Predicting Directions of a Reaction SCH 4 U


![Solution • Qc= [NH 3]2 [N 2][H 2]3 = (0. 20)2 (0. 10)(0. 30)3 Solution • Qc= [NH 3]2 [N 2][H 2]3 = (0. 20)2 (0. 10)(0. 30)3](https://slidetodoc.com/presentation_image_h/a3385bfac25b0ea5260f70bd927154ea/image-9.jpg)







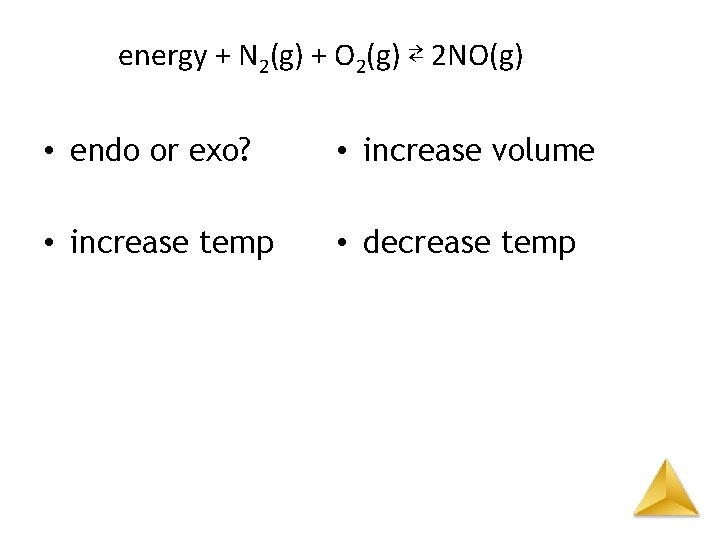
- Slides: 35

Predicting Directions of a Reaction SCH 4 U/ AP

Predicting the Direction of a Reaction • So far, you have worked with reactions that have reached equilibrium. What if a reaction has not yet reached equilibrium? How can we predict the direction in which the reaction must proceed to reach equilibrium?

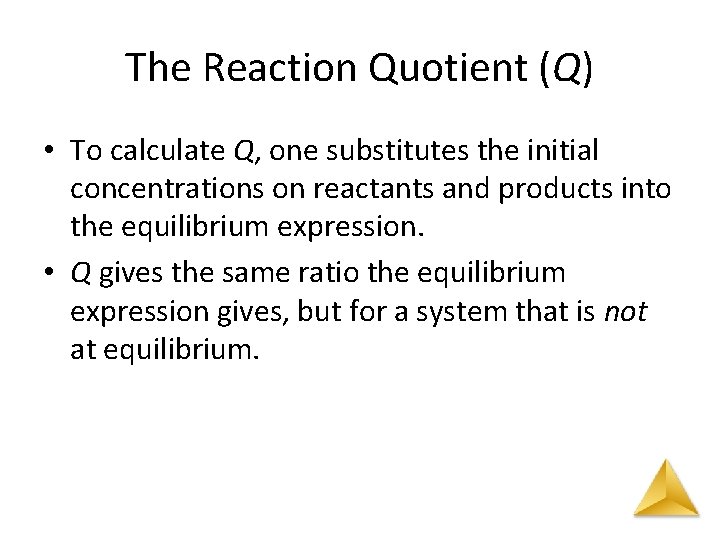
The Reaction Quotient (Q) • To calculate Q, one substitutes the initial concentrations on reactants and products into the equilibrium expression. • Q gives the same ratio the equilibrium expression gives, but for a system that is not at equilibrium.

