Recent Advances in Through Vial Impedance Spectroscopy TVIS








- Slides: 43

Recent Advances in Through Vial Impedance Spectroscopy (TVIS) for Process Parameter Determination Geoff Smith Leicester School of Pharmacy, De Montfort University, United Kingdom ISL-FD Conference April 26 -28 th 2017 Havana, Cuba Through Vial Impedance Spectroscopy

Outline • Through Vial Impedance Spectroscopy (TVIS) o Description of Measurement System • TVIS Application in Freezing o Ice Formation and phase separation • TVIS Application in Annealing o Surrogate Temperature Calibration • TVIS Application in Primary Drying o o o Drying Rate Product Resistance (RP ) Micro-collapse • Acknowledgements Through Vial Impedance Spectroscopy 2

Through Vial Impedance Spectroscopy (TVIS) Description of Measurement System Through Vial Impedance Spectroscopy 3

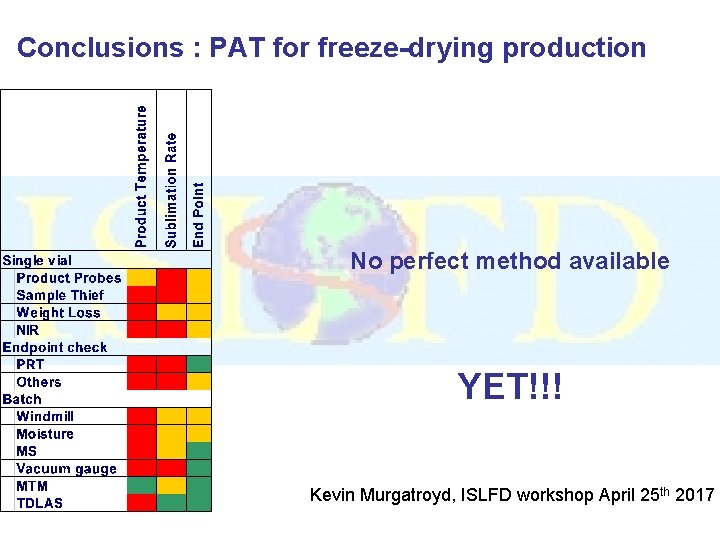
Conclusions : PAT for freeze-drying production No perfect method available YET!!! Kevin Murgatroyd, ISLFD workshop April 25 th 2017

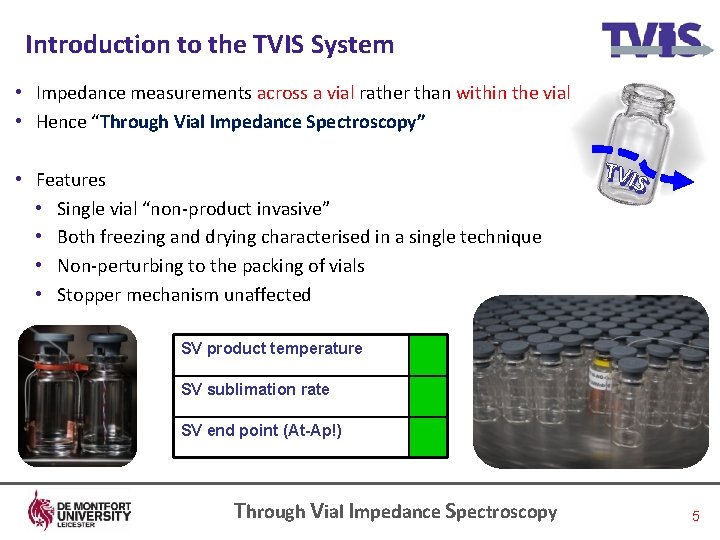
Introduction to the TVIS System • Impedance measurements across a vial rather than within the vial • Hence “Through Vial Impedance Spectroscopy” • Features • Single vial “non-product invasive” • Both freezing and drying characterised in a single technique • Non-perturbing to the packing of vials • Stopper mechanism unaffected SV product temperature SV sublimation rate SV end point (At-Ap!) Through Vial Impedance Spectroscopy 5

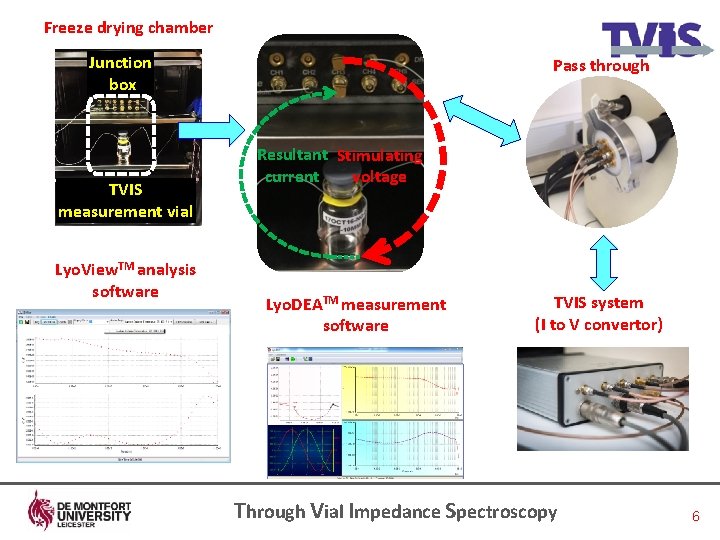
Freeze drying chamber Junction box TVIS measurement vial Lyo. View. TM analysis software Pass through Resultant Stimulating current voltage Lyo. DEATM measurement software TVIS system (I to V convertor) Through Vial Impedance Spectroscopy 6

Through Vial Impedance Spectroscopy (TVIS) Theory Through Vial Impedance Spectroscopy 7

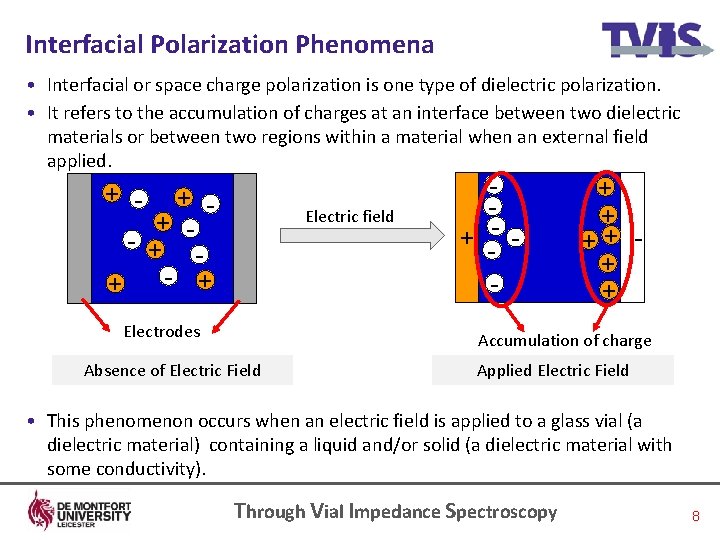
Interfacial Polarization Phenomena • Interfacial or space charge polarization is one type of dielectric polarization. • It refers to the accumulation of charges at an interface between two dielectric materials or between two regions within a material when an external field applied. + - + - + + Electric field Electrodes + - - + + + Accumulation of charge Absence of Electric Field Applied Electric Field • This phenomenon occurs when an electric field is applied to a glass vial (a dielectric material) containing a liquid and/or solid (a dielectric material with some conductivity). Through Vial Impedance Spectroscopy 8

