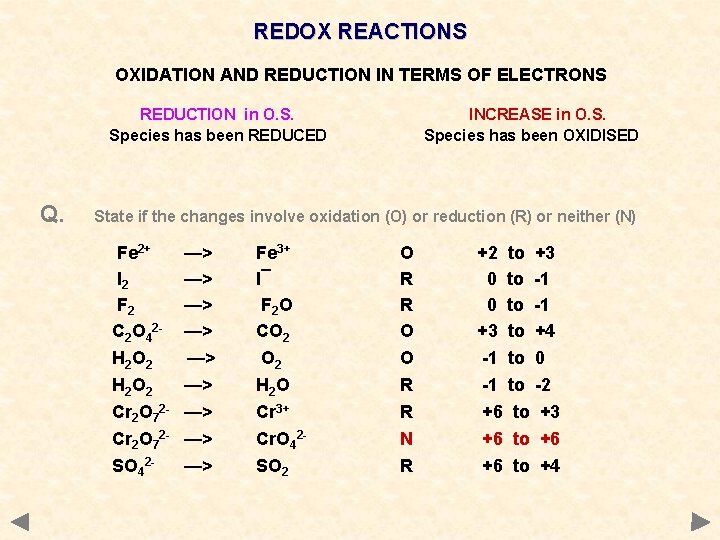
REDOX REACTIONS OXIDATION AND REDUCTION IN TERMS OF ELECTRONS REDUCTION in O. S. Species has been REDUCED Q. INCREASE in O. S. Species has been OXIDISED State if the changes involve oxidation (O) or reduction (R) or neither (N) Fe 2+ I 2 F 2 C 2 O 42 H 2 O 2 Cr 2 O 72 SO 42 - —> —> —> Fe 3+ I¯ F 2 O CO 2 H 2 O Cr 3+ Cr. O 42 SO 2 O R R O O R R N R +2 to +3 0 to -1 +3 to +4 -1 to 0 -1 to -2 +6 to +3 +6 to +6 +6 to +4
 Reduction reaction
Reduction reaction Oxide thickness color chart
Oxide thickness color chart Concentration cell
Concentration cell Cell reaction in electrochemistry
Cell reaction in electrochemistry 2kclo3 2kcl 3o2 oxidation and reduction
2kclo3 2kcl 3o2 oxidation and reduction Explain oxidation
Explain oxidation Oxidising agent example
Oxidising agent example How to know oxidation and reduction
How to know oxidation and reduction Which equation represents an oxidation-reduction reaction
Which equation represents an oxidation-reduction reaction Oxidation reduction quiz
Oxidation reduction quiz Which equation represents an oxidation-reduction reaction
Which equation represents an oxidation-reduction reaction Leo and ger examples
Leo and ger examples Example of redox reaction
Example of redox reaction Chapter 19 review oxidation-reduction reactions
Chapter 19 review oxidation-reduction reactions Reducing agent strength table
Reducing agent strength table Milady chapter 20
Milady chapter 20 Chapter 19 review oxidation reduction reactions answers
Chapter 19 review oxidation reduction reactions answers Leo and ger examples
Leo and ger examples Principles of corrosion
Principles of corrosion Oxidation–reduction reactions
Oxidation–reduction reactions Leo says ger
Leo says ger Oxidation–reduction reactions
Oxidation–reduction reactions Reactions19
Reactions19 Ap chemistry electrochemistry
Ap chemistry electrochemistry Respirometer
Respirometer Red cat an ox chemistry
Red cat an ox chemistry Oxidation number rukes
Oxidation number rukes Galvanic cell cathode
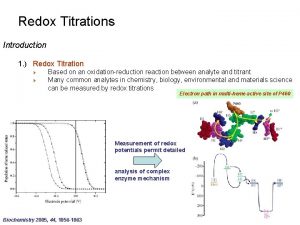
Galvanic cell cathode Introduction of redox titration
Introduction of redox titration Redox
Redox Oxidation of ketones
Oxidation of ketones