LE CHATELIERS PRINCIPLE LE CHATELIERS PRINCIPLE If any






- Slides: 12

LE CHATELIER’S PRINCIPLE

LE CHATELIER’S PRINCIPLE • If any stress is placed on the system, the equilibrium will shift to overcome the stress • Stressors include: –Adding products/reactants, changing temperature, etc. (will go into detail throughout the PPT)

HOW TO PREDICT • Look at which side has gained something from the initial stress –The reaction will then shift to the side opposite the side that gained from the stress

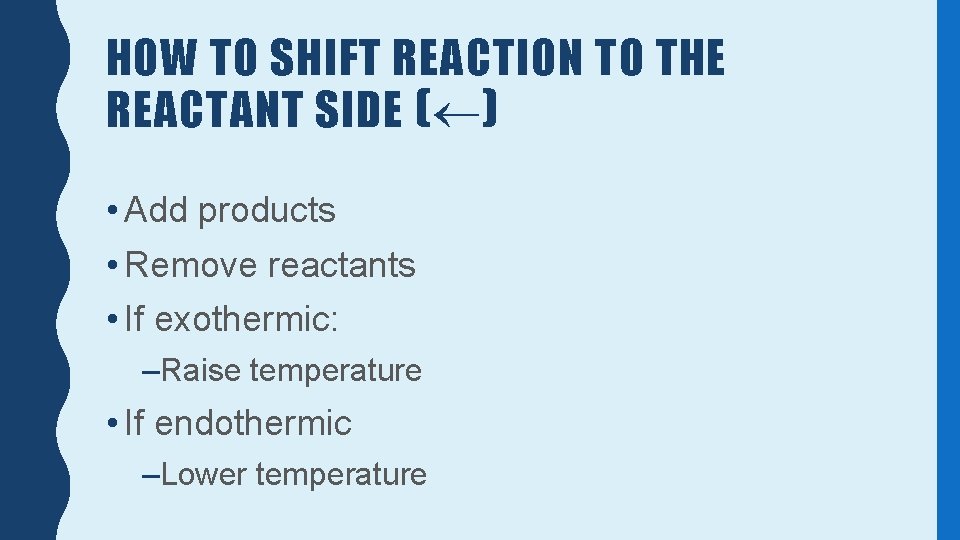
HOW TO SHIFT REACTION TO THE REACTANT SIDE ( ) • Add products • Remove reactants • If exothermic: –Raise temperature • If endothermic –Lower temperature

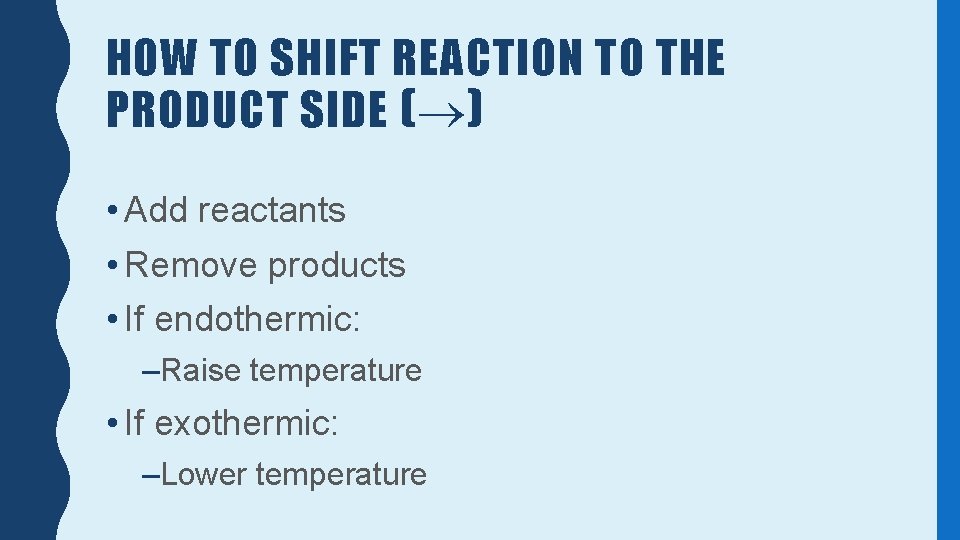
HOW TO SHIFT REACTION TO THE PRODUCT SIDE ( ) • Add reactants • Remove products • If endothermic: –Raise temperature • If exothermic: –Lower temperature