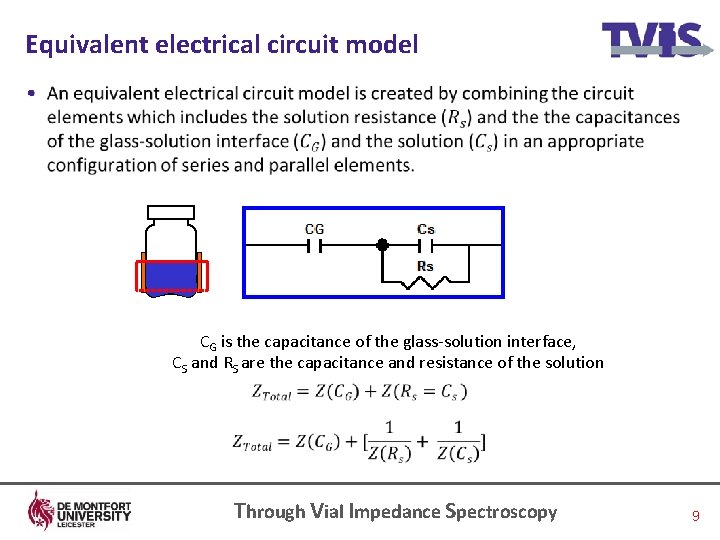
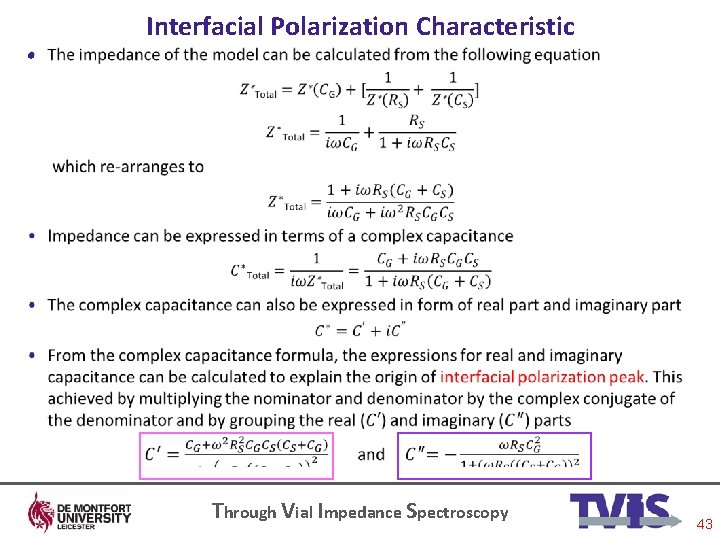
Equivalent electrical circuit model • CG is the capacitance of the glass-solution interface, CS and RS are the capacitance and resistance of the solution Through Vial Impedance Spectroscopy 9

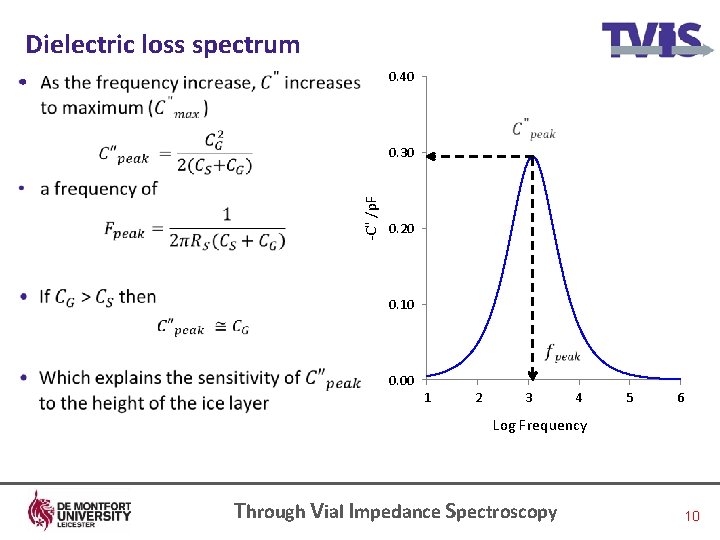
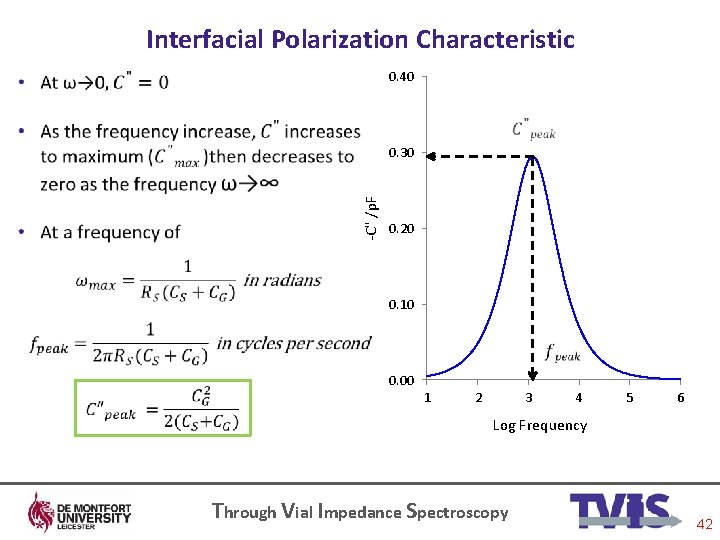
Dielectric loss spectrum 0. 40 • -C" /p. F 0. 30 0. 20 0. 10 0. 00 1 2 3 4 5 6 Log Frequency Through Vial Impedance Spectroscopy 10

TVIS Applications Freezing, Annealing, Primary Drying Through Vial Impedance Spectroscopy 11

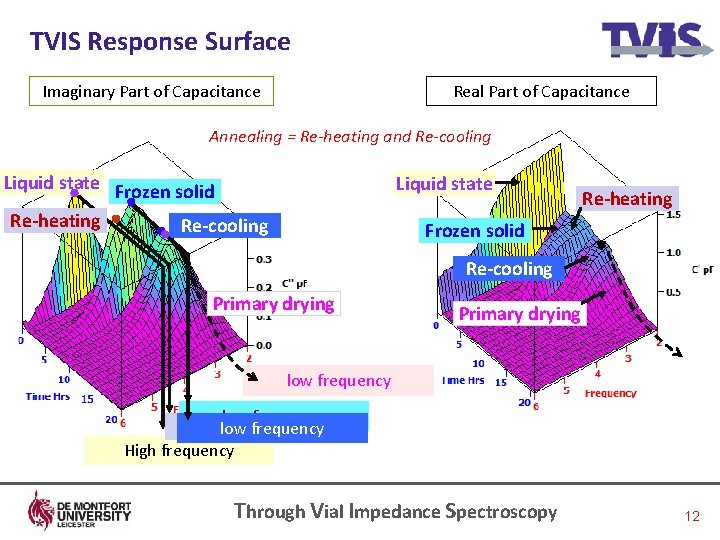
TVIS Response Surface Imaginary Part of Capacitance Real Part of Capacitance Annealing = Re-heating and Re-cooling Liquid state Frozen solid Re-heating Re-cooling Liquid state Re-heating Frozen solid Re-cooling Primary drying low frequency Intermediate frequency low frequency High frequency Through Vial Impedance Spectroscopy 12

