Reaction Rates and Le Chateliers Principle Rate of



- Slides: 32

Reaction Rates and Le Chatelier’s Principle

Rate of Reaction • The rate of a chemical reaction – Describes how rapidly a chemical change takes place • They are determined by measuring changes in physical properties – Volume, Temperature, Color, Mass, or p. H

Five Factors that Affect Reaction Rate • • • 1. Nature of the reactants 2. Temperature 3. Concentration 4. Surface Area 5. Catalysts

Chemical Equilibrium • At equilibrium the forward reaction and the reverse reaction happen at the same time – There is no change in the amount of any substance in the reaction • Le Chatelier’s principle can be used to predict the effect of a change in conditions on a chemical equilibrium

Le Chatelier’s Principle Summarized • If a chemical system at equilibrium experiences a change (also called a stress) in concentration, temperature, volume, or total pressure; the equilibrium will shift in order to minimize that change.

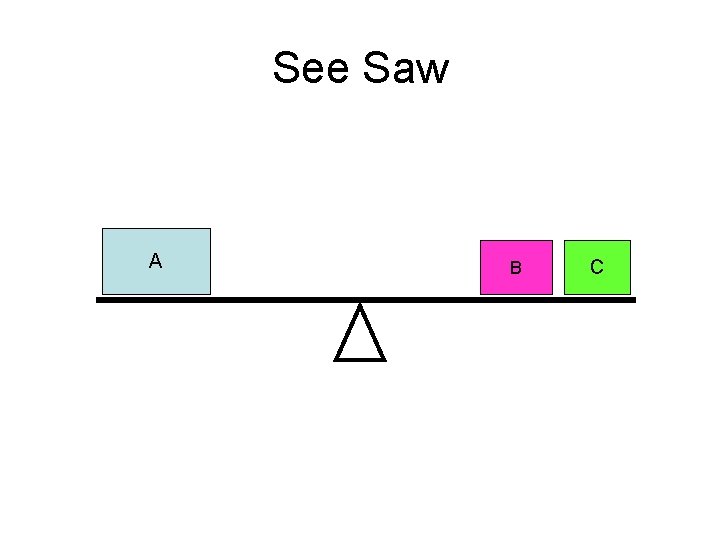
See Saw A B C

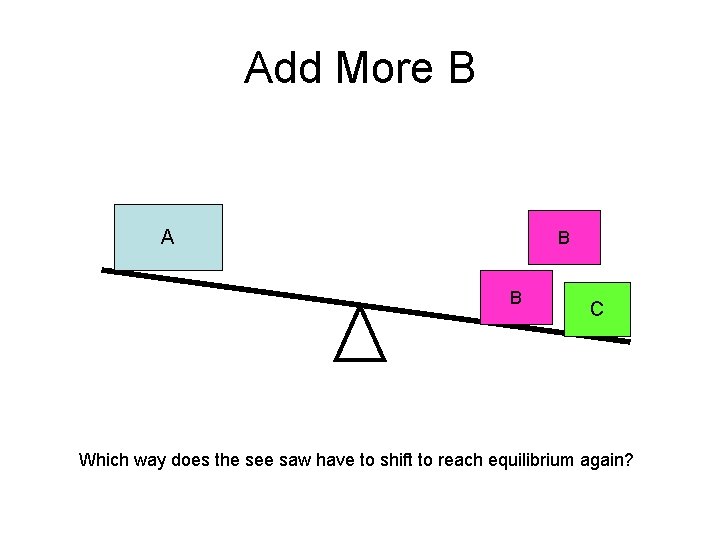
Add More B A B B C Which way does the see saw have to shift to reach equilibrium again?

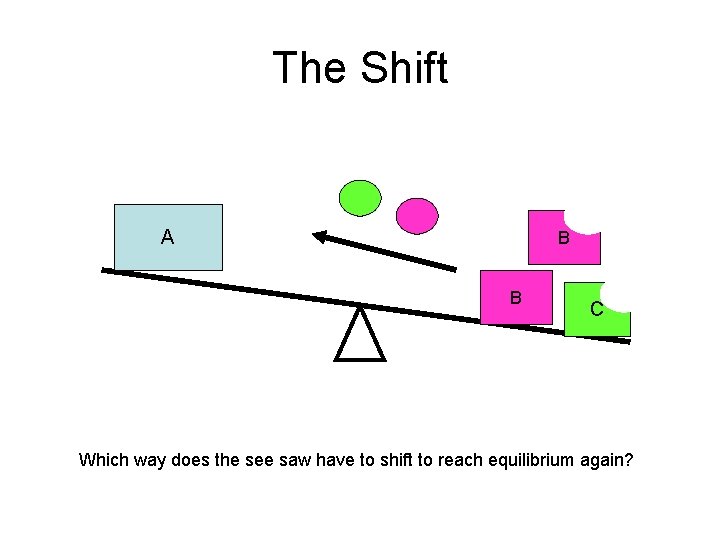
The Shift A B B C Which way does the see saw have to shift to reach equilibrium again?