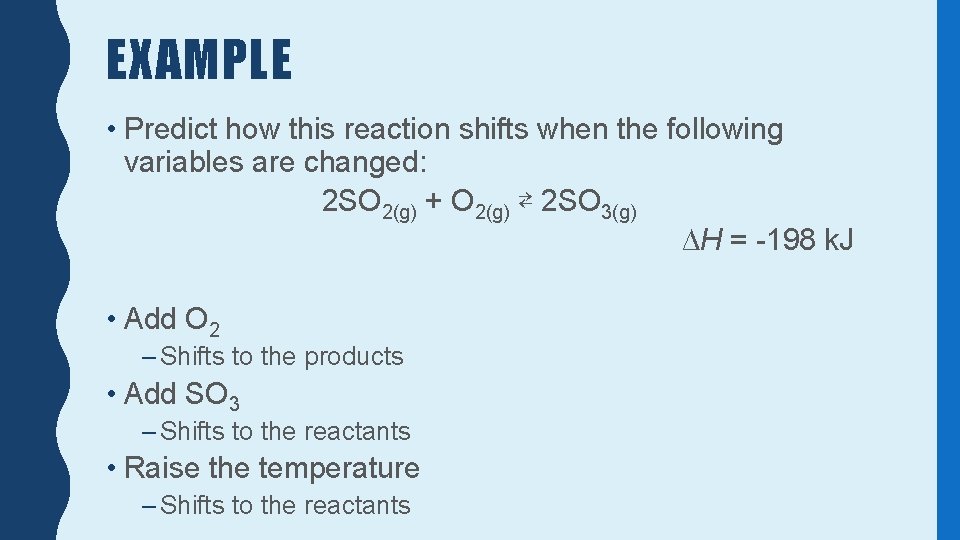
EXAMPLE • Predict how this reaction shifts when the following variables are changed: 2 SO 2(g) + O 2(g) ⇄ 2 SO 3(g) ∆H = -198 k. J • Add O 2 – Shifts to the products • Add SO 3 – Shifts to the reactants • Raise the temperature – Shifts to the reactants

SPECIAL NOTE ABOUT TEMP CHANGES • Temperature changes also affect Keq values: –If a temperature change shifts the equilibrium to the right ( ), then Keq will INCREASE –If a temperature change shifts the equilibrium to the left ( ), then Keq will DECREASE

WITH GASES • If pressure increases, the equilibrium shifts to the side with less total moles of gas • If pressure decreases, the equilibrium shifts to the side with more total moles of gas

CATALYST • If you add a catalyst, there is no effect on equilibrium

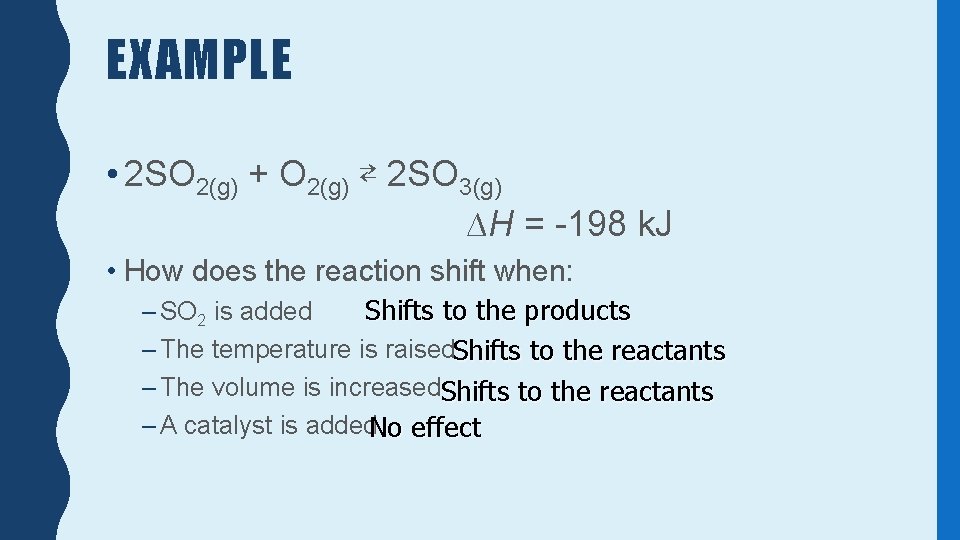
EXAMPLE • 2 SO 2(g) + O 2(g) ⇄ 2 SO 3(g) ∆H = -198 k. J • How does the reaction shift when: Shifts to the products – SO 2 is added – The temperature is raised. Shifts to the reactants – The volume is increased. Shifts to the reactants – A catalyst is added. No effect