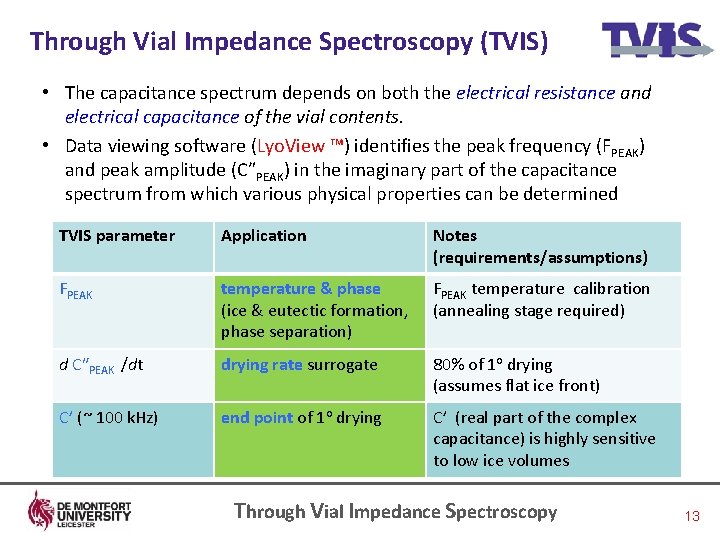
Through Vial Impedance Spectroscopy (TVIS) • The capacitance spectrum depends on both the electrical resistance and electrical capacitance of the vial contents. • Data viewing software (Lyo. View ™) identifies the peak frequency (FPEAK) and peak amplitude (CʺPEAK) in the imaginary part of the capacitance spectrum from which various physical properties can be determined TVIS parameter Application Notes (requirements/assumptions) FPEAK temperature & phase (ice & eutectic formation, phase separation) FPEAK temperature calibration (annealing stage required) d CʺPEAK /dt drying rate surrogate 80% of 1 o drying (assumes flat ice front) C’ (~ 100 k. Hz) end point of 1 o drying C’ (real part of the complex capacitance) is highly sensitive to low ice volumes Through Vial Impedance Spectroscopy 13

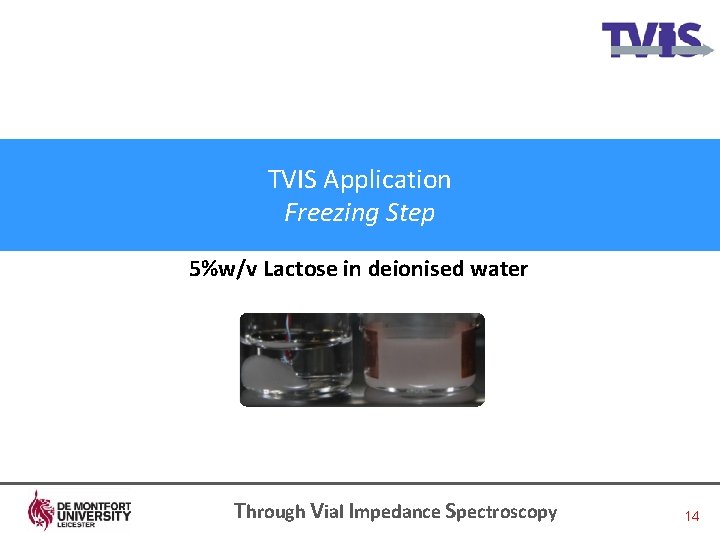
TVIS Application Freezing Step 5%w/v Lactose in deionised water Through Vial Impedance Spectroscopy 14

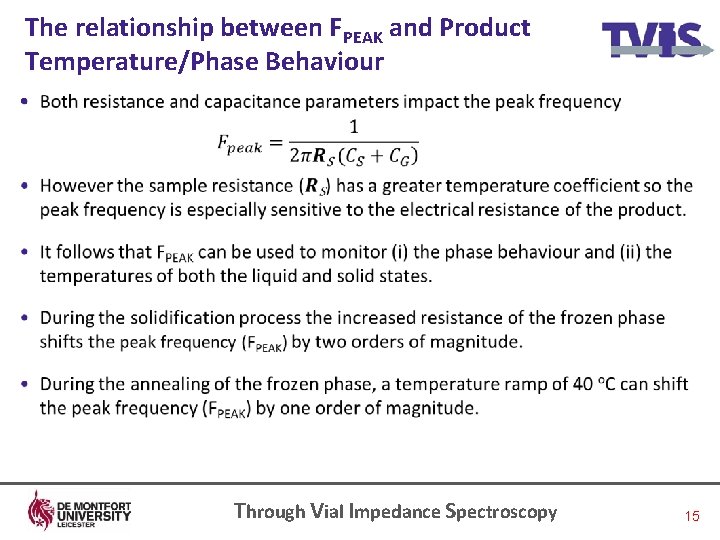
The relationship between FPEAK and Product Temperature/Phase Behaviour • Through Vial Impedance Spectroscopy 15

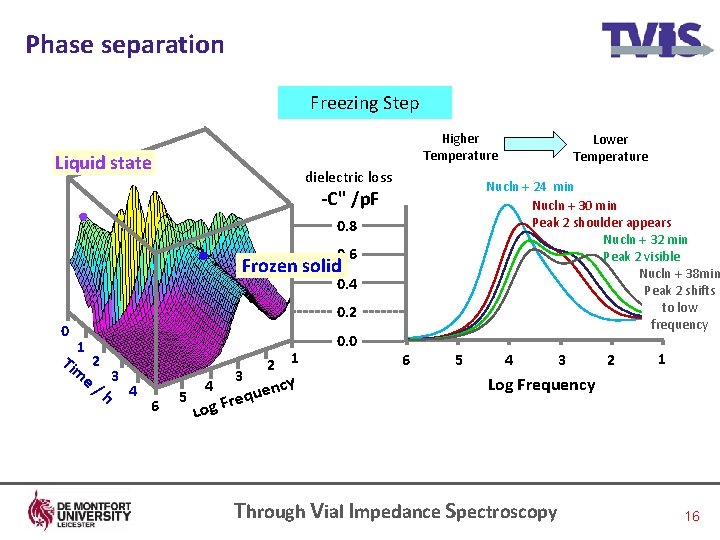
Phase separation Freezing Step Higher Temperature Liquid state dielectric loss Nucln + 24 min Nucln + 30 min Peak 2 shoulder appears Nucln + 32 min Peak 2 visible Nucln + 38 min Peak 2 shifts to low frequency -C" /p. F 0. 8 0. 6 Frozen solid 0. 4 0 0. 2 1 Tim 2 e/ 3 h 4 6 5 4 3 1 2 cy uen q e r g. F 0. 0 Lower Temperature 66 55 44 33 22 11 Log Frequency Lo Through Vial Impedance Spectroscopy 16