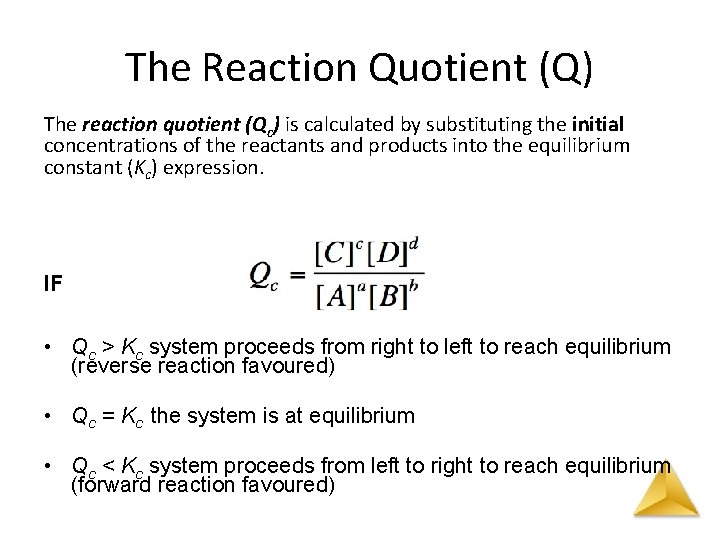
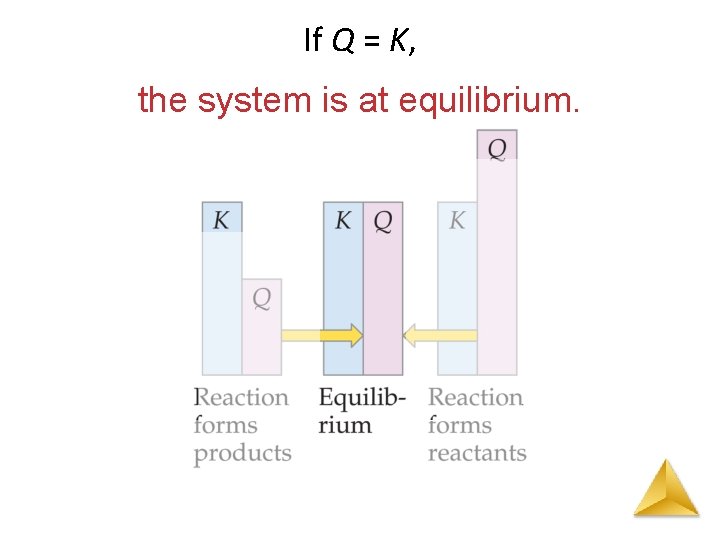
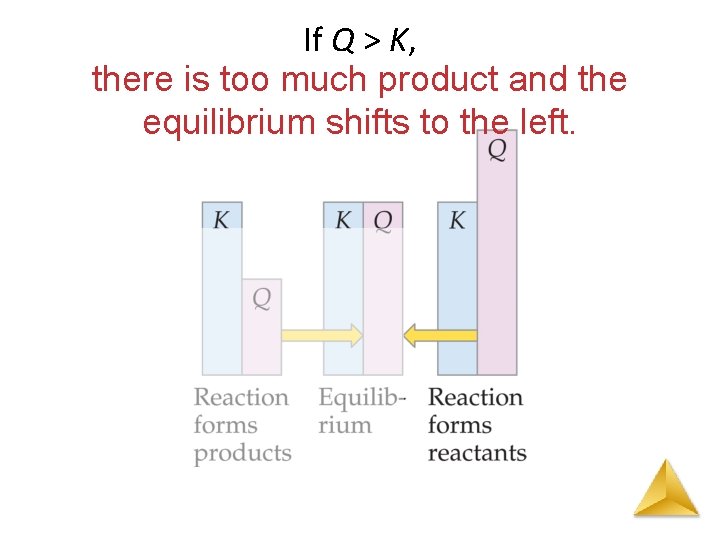
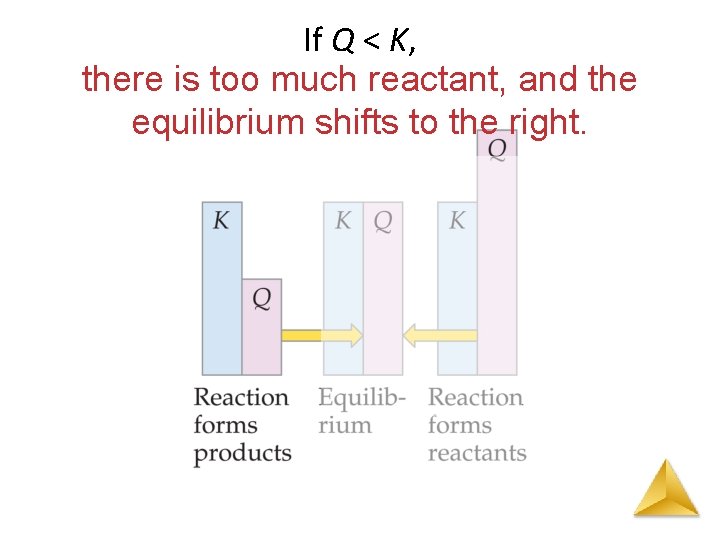
The Reaction Quotient (Q) The reaction quotient (Qc) is calculated by substituting the initial concentrations of the reactants and products into the equilibrium constant (Kc) expression. IF • Qc > Kc system proceeds from right to left to reach equilibrium (reverse reaction favoured) • Qc = Kc the system is at equilibrium • Qc < Kc system proceeds from left to right to reach equilibrium (forward reaction favoured)

If Q = K, the system is at equilibrium.

If Q > K, there is too much product and the equilibrium shifts to the left.

If Q < K, there is too much reactant, and the equilibrium shifts to the right.