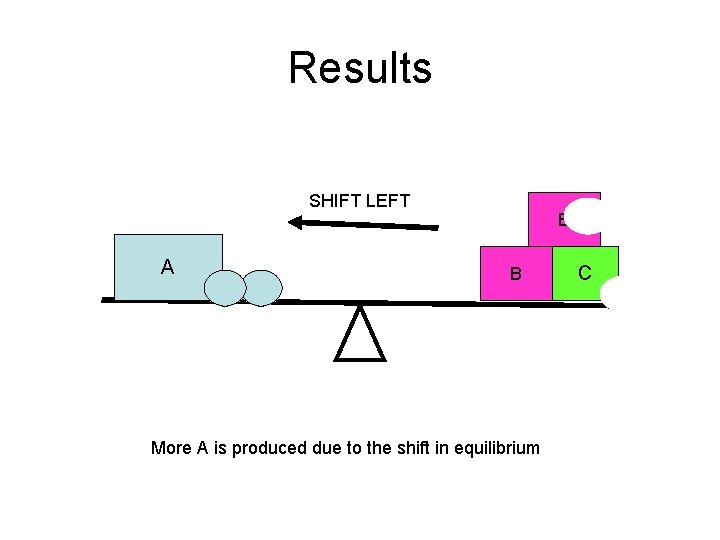
Results SHIFT LEFT A B B More A is produced due to the shift in equilibrium C

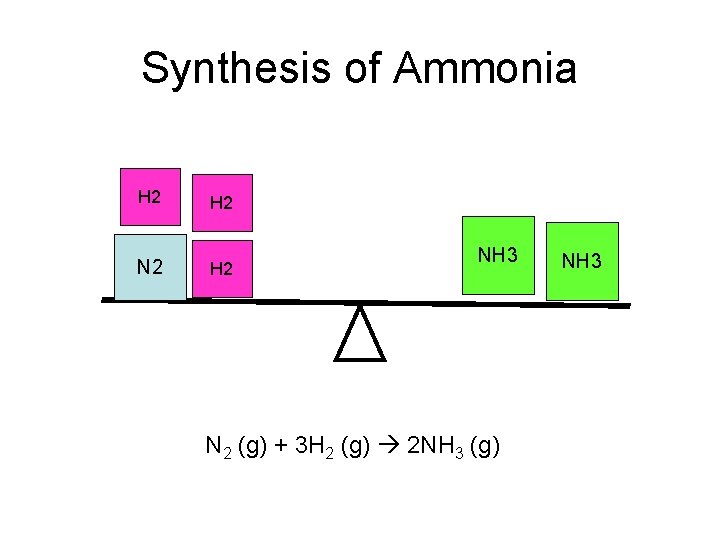
Synthesis of Ammonia H 2 N 2 H 2 NH 3 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) NH 3

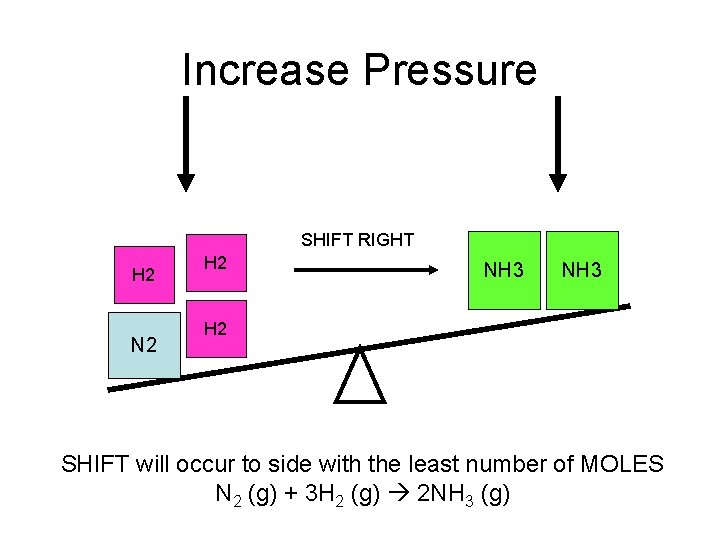
Increase Pressure SHIFT RIGHT H 2 N 2 H 2 NH 3 H 2 SHIFT will occur to side with the least number of MOLES N 2 (g) + 3 H 2 (g) 2 NH 3 (g)