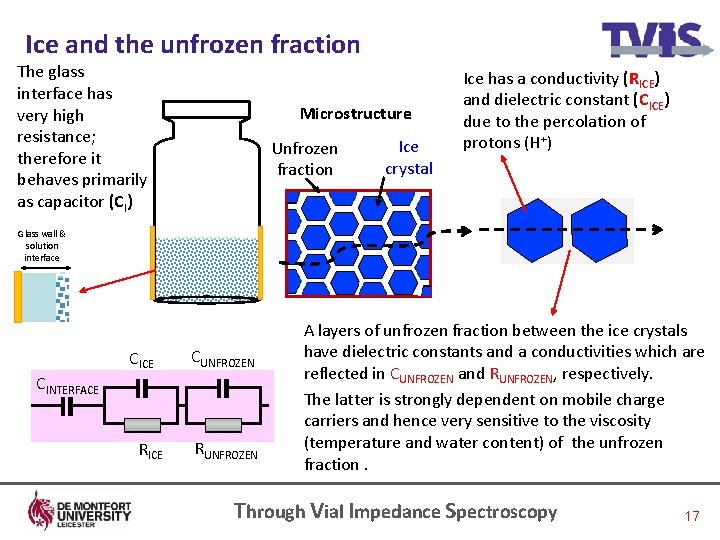
Ice and the unfrozen fraction The glass interface has very high resistance; therefore it behaves primarily as capacitor (CI) Microstructure Unfrozen fraction Ice crystal Ice has a conductivity (RICE) and dielectric constant (CICE) due to the percolation of protons (H+) Glass wall & solution interface CICE CUNFROZEN CINTERFACE CG RICE RUNFROZEN A layers of unfrozen fraction between the ice crystals have dielectric constants and a conductivities which are reflected in CUNFROZEN and RUNFROZEN, respectively. The latter is strongly dependent on mobile charge carriers and hence very sensitive to the viscosity (temperature and water content) of the unfrozen fraction. Through Vial Impedance Spectroscopy 17

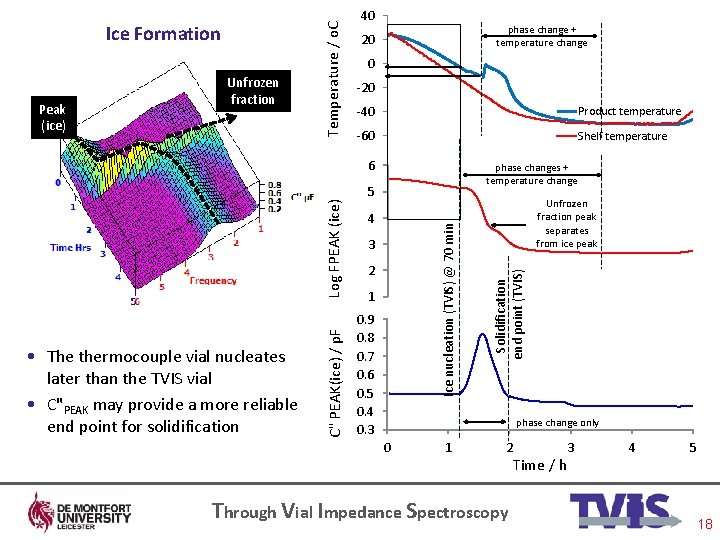
phase change + temperature change 20 0 -20 -40 Product temperature -60 Shelf temperature phase changes + temperature change 5 4 Ice nucleation (TVIS) @ 70 min • The thermocouple vial nucleates later than the TVIS vial • C"PEAK may provide a more reliable end point for solidification C" PEAK(ice) / p. F Log FPEAK (ice) 6 3 2 1 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 Unfrozen fraction peak separates from ice peak Solidification end point (TVIS) Peak (ice) Unfrozen fraction Temperature / o. C Ice Formation 40 phase change only 0 1 2 Through Vial Impedance Spectroscopy Time / h 3 4 5 18

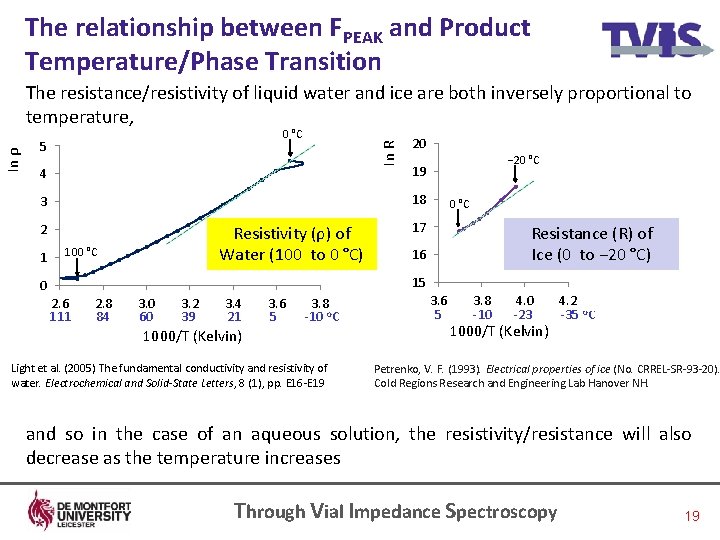
The relationship between FPEAK and Product Temperature/Phase Transition 0 °C 5 4 20 2 100 °C Resistivity (ρ) of Water (100 to 0 °C) − 20 °C 19 18 3 1 In R In ρ The resistance/resistivity of liquid water and ice are both inversely proportional to temperature, 17 16 0 °C Resistance (R) of Ice (0 to − 20 °C) 15 0 2. 6 2. 8 3. 0 3. 2 3. 4 3. 6 3. 8 111 84 60 39 21 5 -10 o. C 1000/T (Kelvin) Light et al. (2005) The fundamental conductivity and resistivity of water. Electrochemical and Solid-State Letters, 8 (1), pp. E 16 -E 19 3. 6 3. 8 4. 0 4. 2 4. 4 5 -10 -23 -35 o. C 4. 6 4. 8 1000/T (Kelvin) Petrenko, V. F. (1993). Electrical properties of ice (No. CRREL-SR-93 -20). Cold Regions Research and Engineering Lab Hanover NH. and so in the case of an aqueous solution, the resistivity/resistance will also decrease as the temperature increases Through Vial Impedance Spectroscopy 19

TVIS Application Annealing for Temperature Calibration 5%w/v Lactose in deionised water Through Vial Impedance Spectroscopy 20

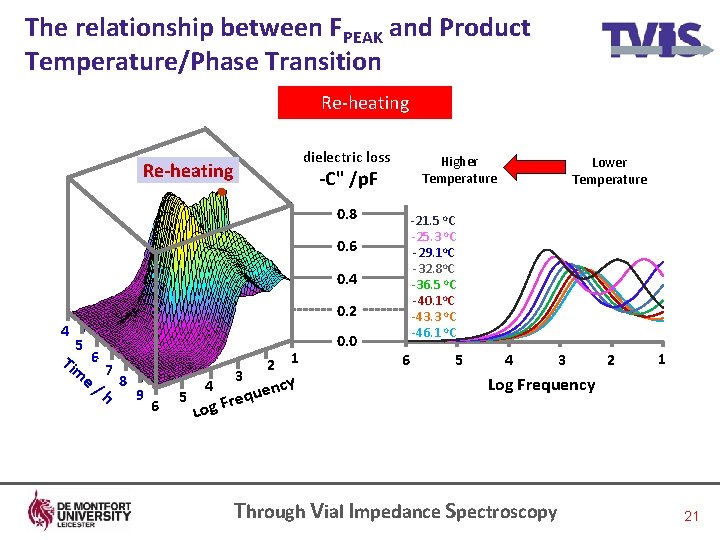
The relationship between FPEAK and Product Temperature/Phase Transition Re-heating dielectric loss Re-heating Higher Temperature -C" /p. F 0. 8 -21. 5 o. C -25. 3 o. C -29. 1 o. C -32. 8 o. C -36. 5 o. C -40. 1 o. C -43. 3 o. C -46. 1 o. C 0. 6 0. 4 0. 2 4 5 Tim 6 e/ 7 8 h 9 6 5 4 3 1 2 cy uen q e r g. F 0. 0 Lower Temperature 6 5 4 3 2 1 Log Frequency Lo Through Vial Impedance Spectroscopy 21