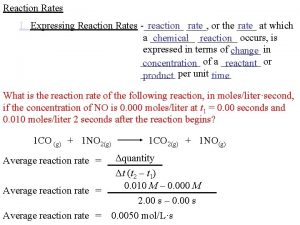
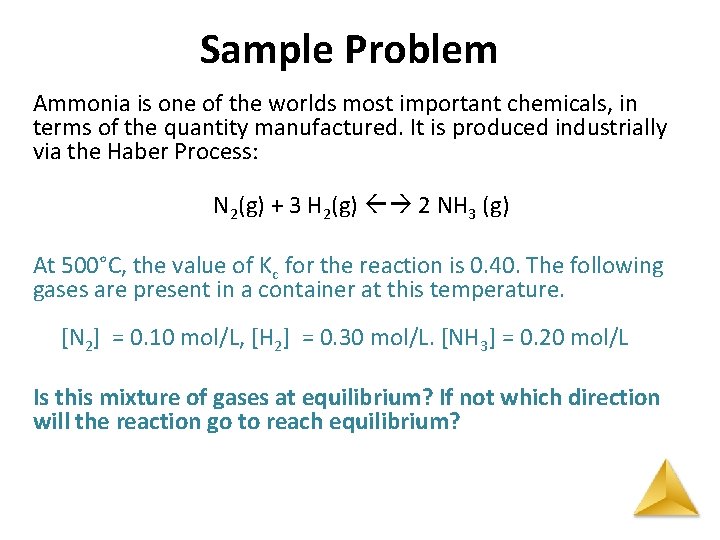
Sample Problem Ammonia is one of the worlds most important chemicals, in terms of the quantity manufactured. It is produced industrially via the Haber Process: N 2(g) + 3 H 2(g) 2 NH 3 (g) At 500°C, the value of Kc for the reaction is 0. 40. The following gases are present in a container at this temperature. [N 2] = 0. 10 mol/L, [H 2] = 0. 30 mol/L. [NH 3] = 0. 20 mol/L Is this mixture of gases at equilibrium? If not which direction will the reaction go to reach equilibrium?
![Solution Qc NH 32 N 2H 23 0 202 0 100 303 Solution • Qc= [NH 3]2 [N 2][H 2]3 = (0. 20)2 (0. 10)(0. 30)3](https://slidetodoc.com/presentation_image_h/a3385bfac25b0ea5260f70bd927154ea/image-9.jpg)
Solution • Qc= [NH 3]2 [N 2][H 2]3 = (0. 20)2 (0. 10)(0. 30)3 = 14. 8 Therefore, Qc > 0. 40 The system is not at equilibrium. The reaction will proceed by moving left (i. e. , the reverse reaction must take place)

Practice Question #1

Practice Question #1 Solution

Practice Question #2

Practice Question #2 Solution

Practice Question #3

Practice Question #3 Solution

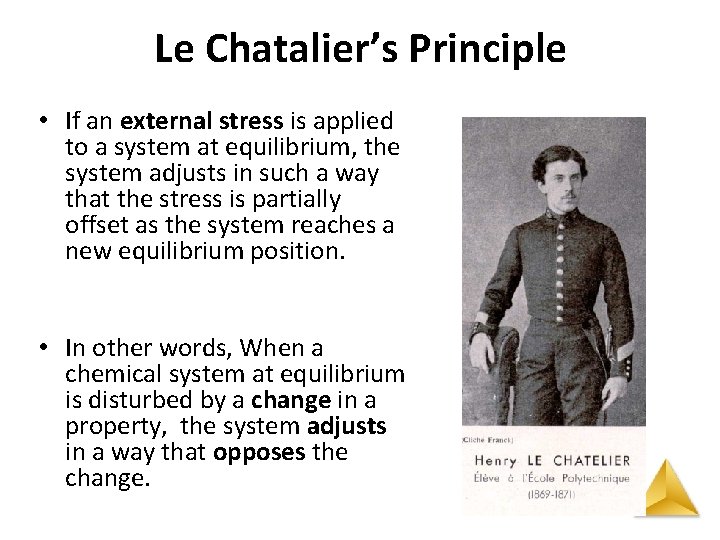
Le Chatalier’s Principle • If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position. • In other words, When a chemical system at equilibrium is disturbed by a change in a property, the system adjusts in a way that opposes the change.

Le Chatalier’s Princple Le Chatelier’s Principle: if you disturb an equilibrium, it will shift to undo the disturbance. Equilibrium shift = movement of a system at equilibrium, resulting in a change in the concentrations of reactants and products • https: //www. youtube. com/watch? v=d. IDg. PFE uc. FM

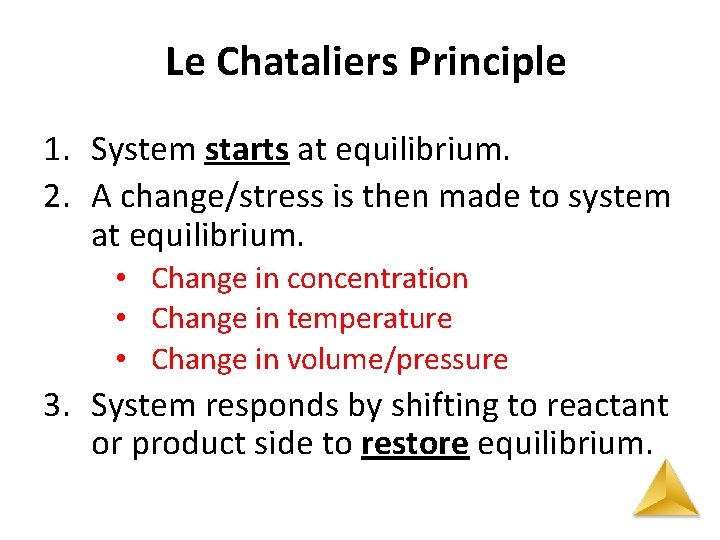
Le Chataliers Principle 1. System starts at equilibrium. 2. A change/stress is then made to system at equilibrium. • Change in concentration • Change in temperature • Change in volume/pressure 3. System responds by shifting to reactant or product side to restore equilibrium.