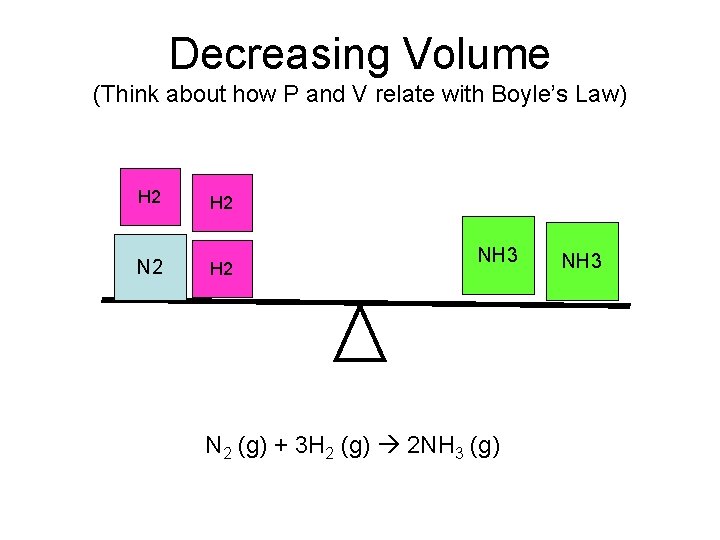
Decreasing Volume (Think about how P and V relate with Boyle’s Law) H 2 N 2 H 2 NH 3 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) NH 3

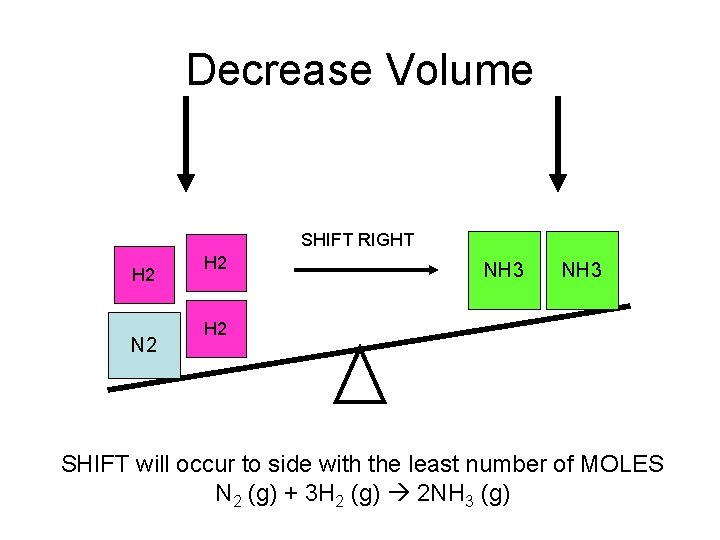
Decrease Volume SHIFT RIGHT H 2 N 2 H 2 NH 3 H 2 SHIFT will occur to side with the least number of MOLES N 2 (g) + 3 H 2 (g) 2 NH 3 (g)

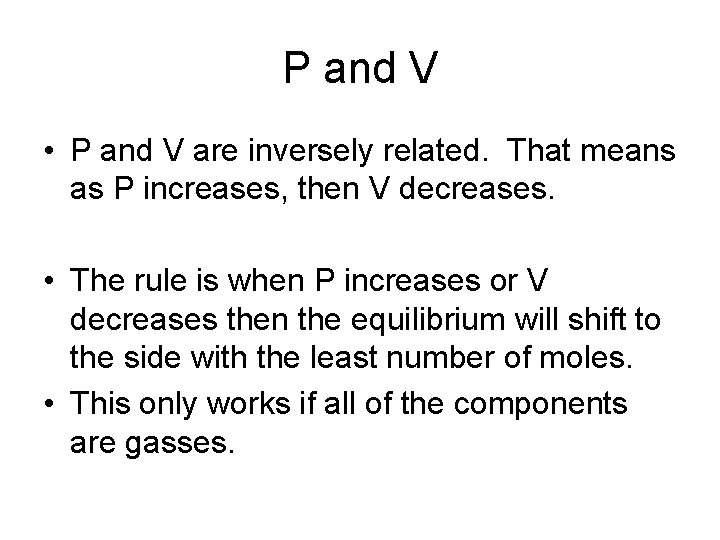
P and V • P and V are inversely related. That means as P increases, then V decreases. • The rule is when P increases or V decreases then the equilibrium will shift to the side with the least number of moles. • This only works if all of the components are gasses.