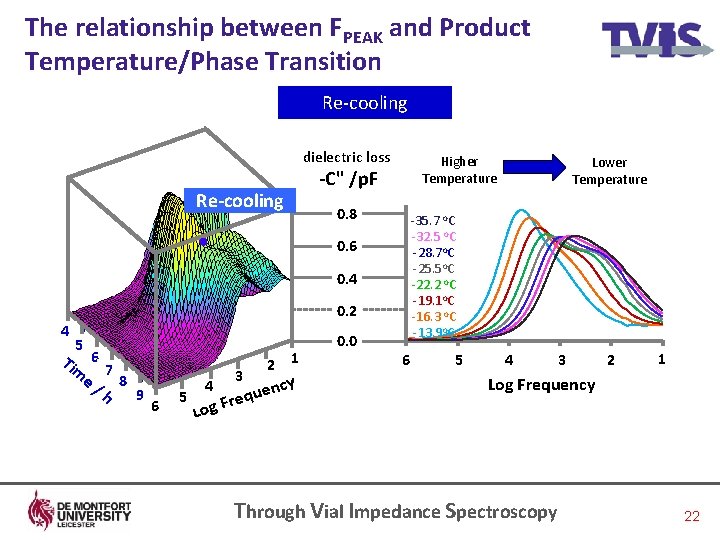
The relationship between FPEAK and Product Temperature/Phase Transition Re-cooling dielectric loss Higher Temperature -C" /p. F Re-cooling 0. 8 -35. 7 o. C -32. 5 o. C -28. 7 o. C -25. 5 o. C -22. 2 o. C -19. 1 o. C -16. 3 o. C -13. 9 o. C 0. 6 0. 4 0. 2 4 5 Tim 6 e/ 7 8 h 9 6 5 4 3 1 2 cy uen q e r g. F 0. 0 Lower Temperature 6 5 4 3 2 1 Log Frequency Lo Through Vial Impedance Spectroscopy 22

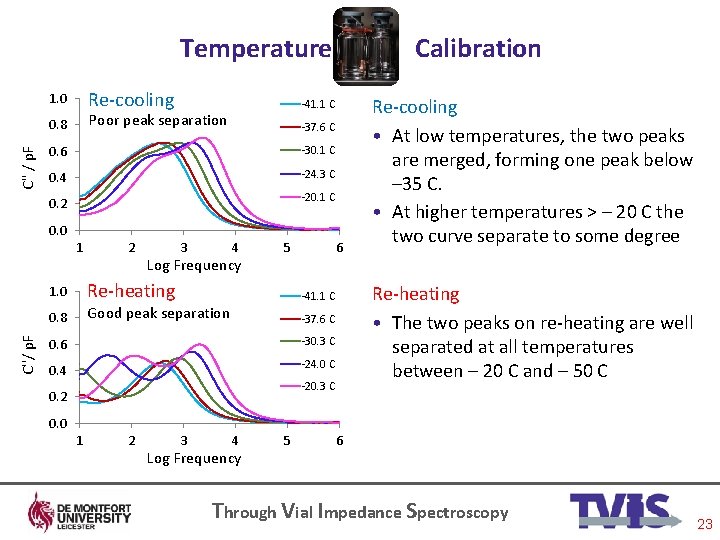
Temperature Re-cooling 1. 0 C'' / p. F -41. 1 C Poor peak separation 0. 8 Calibration -37. 6 C 0. 6 -30. 1 C 0. 4 -24. 3 C 0. 2 -20. 1 C 0. 0 C''/ p. F 1 2 3 4 Log Frequency 1. 0 Re-heating 0. 8 Good peak separation 5 6 Re-heating • The two peaks on re-heating are well separated at all temperatures between – 20 C and – 50 C -41. 1 C -37. 6 C 0. 6 -30. 3 C 0. 4 -24. 0 C -20. 3 C 0. 2 Re-cooling • At low temperatures, the two peaks are merged, forming one peak below – 35 C. • At higher temperatures > – 20 C the two curve separate to some degree 0. 0 1 2 3 4 Log Frequency 5 6 Through Vial Impedance Spectroscopy 23

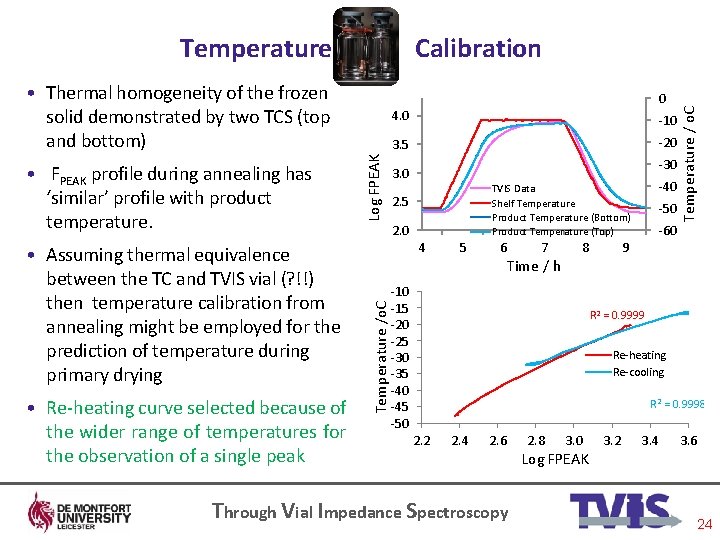
Calibration • Thermal homogeneity of the frozen solid demonstrated by two TCS (top and bottom) • Assuming thermal equivalence between the TC and TVIS vial (? !!) then temperature calibration from annealing might be employed for the prediction of temperature during primary drying • Re-heating curve selected because of the wider range of temperatures for the observation of a single peak Log FPEAK 4. 0 -10 3. 5 -20 -30 3. 0 -40 TVIS Data Shelf Temperature Product Temperature (Bottom) Product Temperature (Top) 2. 5 2. 0 4 Temperature /o. C • FPEAK profile during annealing has ‘similar’ profile with product temperature. 0 5 6 7 Time / h 8 -10 -15 -20 -25 -30 -35 -40 -45 -50 -60 Temperature / o. C Temperature 9 R 2 = 0. 9999 Re-heating Re-cooling R 2 = 0. 9998 2. 2 2. 4 2. 6 Through Vial Impedance Spectroscopy 2. 8 3. 0 Log FPEAK 3. 2 3. 4 3. 6 24

5%w/v Lactose in deionised water TVIS Application Primary Drying (1) Product temperature prediction Through Vial Impedance Spectroscopy 25