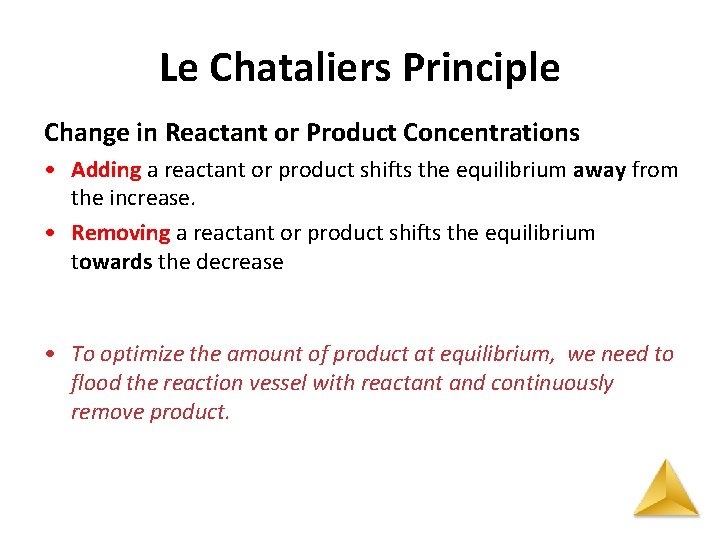
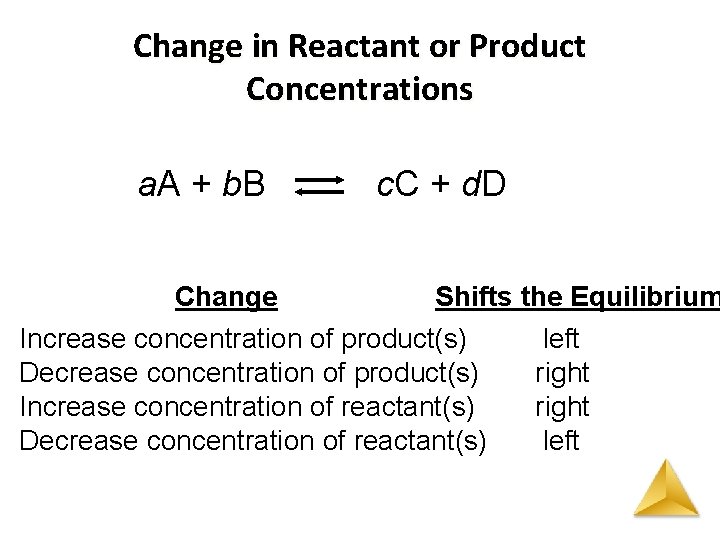
Le Chataliers Principle Change in Reactant or Product Concentrations • Adding a reactant or product shifts the equilibrium away from the increase. • Removing a reactant or product shifts the equilibrium towards the decrease • To optimize the amount of product at equilibrium, we need to flood the reaction vessel with reactant and continuously remove product.

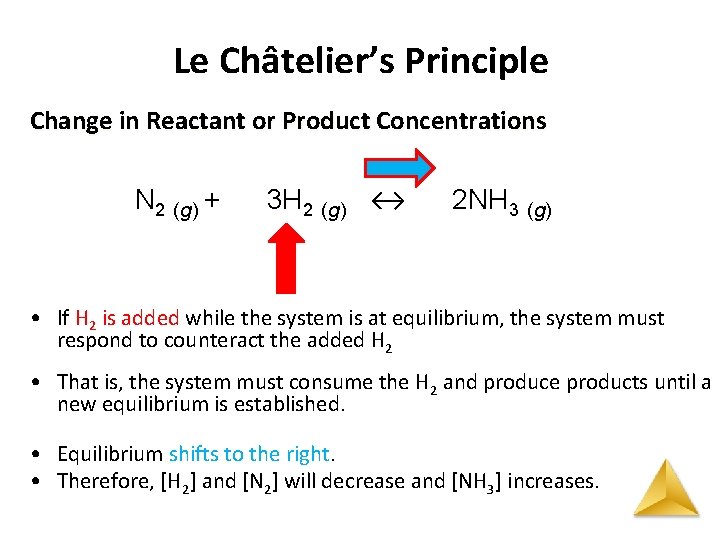
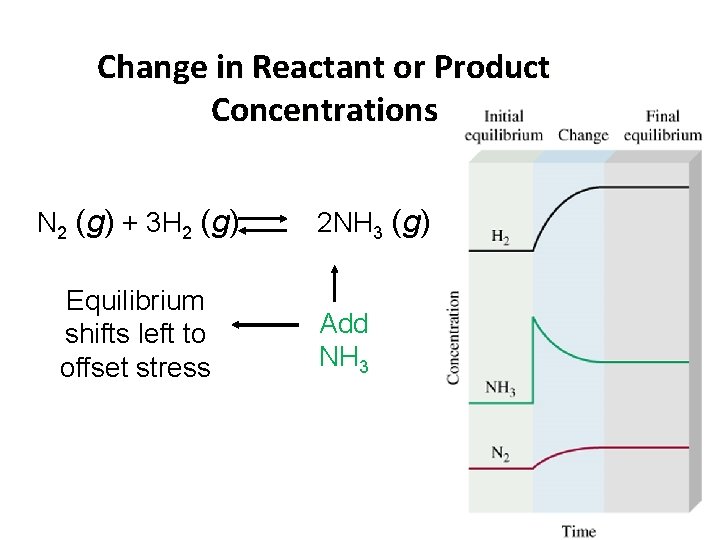
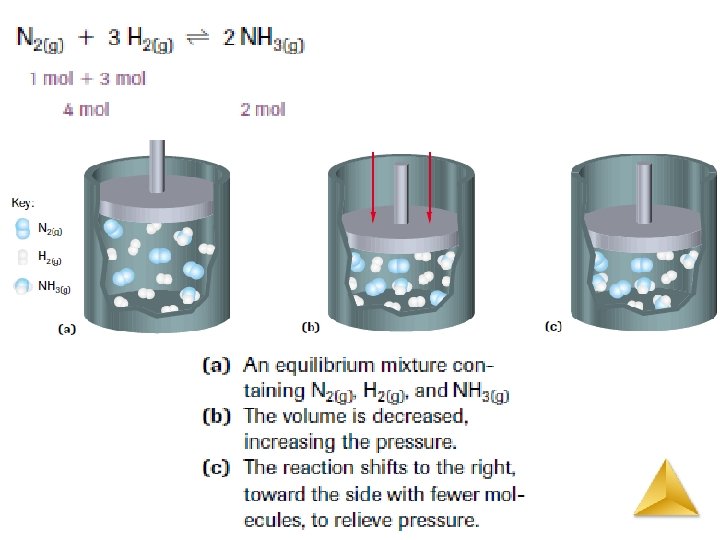
Le Châtelier’s Principle Change in Reactant or Product Concentrations N 2 (g) + 3 H 2 (g) ↔ 2 NH 3 (g) • If H 2 is added while the system is at equilibrium, the system must respond to counteract the added H 2 • That is, the system must consume the H 2 and produce products until a new equilibrium is established. • Equilibrium shifts to the right. • Therefore, [H 2] and [N 2] will decrease and [NH 3] increases.