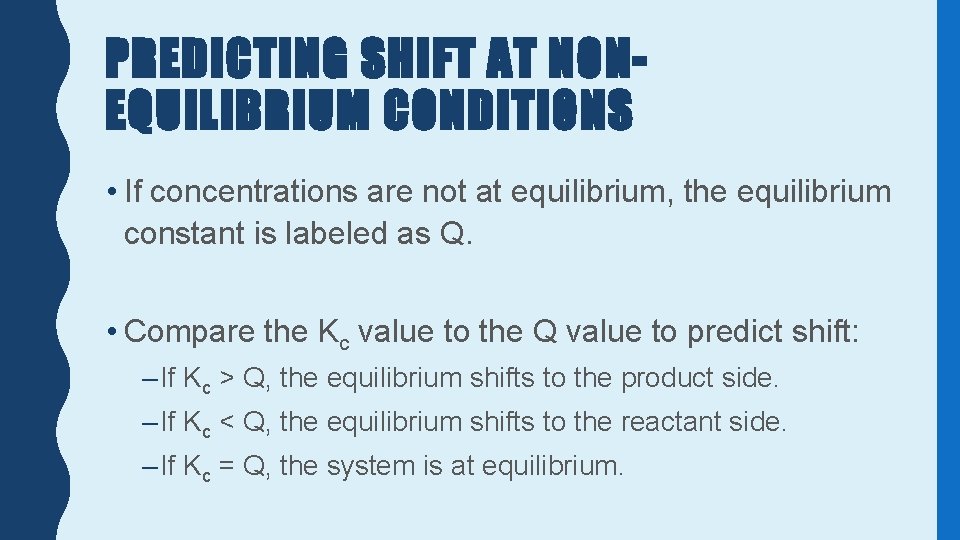
PREDICTING SHIFT AT NONEQUILIBRIUM CONDITIONS • If concentrations are not at equilibrium, the equilibrium constant is labeled as Q. • Compare the Kc value to the Q value to predict shift: – If Kc > Q, the equilibrium shifts to the product side. – If Kc < Q, the equilibrium shifts to the reactant side. – If Kc = Q, the system is at equilibrium.

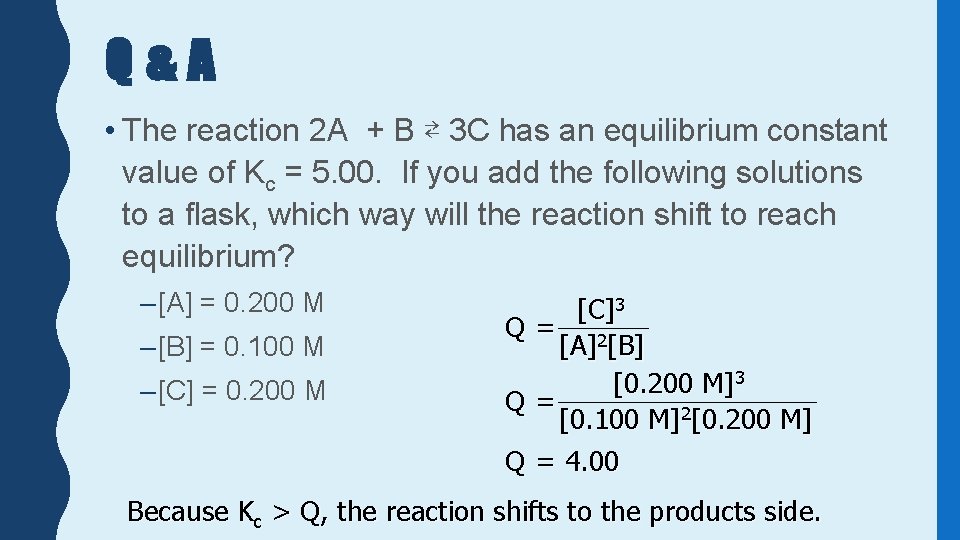
Q&A • The reaction 2 A + B ⇄ 3 C has an equilibrium constant value of Kc = 5. 00. If you add the following solutions to a flask, which way will the reaction shift to reach equilibrium? – [A] = 0. 200 M – [B] = 0. 100 M – [C] = 0. 200 M [C]3 Q= 2 [A] [B] [0. 200 M]3 Q= [0. 100 M]2[0. 200 M] Q = 4. 00 Because Kc > Q, the reaction shifts to the products side.