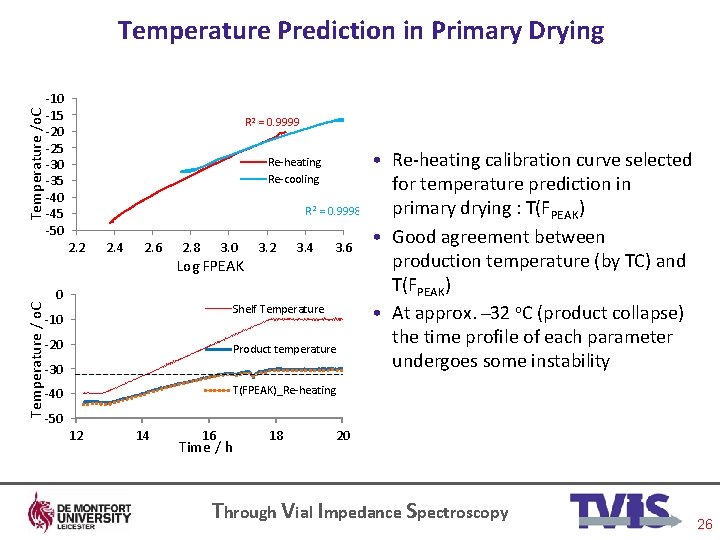
Temperature /o. C Temperature Prediction in Primary Drying -10 -15 -20 -25 -30 -35 -40 -45 -50 R 2 = 0. 9999 Re-heating Re-cooling R 2 = 0. 9998 Temperature / o. C 2. 2 2. 4 2. 6 2. 8 3. 0 Log FPEAK 0 3. 2 3. 4 3. 6 Shelf Temperature -10 -20 Product temperature -30 • Re-heating calibration curve selected for temperature prediction in primary drying : T(FPEAK) • Good agreement between production temperature (by TC) and T(FPEAK) • At approx. 32 o. C (product collapse) the time profile of each parameter undergoes some instability T(FPEAK)_Re-heating -40 -50 12 14 16 Time / h 18 20 Through Vial Impedance Spectroscopy 26

5%w/v Lactose in deionised water TVIS Application Primary Drying (2) Drying Rate Prediction Through Vial Impedance Spectroscopy 27

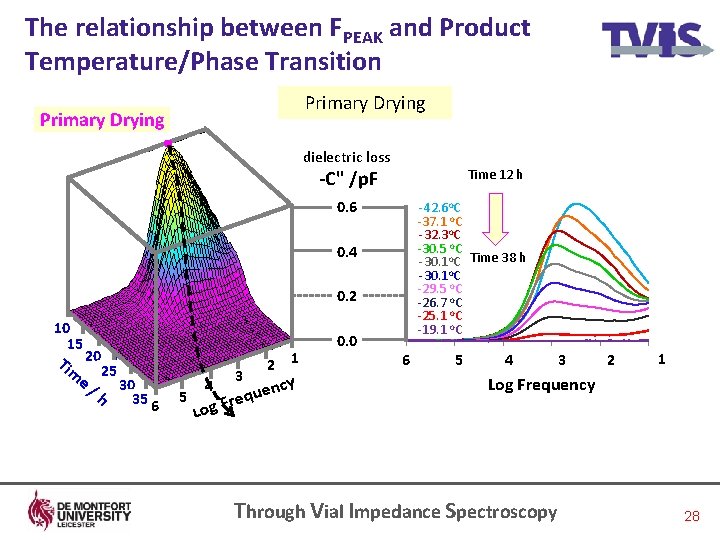
The relationship between FPEAK and Product Temperature/Phase Transition Primary Drying dielectric loss Time 12 h -C" /p. F 0. 6 -42. 6 o. C -37. 1 o. C -32. 3 o. C -30. 5 o. C -30. 1 o. C Time 38 h -30. 1 o. C -29. 5 o. C -26. 7 o. C -25. 1 o. C -19. 1 o. C 0. 4 0. 2 10 15 Tim 20 e / 25 30 h 35 6 5 4 3 1 2 cy uen q e r g. F 0. 0 6 5 4 3 2 1 Log Frequency Lo Through Vial Impedance Spectroscopy 28

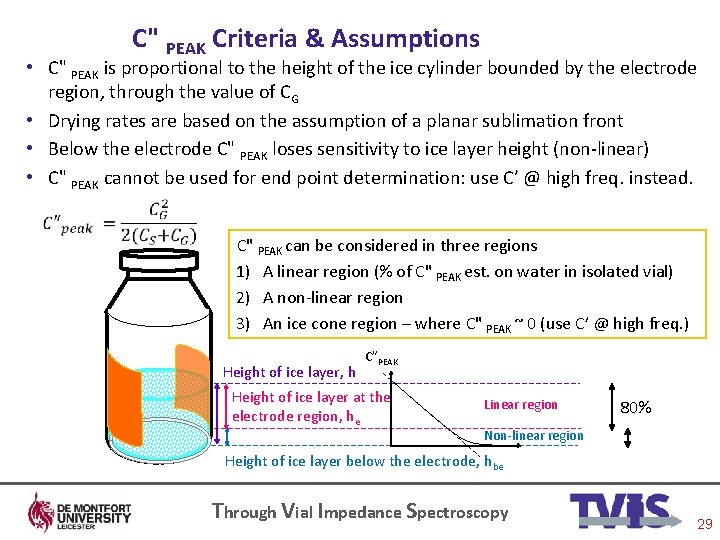
C" PEAK Criteria & Assumptions • C" PEAK is proportional to the height of the ice cylinder bounded by the electrode region, through the value of CG • Drying rates are based on the assumption of a planar sublimation front • Below the electrode C" PEAK loses sensitivity to ice layer height (non-linear) • C" PEAK cannot be used for end point determination: use C’ @ high freq. instead. C" PEAK can be considered in three regions 1) A linear region (% of C" PEAK est. on water in isolated vial) 2) A non-linear region 3) An ice cone region – where C" PEAK ~ 0 (use C’ @ high freq. ) Height of ice layer, h C”PEAK Height of ice layer at the electrode region, he Linear region 80% Non-linear region Height of ice layer below the electrode, hbe Through Vial Impedance Spectroscopy 29

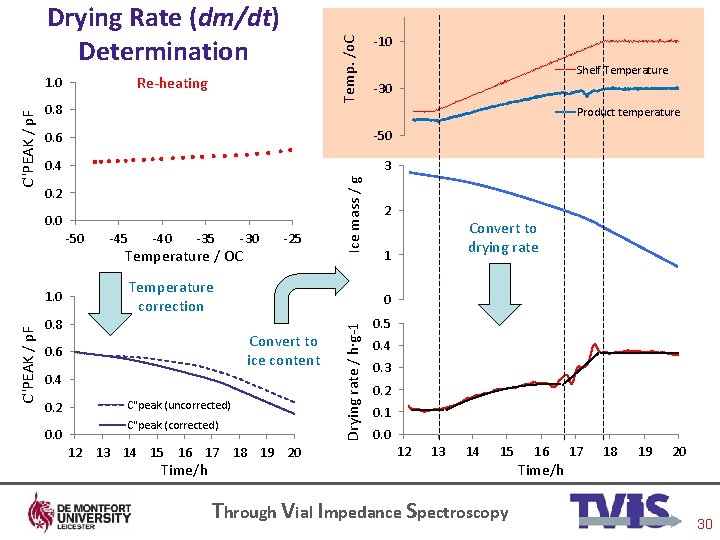
Re-heating 1. 0 0. 8 -10 Shelf Temperature -30 Product temperature -50 0. 6 0. 4 0. 2 0. 0 -50 -45 -40 -35 -30 Temperature / OC -25 Ice mass / g C''PEAK / p. F Temp. /o. C Drying Rate (dm/dt) Determination C''PEAK / p. F 1. 0 0. 8 0. 6 0. 4 0. 2 0. 0 C"peak (uncorrected) C"peak (corrected) 12 13 14 15 16 17 18 19 20 Time/h Through Vial Impedance Spectroscopy 30

5%w/v Lactose in deionised water TVIS Application Primary Drying: Rp determination Through Vial Impedance Spectroscopy 31