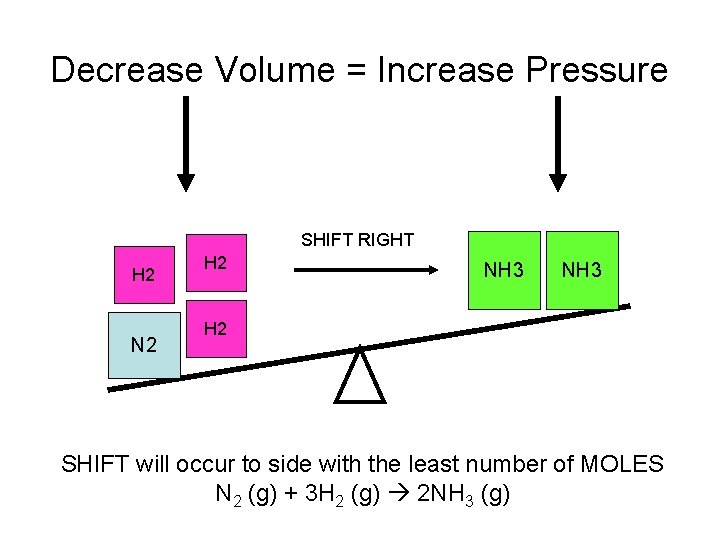
Decrease Volume = Increase Pressure SHIFT RIGHT H 2 N 2 H 2 NH 3 H 2 SHIFT will occur to side with the least number of MOLES N 2 (g) + 3 H 2 (g) 2 NH 3 (g)

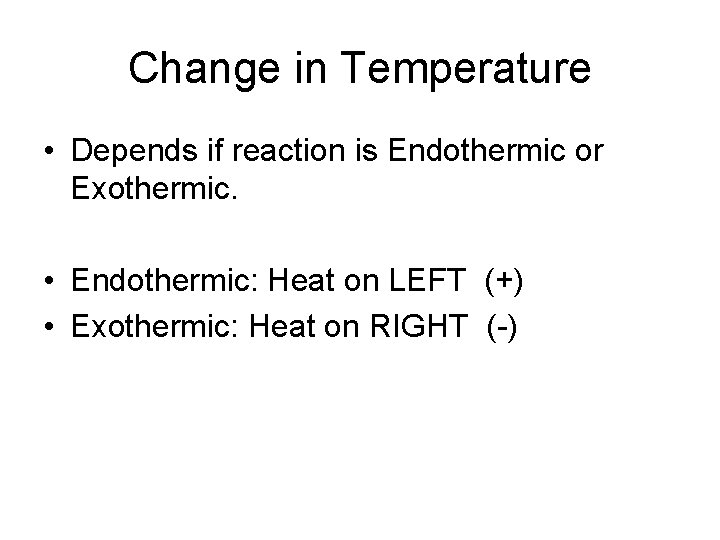
Change in Temperature • Depends if reaction is Endothermic or Exothermic. • Endothermic: Heat on LEFT (+) • Exothermic: Heat on RIGHT (-)