Change in Reactant or Product Concentrations N 2 (g) + 3 H 2 (g) Equilibrium shifts left to offset stress 2 NH 3 (g) Add NH 3

Change in Reactant or Product Concentrations a. A + b. B c. C + d. D Change Shifts the Equilibrium Increase concentration of product(s) left Decrease concentration of product(s) right Increase concentration of reactant(s) right Decrease concentration of reactant(s) left

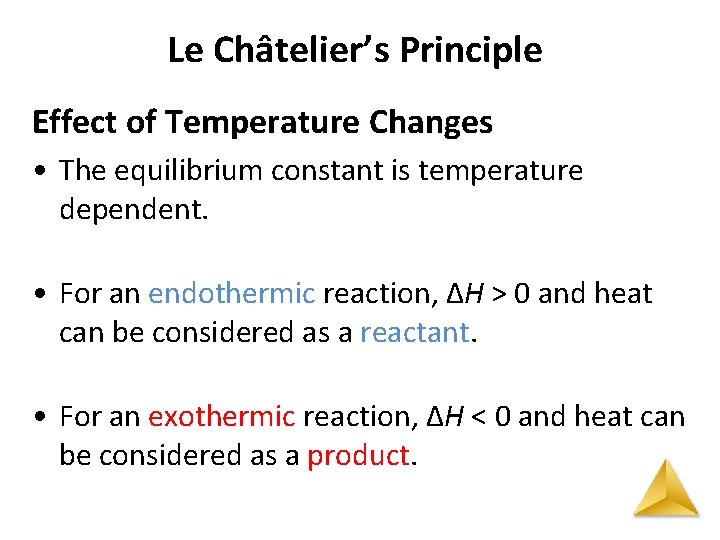
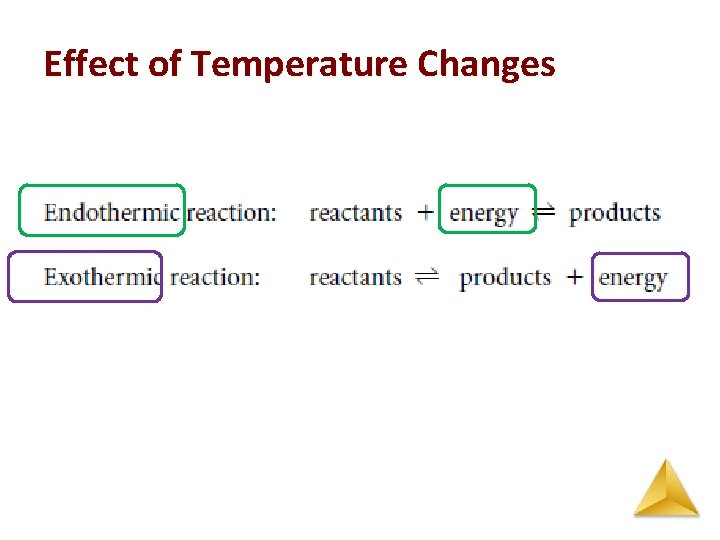
Le Châtelier’s Principle Effect of Temperature Changes • The equilibrium constant is temperature dependent. • For an endothermic reaction, ΔH > 0 and heat can be considered as a reactant. • For an exothermic reaction, ΔH < 0 and heat can be considered as a product.