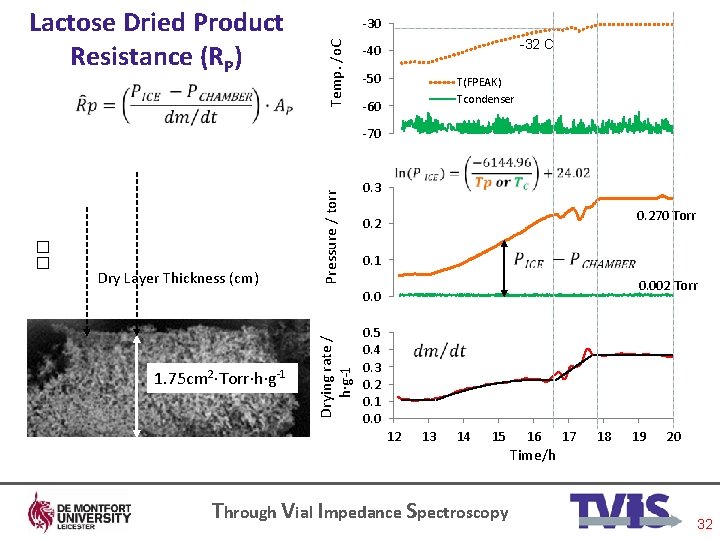
-30 Temp. /o. C Lactose Dried Product Resistance (RP) -32 C -40 -50 -60 T(FPEAK) Tcondenser Pressure / torr -70 Through Vial Impedance Spectroscopy 32

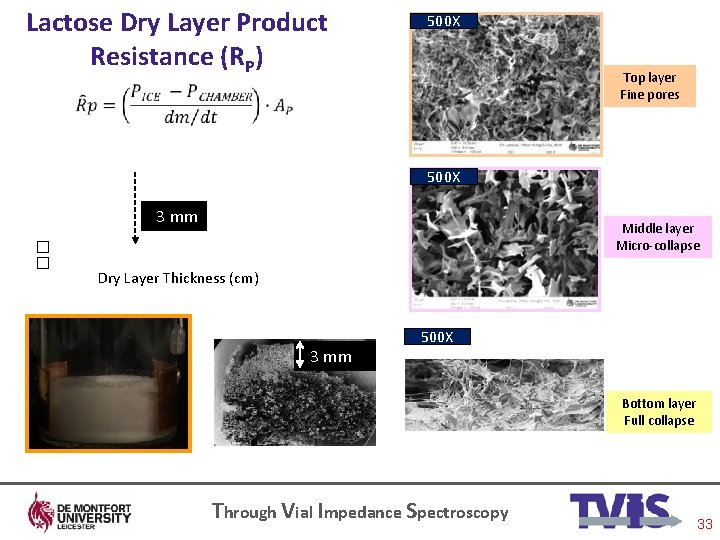
Lactose Dry Layer Product Resistance (RP) 500 X Top layer Fine pores 500 X �� 3 mm Middle layer Micro-collapse Dry Layer Thickness (cm) 3 mm 500 X Bottom layer Full collapse Through Vial Impedance Spectroscopy 33

Acknowledgements, Recent Projects & Collaborators • De Montfort University o o Evgeny Polygalov. Senior Research Fellow Irina Ermolina. Senior Lecturer Yowwares Jeerarunangrattana. Ph. D student Bhaskar Pandya. Ph. D student • GEA Process Engineering o o Trevor Page & Julian Taylor Daniela Buchmeyer & Thomas Beutler • Blue. Frog : Chris Samwell Ben Irvin • NIBSC : Paul Matejtschuk • Sanofi : Tim Mc. Coy Through Vial Impedance Spectroscopy 34

Annex : TVIS Theory Through Vial Impedance Spectroscopy 35

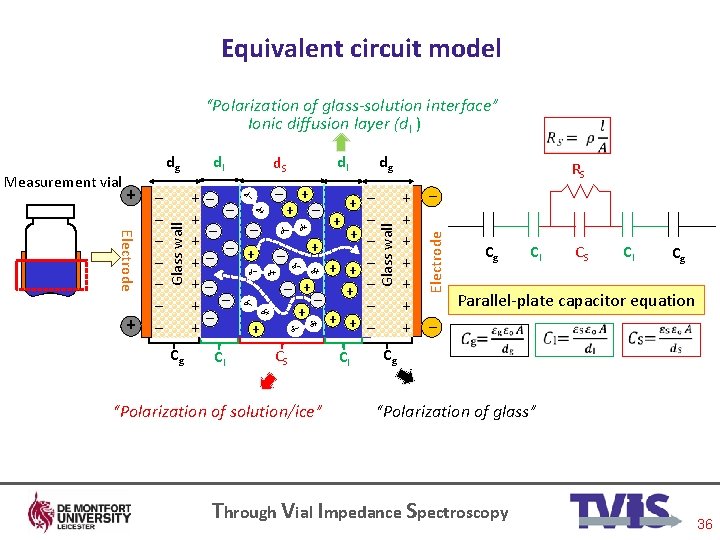
Equivalent circuit model “Polarization of glass-solution interface” Ionic diffusion layer (d. I ) δ− δ+ + δ− − − δ+ δ+ + − + − + + − − − + + + − δ+ δ− δ− δ+ CS − − − + “Polarization of solution/ice” CI RS + − + + + − Electrode − δ− CI + Glass wall − + Cg − + δ+ Electrode + + + + dg dl d. S δ− − − − dl − Measurement vial dg Cg CI CS CI Cg Parallel-plate capacitor equation Cg “Polarization of glass” Through Vial Impedance Spectroscopy 36

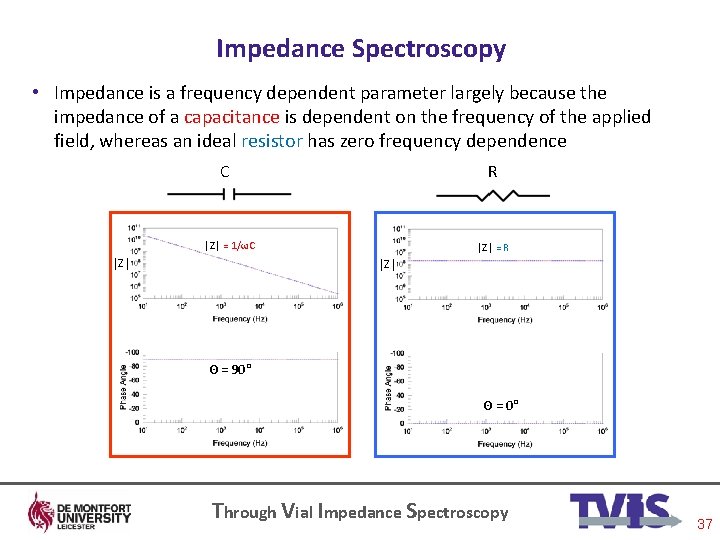
Impedance Spectroscopy • Impedance is a frequency dependent parameter largely because the impedance of a capacitance is dependent on the frequency of the applied field, whereas an ideal resistor has zero frequency dependence C R │Z│ = 1/ωC │Z│ = R │Z│ Θ = 90° Θ = 0° Through Vial Impedance Spectroscopy 37