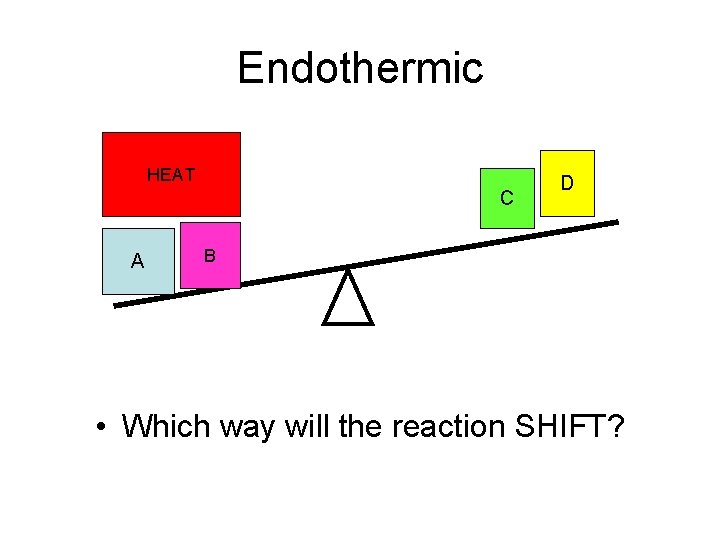
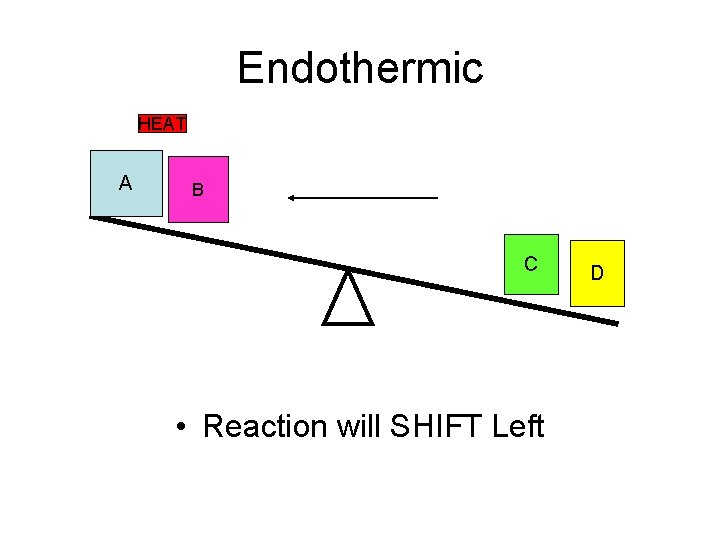
Endothermic HEAT A B C D • Increase Temperature… which way will the see saw tip?

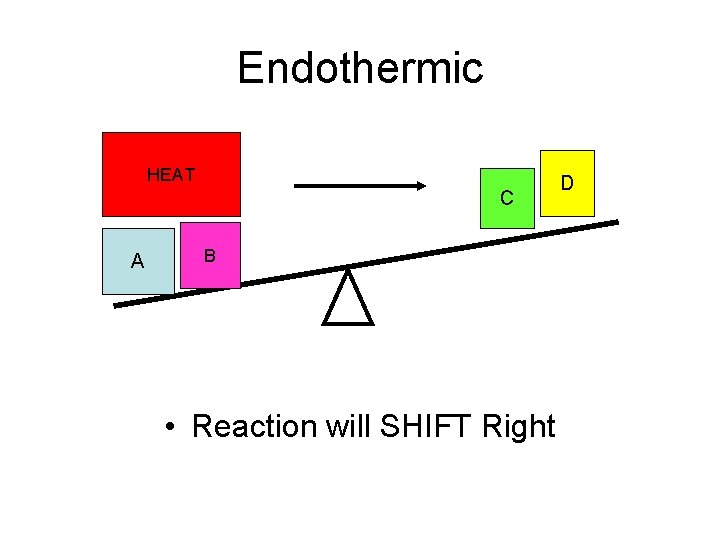
Endothermic HEAT C A D B • Which way will the reaction SHIFT?

Endothermic HEAT C A B • Reaction will SHIFT Right D

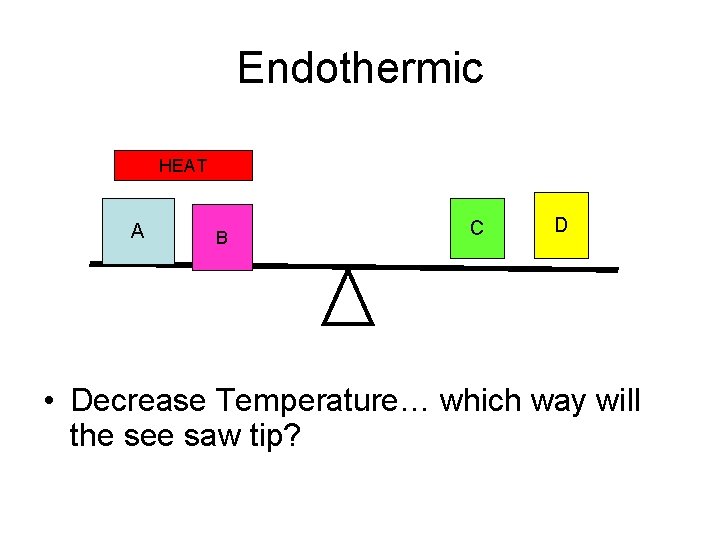
Endothermic HEAT A B C D • Decrease Temperature… which way will the see saw tip?