Effect of Temperature Changes

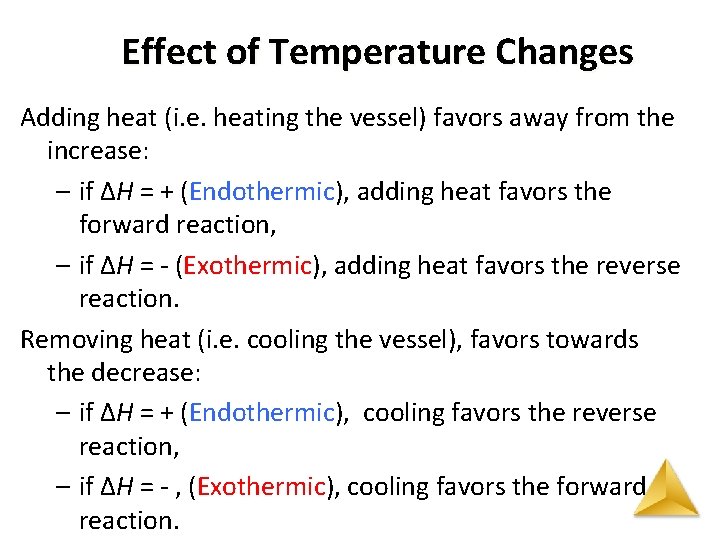
Effect of Temperature Changes Adding heat (i. e. heating the vessel) favors away from the increase: – if ΔH = + (Endothermic), adding heat favors the forward reaction, – if ΔH = - (Exothermic), adding heat favors the reverse reaction. Removing heat (i. e. cooling the vessel), favors towards the decrease: – if ΔH = + (Endothermic), cooling favors the reverse reaction, – if ΔH = - , (Exothermic), cooling favors the forward reaction.

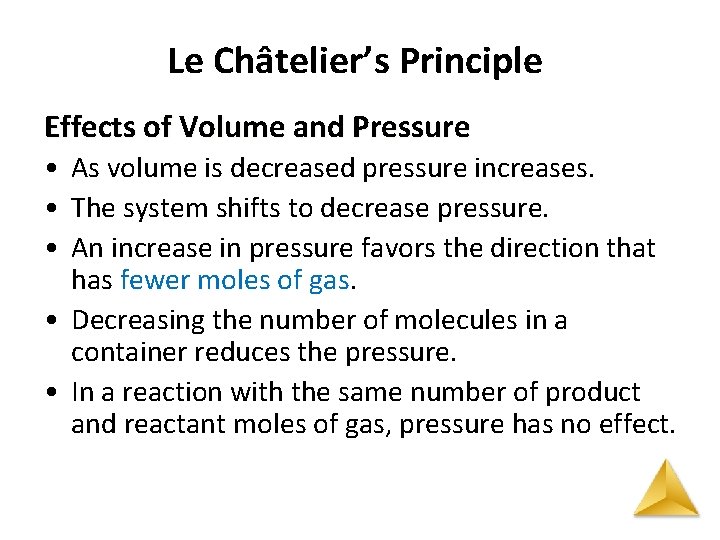
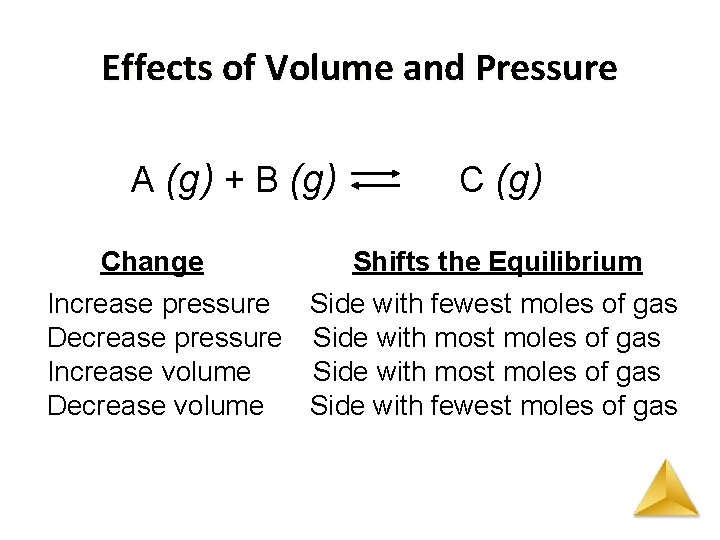
Le Châtelier’s Principle Effects of Volume and Pressure • As volume is decreased pressure increases. • The system shifts to decrease pressure. • An increase in pressure favors the direction that has fewer moles of gas. • Decreasing the number of molecules in a container reduces the pressure. • In a reaction with the same number of product and reactant moles of gas, pressure has no effect.


Effects of Volume and Pressure A (g) + B (g) Change C (g) Shifts the Equilibrium Increase pressure Side with fewest moles of gas Decrease pressure Side with most moles of gas Increase volume Side with most moles of gas Decrease volume Side with fewest moles of gas

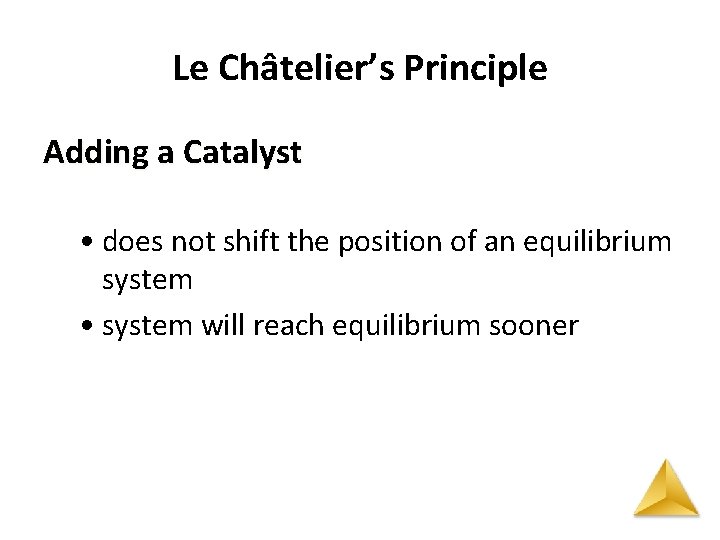
Le Châtelier’s Principle Adding a Catalyst • does not shift the position of an equilibrium system • system will reach equilibrium sooner