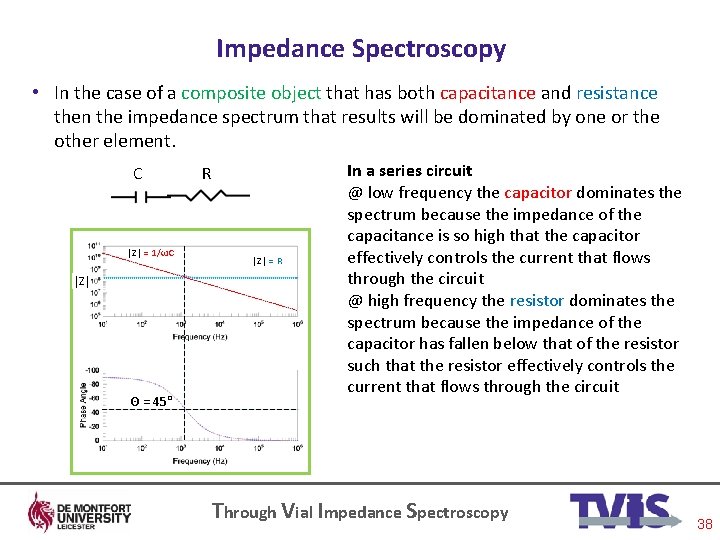
Impedance Spectroscopy • In the case of a composite object that has both capacitance and resistance then the impedance spectrum that results will be dominated by one or the other element. C │Z│ = 1/ωC │Z│ Θ = 45° R │Z│ = R In a series circuit @ low frequency the capacitor dominates the spectrum because the impedance of the capacitance is so high that the capacitor effectively controls the current that flows through the circuit @ high frequency the resistor dominates the spectrum because the impedance of the capacitor has fallen below that of the resistor such that the resistor effectively controls the current that flows through the circuit Through Vial Impedance Spectroscopy 38

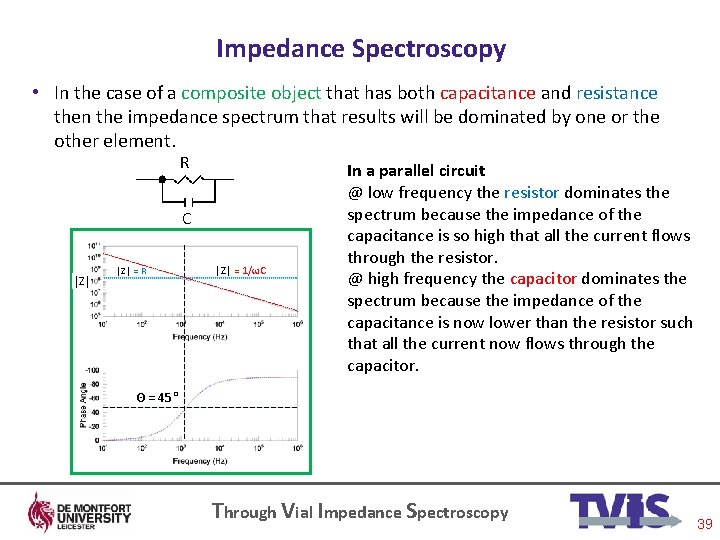
Impedance Spectroscopy • In the case of a composite object that has both capacitance and resistance then the impedance spectrum that results will be dominated by one or the other element. R C │Z│ = R │Z│ = 1/ωC In a parallel circuit @ low frequency the resistor dominates the spectrum because the impedance of the capacitance is so high that all the current flows through the resistor. @ high frequency the capacitor dominates the spectrum because the impedance of the capacitance is now lower than the resistor such that all the current now flows through the capacitor. Θ = 45° Through Vial Impedance Spectroscopy 39

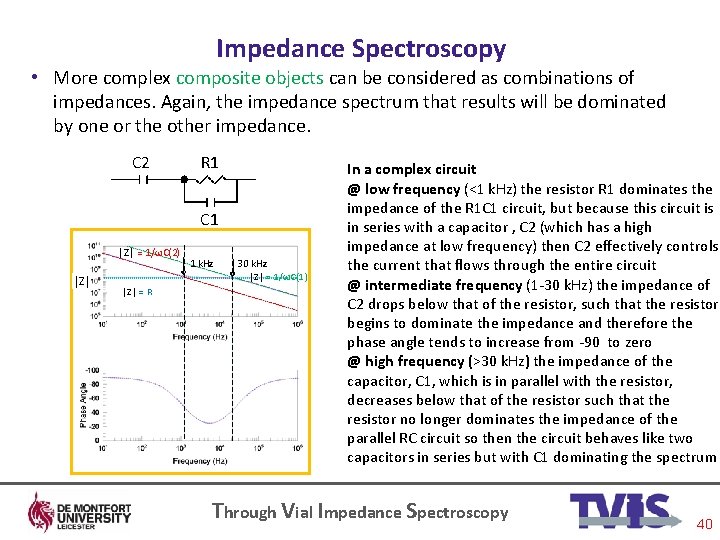
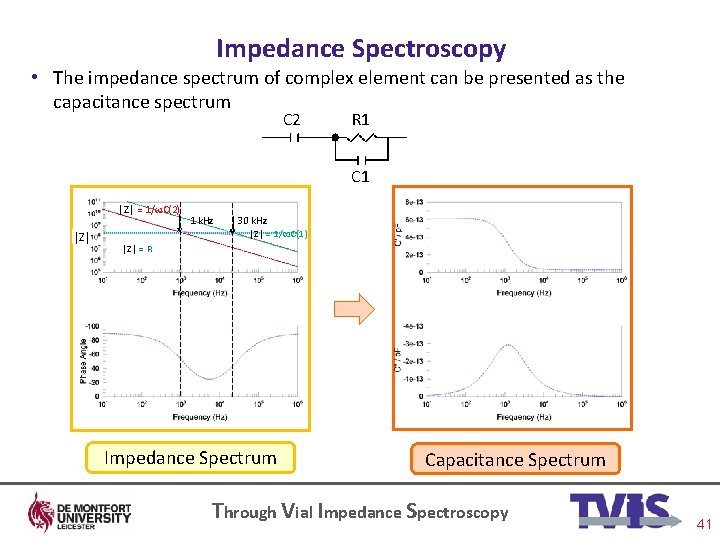
Impedance Spectroscopy • More complex composite objects can be considered as combinations of impedances. Again, the impedance spectrum that results will be dominated by one or the other impedance. C 2 R 1 C 1 │Z│ = 1/ωC(2) │Z│ = R 1 k. Hz 30 k. Hz │Z│ = 1/ωC(1) In a complex circuit @ low frequency (<1 k. Hz) the resistor R 1 dominates the impedance of the R 1 C 1 circuit, but because this circuit is in series with a capacitor , C 2 (which has a high impedance at low frequency) then C 2 effectively controls the current that flows through the entire circuit @ intermediate frequency (1 -30 k. Hz) the impedance of C 2 drops below that of the resistor, such that the resistor begins to dominate the impedance and therefore the phase angle tends to increase from -90 to zero @ high frequency (>30 k. Hz) the impedance of the capacitor, C 1, which is in parallel with the resistor, decreases below that of the resistor such that the resistor no longer dominates the impedance of the parallel RC circuit so then the circuit behaves like two capacitors in series but with C 1 dominating the spectrum Through Vial Impedance Spectroscopy 40

Impedance Spectroscopy • The impedance spectrum of complex element can be presented as the capacitance spectrum C 2 R 1 C 1 │Z│ = 1/ωC(2) │Z│ 1 k. Hz 30 k. Hz │Z│ = 1/ωC(1) │Z│ = R Impedance Spectrum Capacitance Spectrum Through Vial Impedance Spectroscopy 41

Interfacial Polarization Characteristic 0. 40 • -C" /p. F 0. 30 0. 20 0. 10 0. 00 1 2 3 4 5 6 Log Frequency Through Vial Impedance Spectroscopy 42

Interfacial Polarization Characteristic • Through Vial Impedance Spectroscopy 43