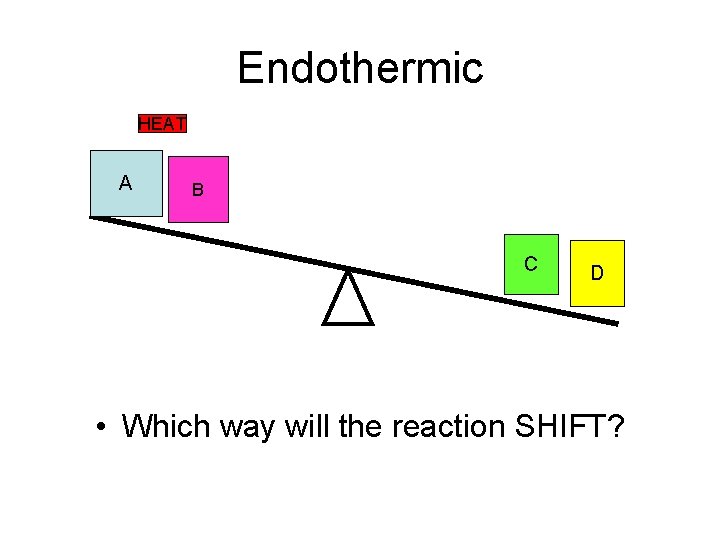
Endothermic HEAT A B C D • Which way will the reaction SHIFT?

Endothermic HEAT A B C • Reaction will SHIFT Left D

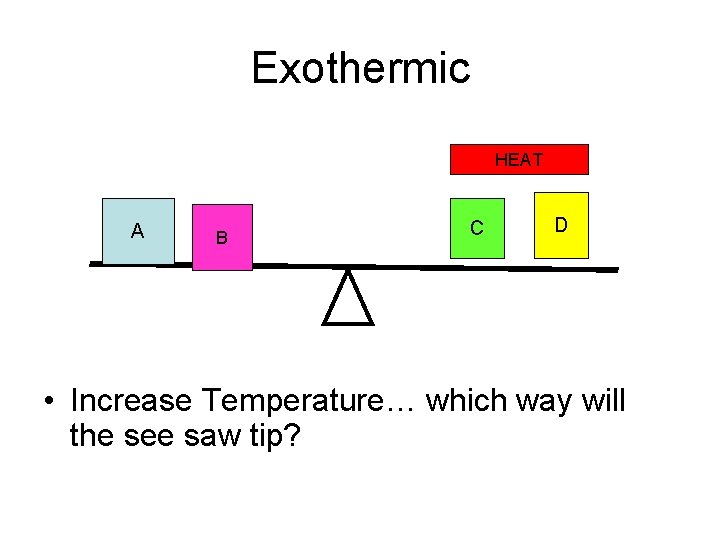
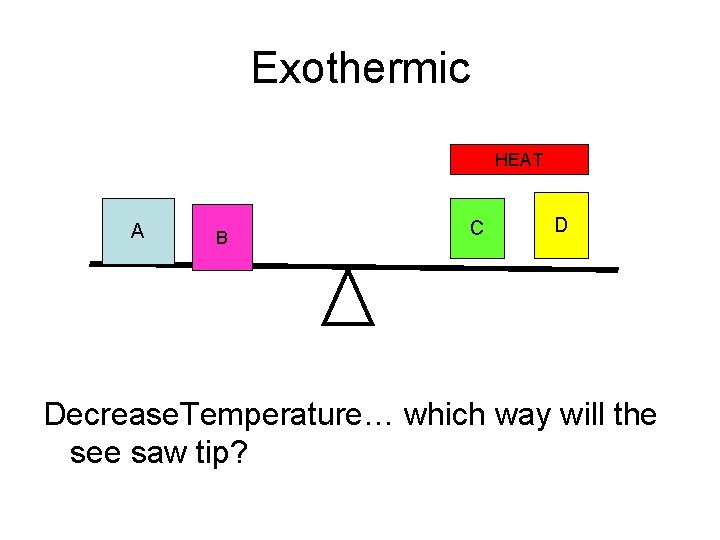
Exothermic HEAT A B C D • Increase Temperature… which way will the see saw tip?

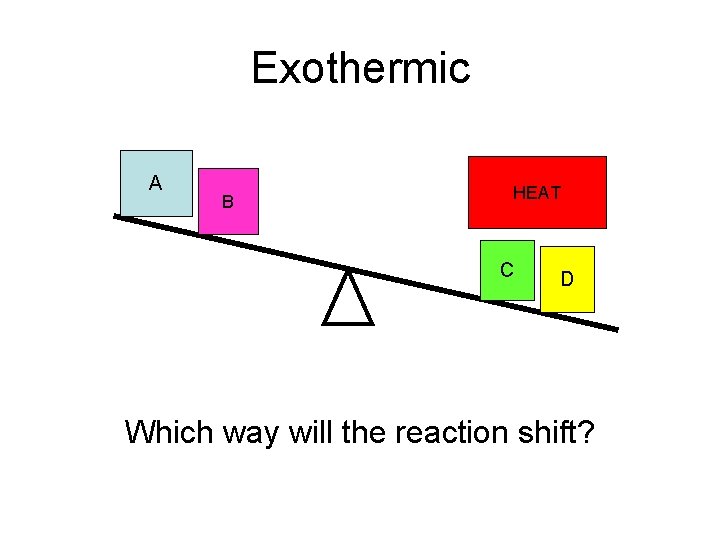
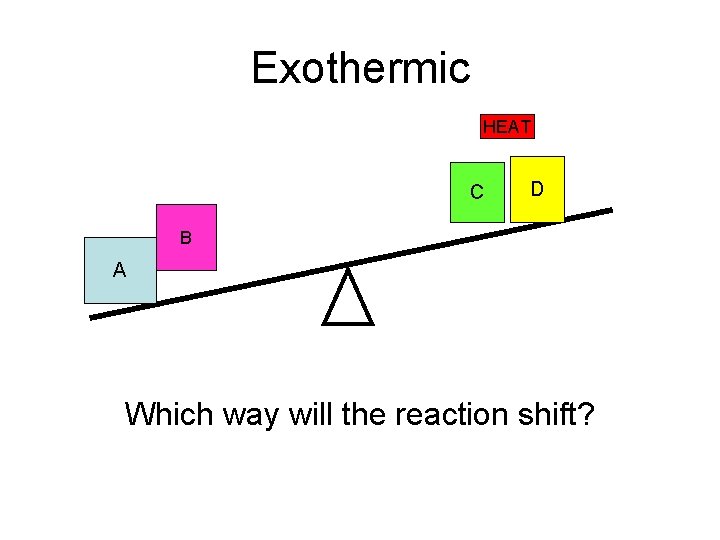
Exothermic A B HEAT C D Which way will the reaction shift?

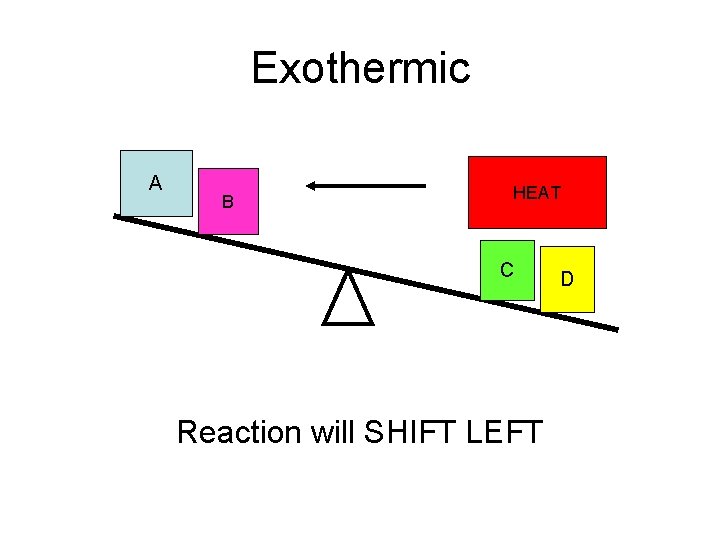
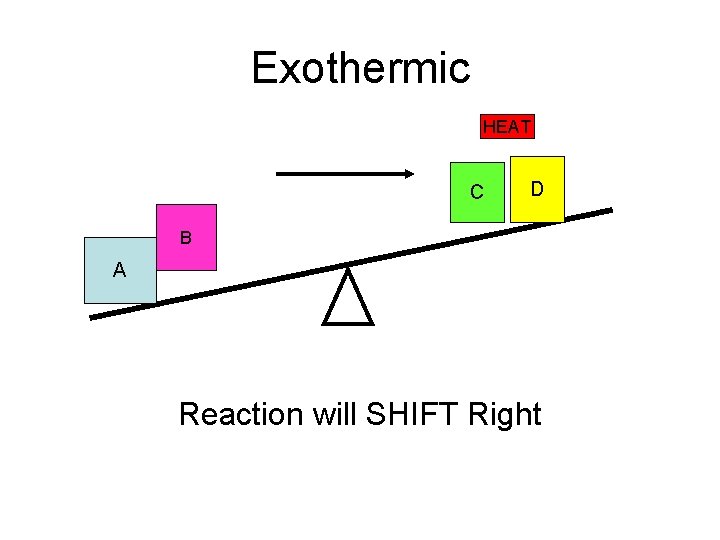
Exothermic A B HEAT C Reaction will SHIFT LEFT D

Exothermic HEAT A B C D Decrease. Temperature… which way will the see saw tip?

Exothermic HEAT C D B A Which way will the reaction shift?

Exothermic HEAT C D B A Reaction will SHIFT Right

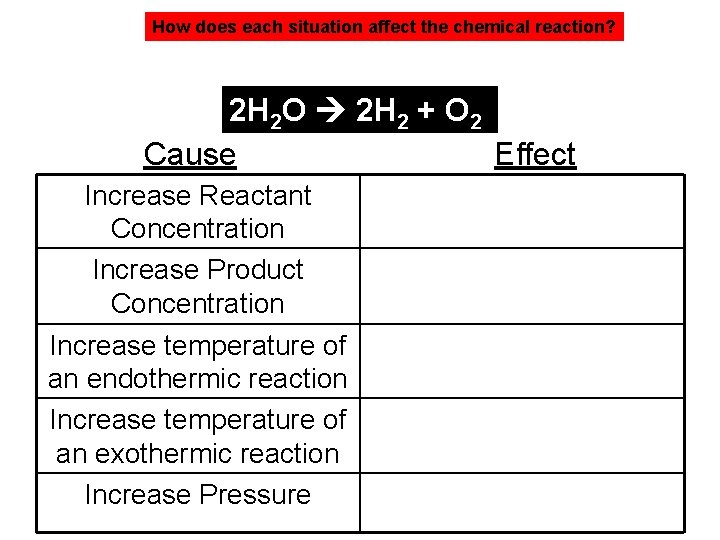
How does each situation affect the chemical reaction? 2 H 2 O 2 H 2 + O 2 Cause Effect Increase Reactant Concentration Increase Product Concentration Increase temperature of an endothermic reaction Increase temperature of an exothermic reaction Increase Pressure

Which INCREASE Reaction Rates? • • Increasing Temperature Increasing Concentration Increasing Surface Area Adding a Catalyst. • How are these factors related to Reaction Rates? – DIRECTLY

Nature of Reactants • Reaction rates are affected by the complexity of the bonds that must be broken and formed in the chemical reaction • The state of a reactant can also affect the reaction rate – Gases have the fastest and solids the slowest reaction rats • Because the frequency at which particles collide and the amount of energy they possess increase with increasing motion.

Continued • The faster particles are moving, the more frequent they will collide – Collide with greater kinetic energy. • Because of this TEMPERATURE and REACTION RATE are directly related
 Le chatelier's principle
Le chatelier's principle Qc in chemistry
Qc in chemistry Le chatelier principle
Le chatelier principle Unit rate
Unit rate Ratios and proportions guided notes
Ratios and proportions guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
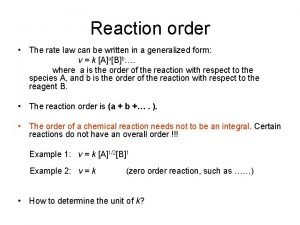
Ratios rates and unit rates Order of reaction
Order of reaction Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rates of reaction can be positive or negative. true false
Rates of reaction can be positive or negative. true false Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur Addition reaction and substitution reaction
Addition reaction and substitution reaction Bomb power
Bomb power What is catalyst and how it affects reaction rate
What is catalyst and how it affects reaction rate Reaction rate and stoichiometry
Reaction rate and stoichiometry Nominal v. real interest rates
Nominal v. real interest rates Define growth analysis
Define growth analysis Spot forward rate formula
Spot forward rate formula 1 year forward rate formula
1 year forward rate formula What is the difference between rate and unit rate
What is the difference between rate and unit rate Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Equilibrium reaction rate
Equilibrium reaction rate First order rate law
First order rate law Molecularity of reaction
Molecularity of reaction How to calculate rate of reaction
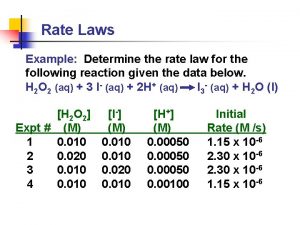
How to calculate rate of reaction How to find the rate law
How to find the rate law