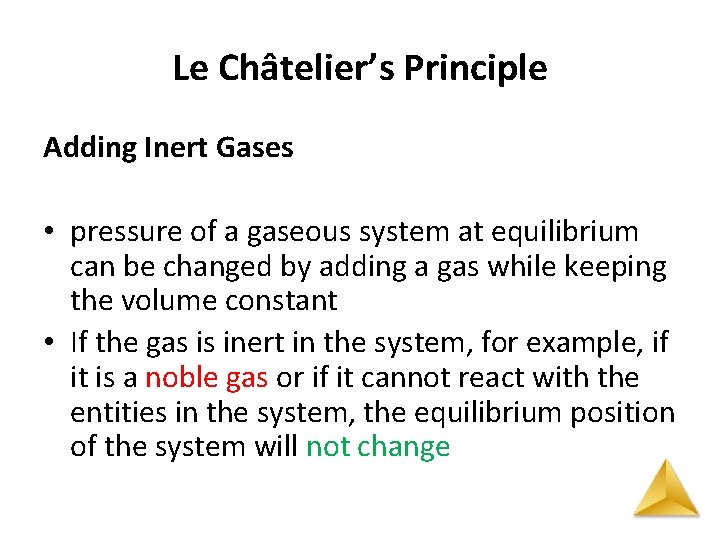
Le Châtelier’s Principle Adding Inert Gases • pressure of a gaseous system at equilibrium can be changed by adding a gas while keeping the volume constant • If the gas is inert in the system, for example, if it is a noble gas or if it cannot react with the entities in the system, the equilibrium position of the system will not change

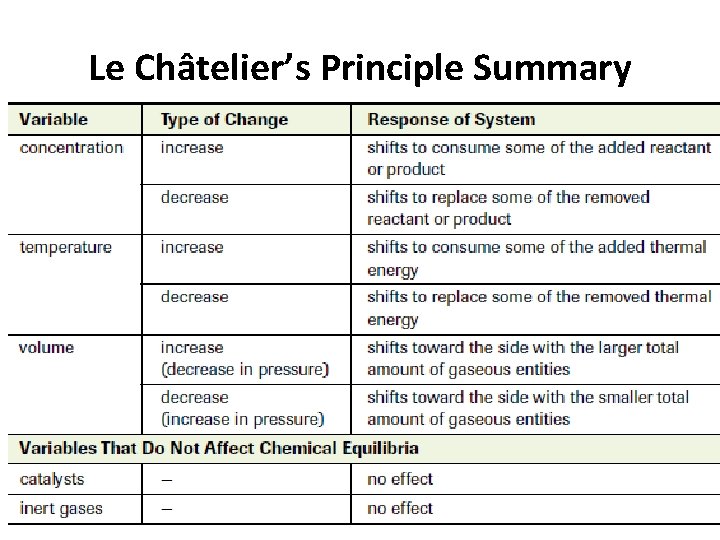
Le Châtelier’s Principle Summary

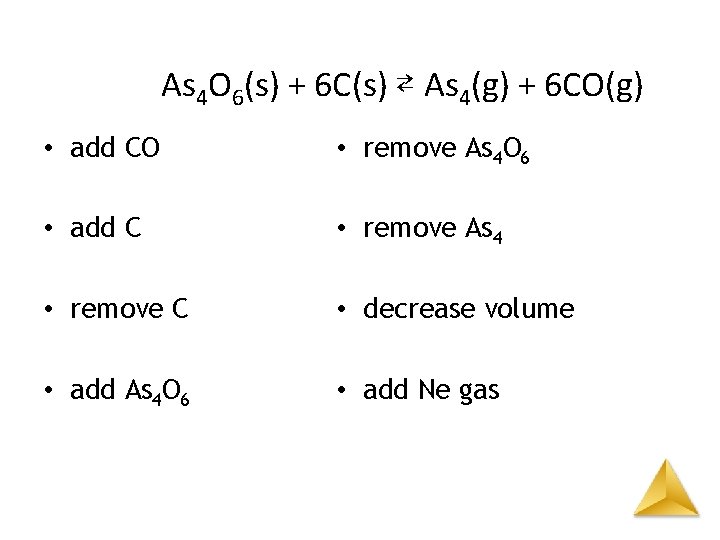
As 4 O 6(s) + 6 C(s) ⇄ As 4(g) + 6 CO(g) • add CO – to left • add C – no shift • remove C – no shift • add As 4 O 6 – no shift • remove As 4 O 6 – no shift • remove As 4 – to right • decrease volume – to left • add Ne gas – no shift

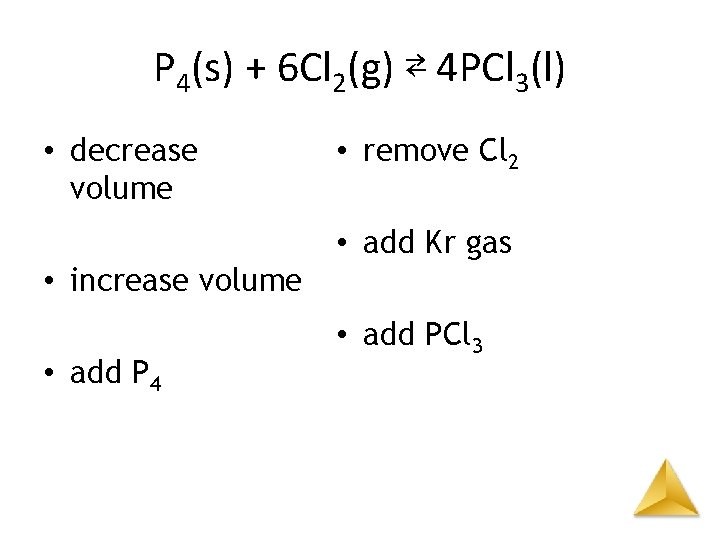
P 4(s) + 6 Cl 2(g) ⇄ 4 PCl 3(l) • decrease volume – to right • increase volume – to left • add P 4 – no shift • remove Cl 2 – to left • add Kr gas – no shift • add PCl 3 – no shift
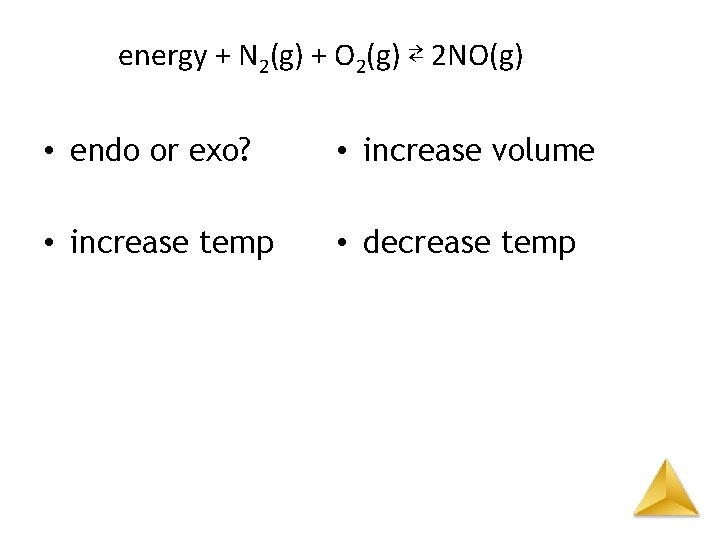
energy + N 2(g) + O 2(g) ⇄ 2 NO(g) • endo or exo? – endothermic • increase temp – to right • increase volume – no shift • decrease temp – to left

Homework • SCH 4 U – – – Read 350 - 369 pg 352 - # 21 -25 pg 353 # 1 -5 Pg 356 # 26 -28 Pg 366 # 28 -33 Pg 370 # 1 -5 • SCH 4 U AP – Read 15. 6 – 15. 7 – Q 51 – 72 • Virtual Labhttp: //dept. harperc ollege. edu/chemistry/c hm/100/dgodambe/the disk/equil. htm
 Predicting synthesis reactions
Predicting synthesis reactions Viewgrade 5 sch
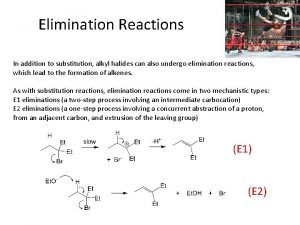
Viewgrade 5 sch E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Neutron emission
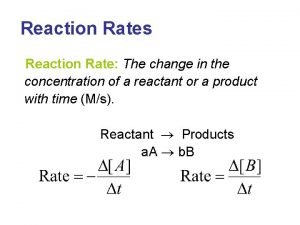
Neutron emission Rate of reaction formula
Rate of reaction formula Predicting products of chemical reactions
Predicting products of chemical reactions Potassium chloride precipitate
Potassium chloride precipitate Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Predicting science process skills
Predicting science process skills Highest braden score
Highest braden score How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs Paragraph on scientist
Paragraph on scientist Predicting pip
Predicting pip Predicting pip
Predicting pip Single displacement activity series
Single displacement activity series Predicting products of chemical reactions
Predicting products of chemical reactions Predicting content in listening
Predicting content in listening Predicting spontaneity
Predicting spontaneity Makalah previewing and predicting
Makalah previewing and predicting Predicting nba games using neural networks
Predicting nba games using neural networks Science process skills predicting
Science process skills predicting Vsepr theory is a model for predicting
Vsepr theory is a model for predicting Redox table
Redox table The evolution of crm is reporting analyzing and predicting
The evolution of crm is reporting analyzing and predicting Alexandru niculescu-mizil
Alexandru niculescu-mizil Orderly search pattern
Orderly search pattern Pleasure predicting sheet
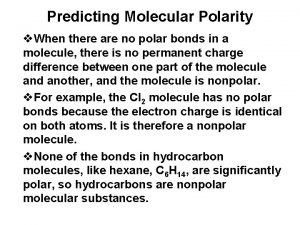
Pleasure predicting sheet Predicting molecular polarity
Predicting molecular polarity Braden scale for predicting pressure sore risk
Braden scale for predicting pressure sore risk Predicting products
Predicting products Single replacement products
Single replacement products Predicting fraud
Predicting fraud 6 manipulative skills
6 manipulative skills Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions