Quantum Mechanics Radiation Amplitude emission and transmission of














![Noble Gas Configuration Shorthand electron configuration [Ar] 4 s 23 d 6 Noble Gas Configuration Shorthand electron configuration [Ar] 4 s 23 d 6](https://slidetodoc.com/presentation_image_h2/7d2b04b74a86a213d752843fc4594466/image-37.jpg)
![Noble Gas Orbital Diagram [Ne] Noble Gas Orbital Diagram [Ne]](https://slidetodoc.com/presentation_image_h2/7d2b04b74a86a213d752843fc4594466/image-39.jpg)
- Slides: 39


Quantum Mechanics

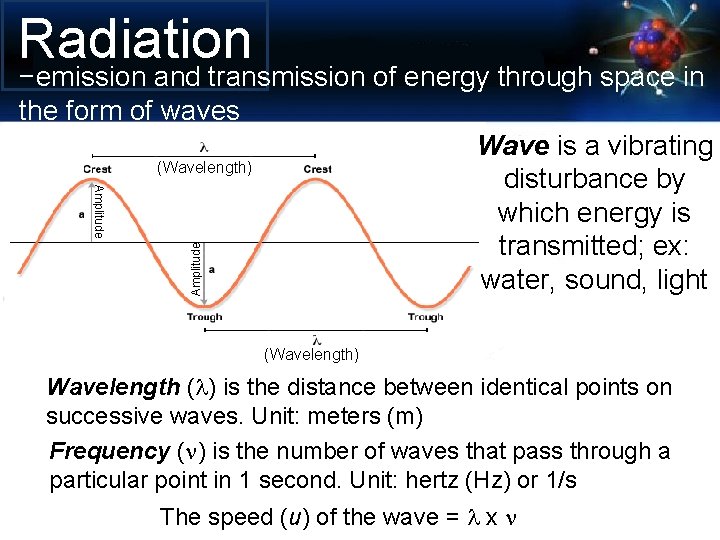
Radiation Amplitude −emission and transmission of energy through space in the form of waves Wave is a vibrating (Wavelength) disturbance by which energy is transmitted; ex: water, sound, light (Wavelength) Wavelength (l) is the distance between identical points on successive waves. Unit: meters (m) Frequency (n) is the number of waves that pass through a particular point in 1 second. Unit: hertz (Hz) or 1/s The speed (u) of the wave = l x n

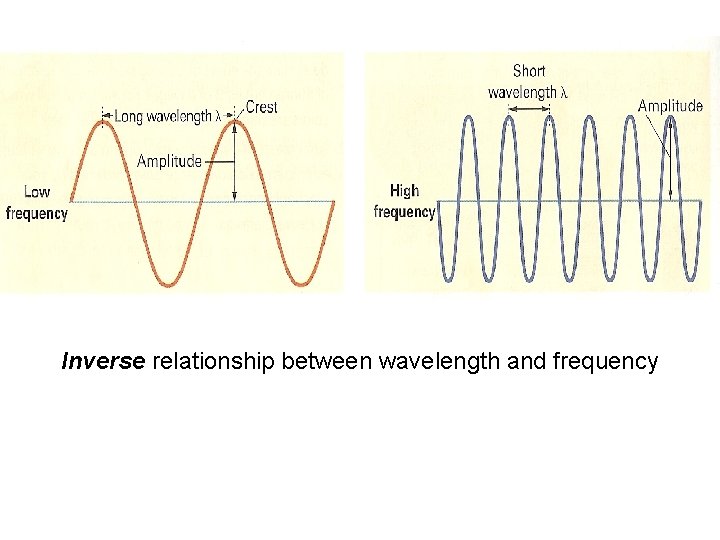
Inverse relationship between wavelength and frequency

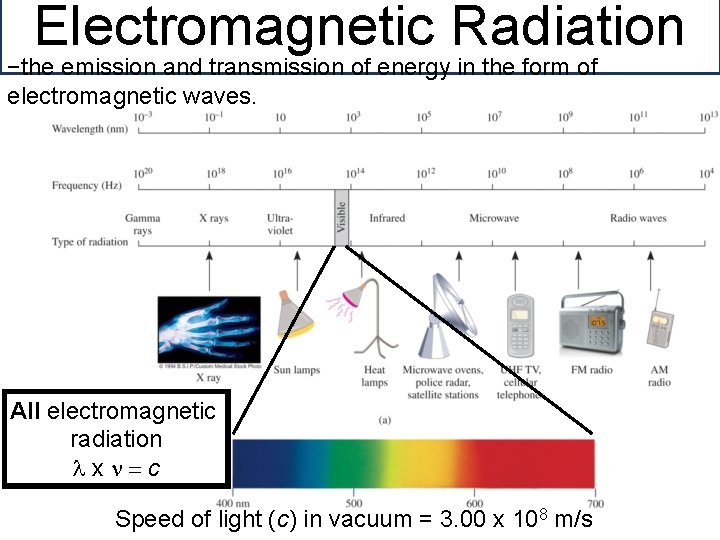
Electromagnetic Radiation −the emission and transmission of energy in the form of electromagnetic waves. All electromagnetic radiation lxn=c Speed of light (c) in vacuum = 3. 00 x 108 m/s


Examples 1. Calculate the wavelength of orange light if its frequency is 4. 84× 10− 4 s− 1. 1. Calculate the frequency of microwaves that have a wavelength of 0. 0872 m.

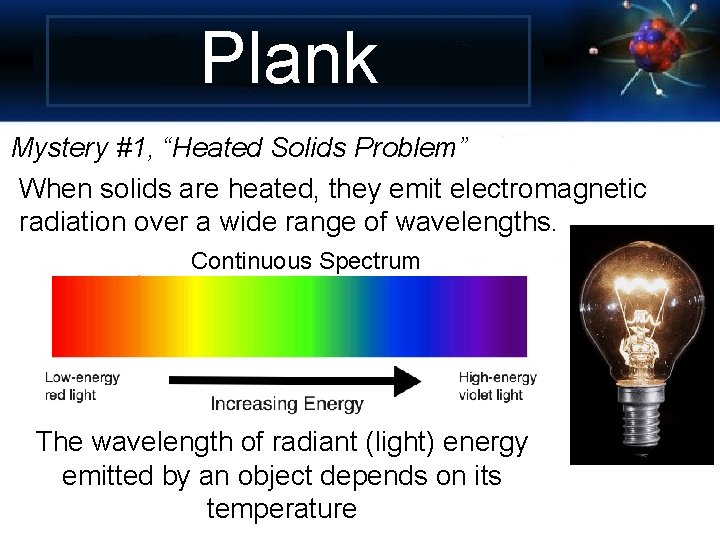
Plank Mystery #1, “Heated Solids Problem” When solids are heated, they emit electromagnetic radiation over a wide range of wavelengths. Continuous Spectrum The wavelength of radiant (light) energy emitted by an object depends on its temperature


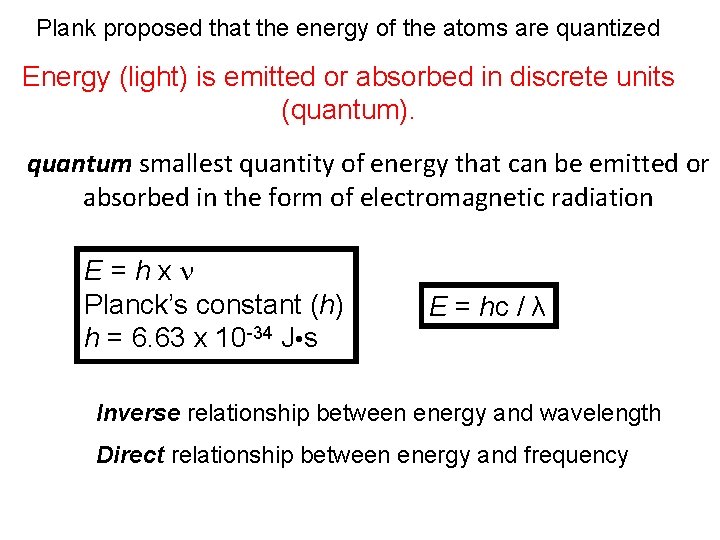
Plank proposed that the energy of the atoms are quantized Energy (light) is emitted or absorbed in discrete units (quantum). quantum smallest quantity of energy that can be emitted or absorbed in the form of electromagnetic radiation E=hxn Planck’s constant (h) h = 6. 63 x 10 -34 J • s E = hc / λ Inverse relationship between energy and wavelength Direct relationship between energy and frequency

Example A red laser pointer emits light with a wavelength of 700 nm. A fancy green laser pointer emits light with a wavelength of 500 nm. Which emits more energy per photon?


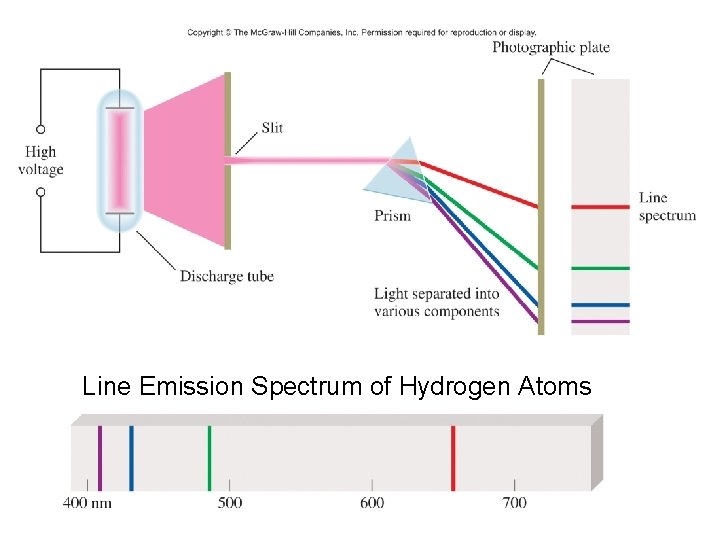
Line Emission Spectrum of Hydrogen Atoms

Ground state lowest energy state of the electron Excited state higher energy state than the ground state of the electron

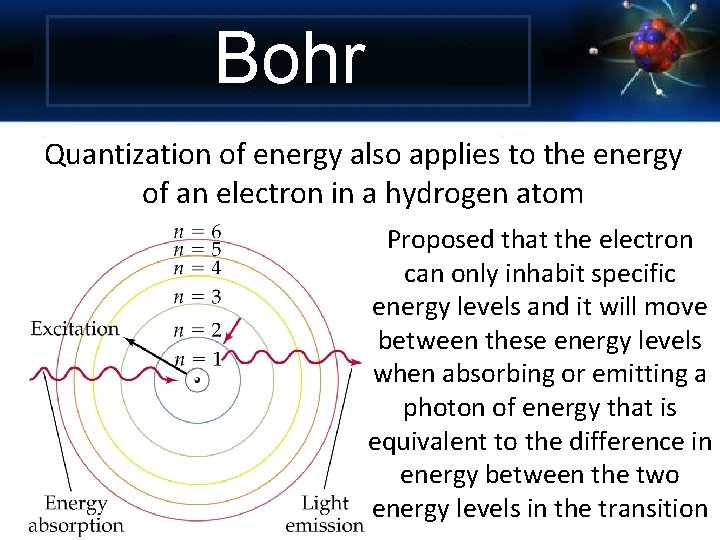
Bohr Quantization of energy also applies to the energy of an electron in a hydrogen atom Proposed that the electron can only inhabit specific energy levels and it will move between these energy levels when absorbing or emitting a photon of energy that is equivalent to the difference in energy between the two energy levels in the transition

Energy levels are like rungs of a ladder. You cannot be in between a rung Energy levels in an atom’s electron are unequally spaced. The higher energy levels are closer together.

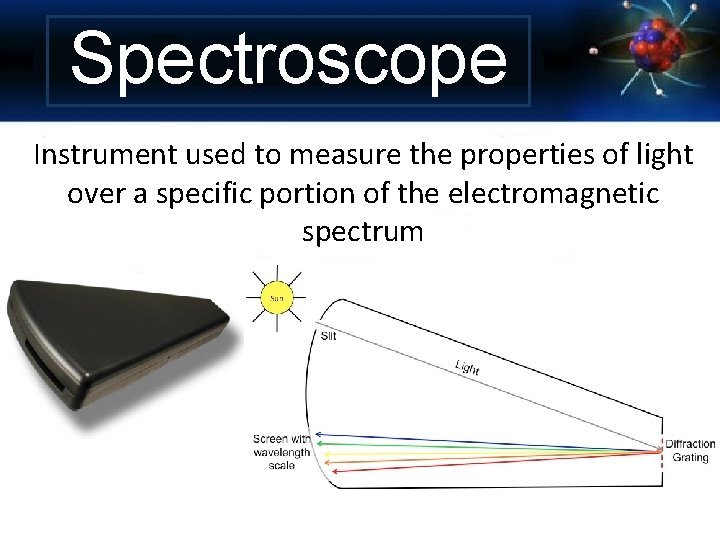
Spectroscope Instrument used to measure the properties of light over a specific portion of the electromagnetic spectrum


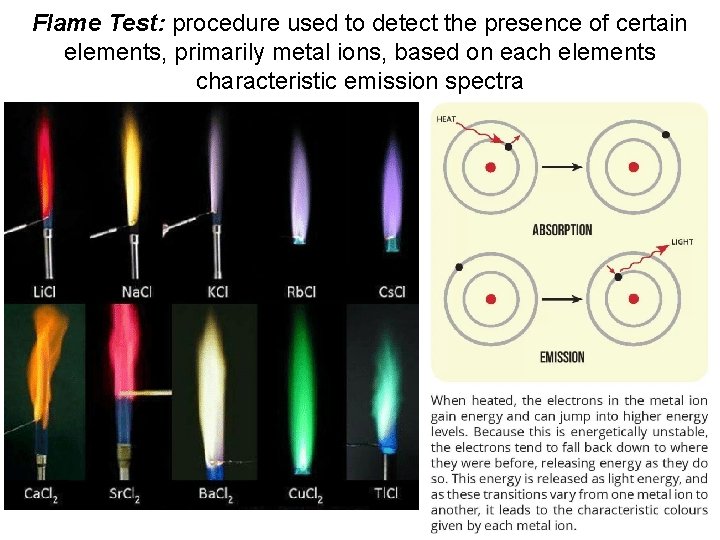
Flame Test: procedure used to detect the presence of certain elements, primarily metal ions, based on each elements characteristic emission spectra

Chemistry In Action

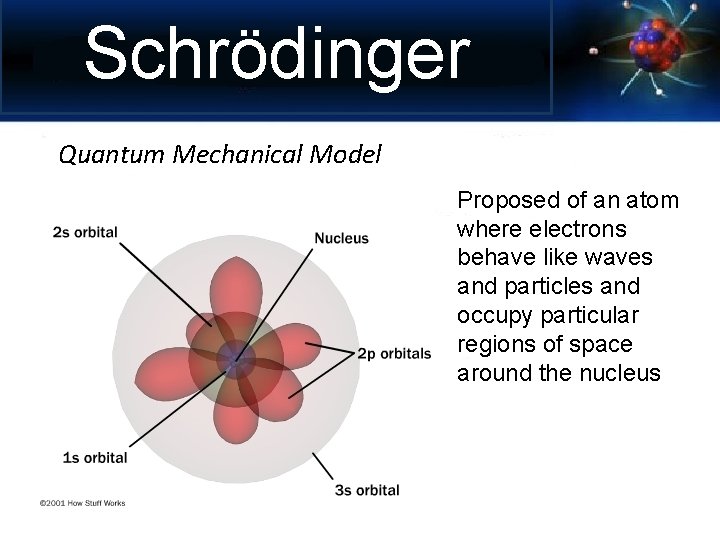
Schrödinger Quantum Mechanical Model Proposed of an atom where electrons behave like waves and particles and occupy particular regions of space around the nucleus

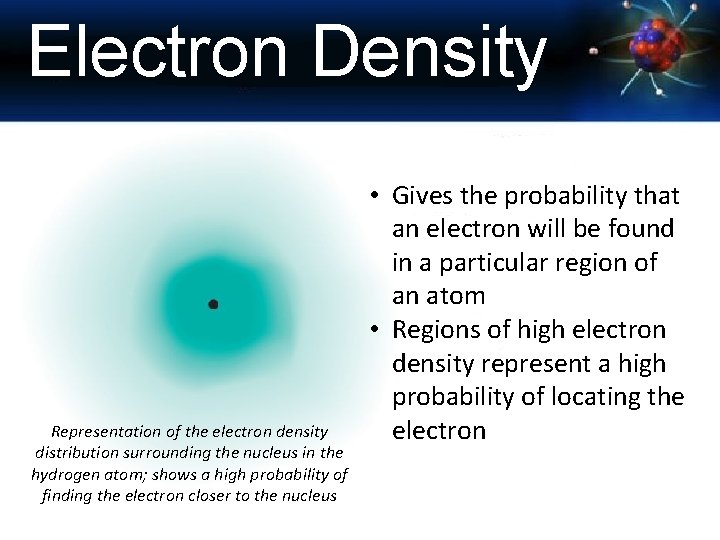
Electron Density Representation of the electron density distribution surrounding the nucleus in the hydrogen atom; shows a high probability of finding the electron closer to the nucleus • Gives the probability that an electron will be found in a particular region of an atom • Regions of high electron density represent a high probability of locating the electron

Atomic Orbital • Way to distinguish Bohr’s model from the current quantum mechanical model • Probability of locating the electron in 3 D space around the nucleus • Has a characteristic energy

Quantum numbers • Used to describe the characteristics of an electron in an atom (probable location) • Total of 4 • Each electron in an atom has a unique set of quantum numbers • Used to determine electron configuration of an atom

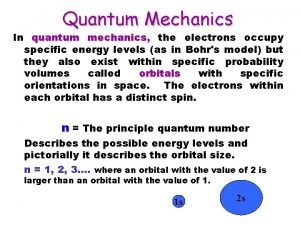
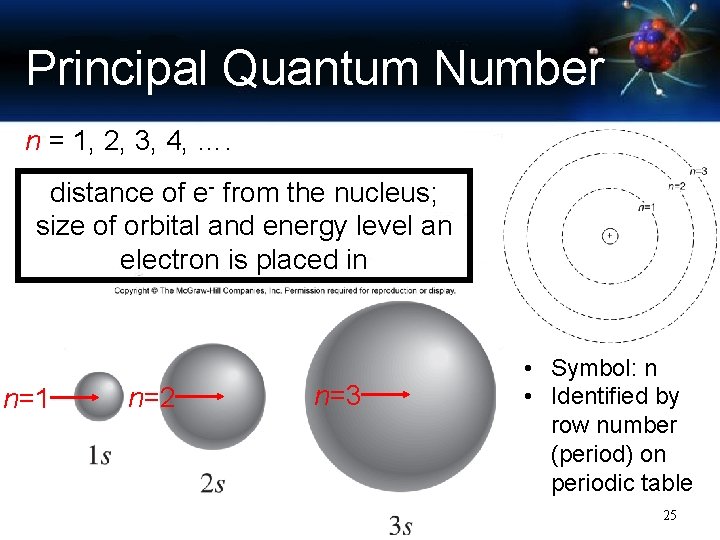
Principal Quantum Number n = 1, 2, 3, 4, …. distance of e- from the nucleus; size of orbital and energy level an electron is placed in n=1 n=2 n=3 • Symbol: n • Identified by row number (period) on periodic table 25

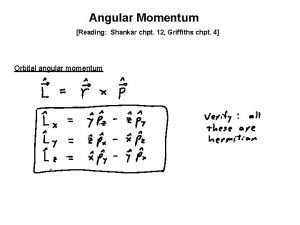
Angular Momentum Quantum Number Shape of the “volume” of space that the e- occupies s orbital p orbital d orbital f orbital *also called sublevels

Magnetic Quantum Number Describes the orientation of the orbitals in space

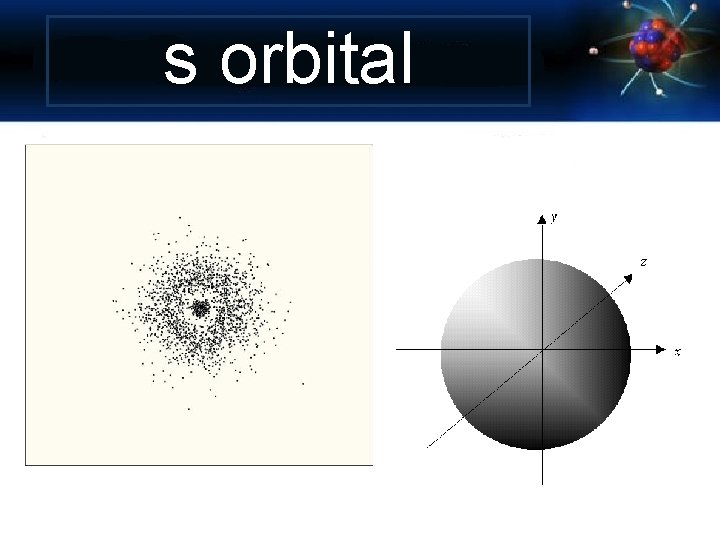
s orbital

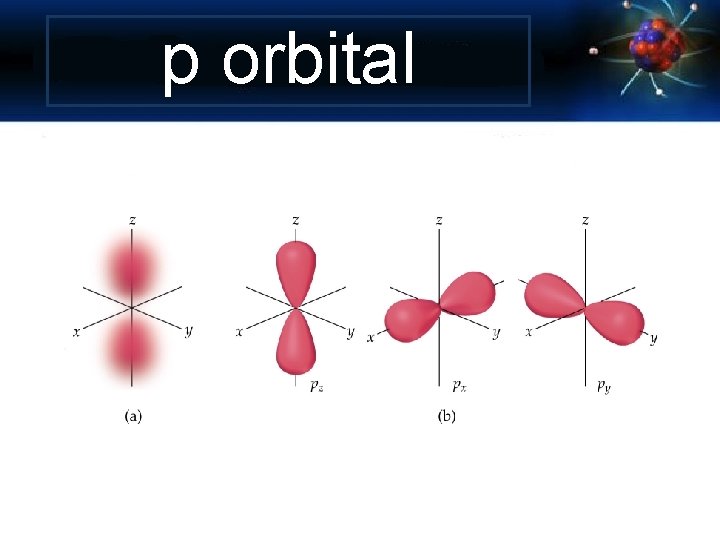
p orbital


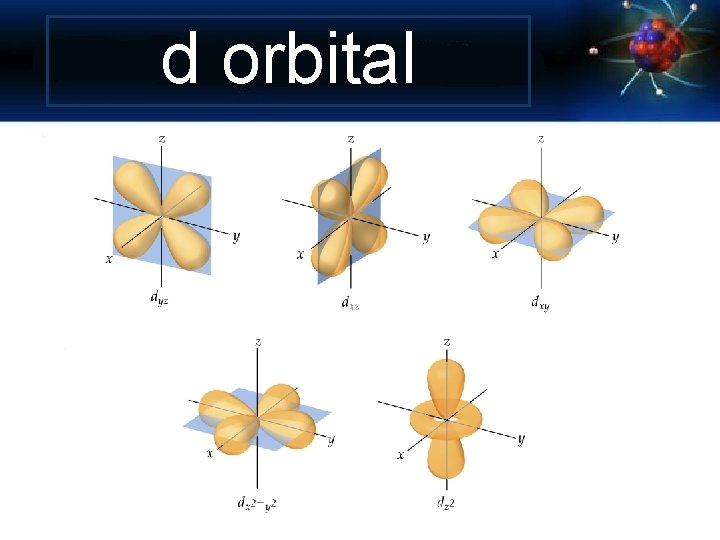
d orbital

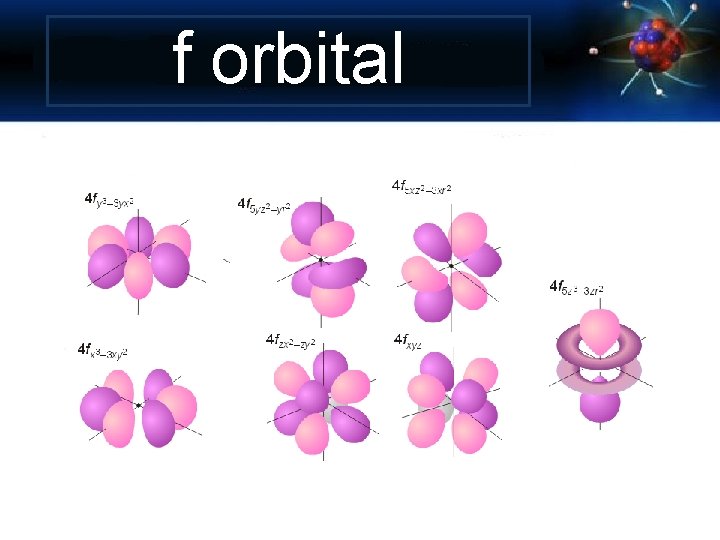
f orbital

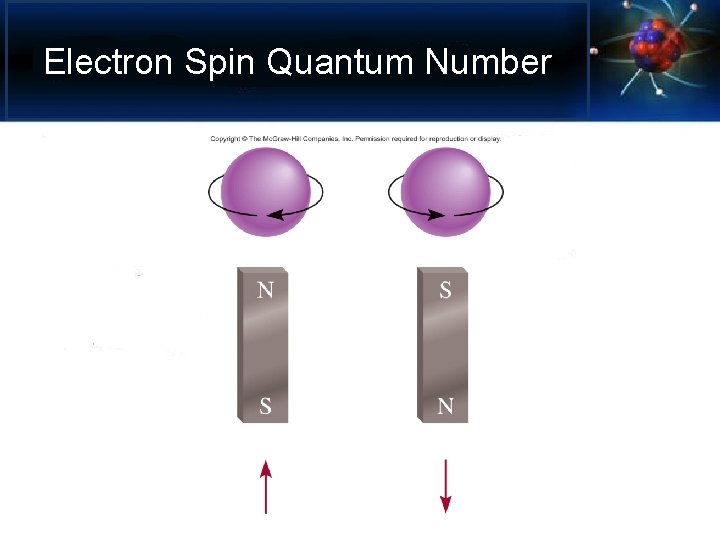
Electron Spin Quantum Number


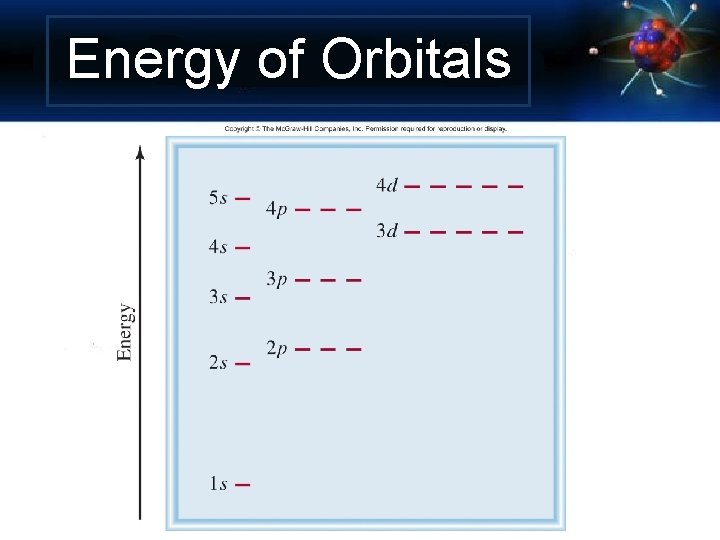
Energy of Orbitals

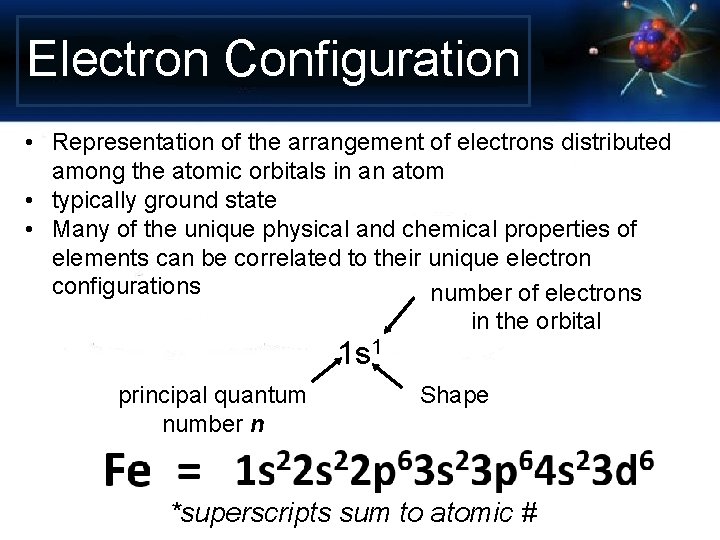
Electron Configuration • Representation of the arrangement of electrons distributed among the atomic orbitals in an atom • typically ground state • Many of the unique physical and chemical properties of elements can be correlated to their unique electron configurations number of electrons in the orbital 1 s 1 principal quantum number n Shape *superscripts sum to atomic #
![Noble Gas Configuration Shorthand electron configuration Ar 4 s 23 d 6 Noble Gas Configuration Shorthand electron configuration [Ar] 4 s 23 d 6](https://slidetodoc.com/presentation_image_h2/7d2b04b74a86a213d752843fc4594466/image-37.jpg)
Noble Gas Configuration Shorthand electron configuration [Ar] 4 s 23 d 6

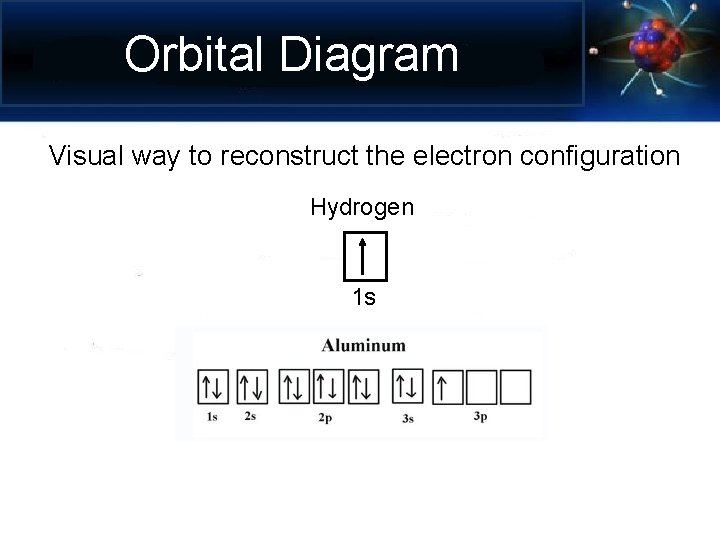
Orbital Diagram Visual way to reconstruct the electron configuration Hydrogen 1 s
![Noble Gas Orbital Diagram Ne Noble Gas Orbital Diagram [Ne]](https://slidetodoc.com/presentation_image_h2/7d2b04b74a86a213d752843fc4594466/image-39.jpg)
Noble Gas Orbital Diagram [Ne]
 Quantum physics vs mechanics
Quantum physics vs mechanics Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Atomic emission spectra and the quantum mechanical model
Atomic emission spectra and the quantum mechanical model Atomic emission spectra and the quantum mechanical model
Atomic emission spectra and the quantum mechanical model Maser vs laser
Maser vs laser Metastable state
Metastable state For distortionless transmission the amplitude response is
For distortionless transmission the amplitude response is Schrodingers cay
Schrodingers cay Borns interpretation of wave function
Borns interpretation of wave function Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics Expectation value in quantum mechanics
Expectation value in quantum mechanics Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics Quantum mechanics in your face
Quantum mechanics in your face Operators in quantum chemistry
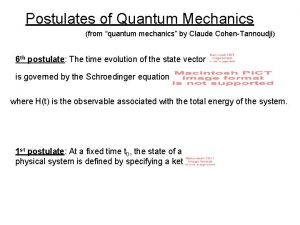
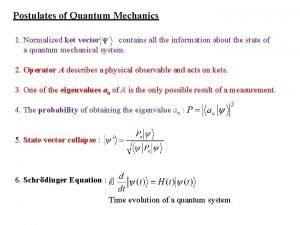
Operators in quantum chemistry Postulates of quantum mechanics
Postulates of quantum mechanics Normalized ket
Normalized ket Operators in quantum mechanics
Operators in quantum mechanics Dr susan cartwright
Dr susan cartwright Operator formalism in quantum mechanics
Operator formalism in quantum mechanics Schroendiger
Schroendiger Beta decay
Beta decay Instantons in quantum mechanics
Instantons in quantum mechanics Expectation value in quantum mechanics
Expectation value in quantum mechanics Mathematical tools of quantum mechanics
Mathematical tools of quantum mechanics Quantum mechanics in three dimensions
Quantum mechanics in three dimensions The basics of quantum mechanics
The basics of quantum mechanics Schrodinger cat
Schrodinger cat Spin angular momentum formula
Spin angular momentum formula Introduction to quantum statistical mechanics
Introduction to quantum statistical mechanics Mark tame
Mark tame Commutation relation in quantum mechanics
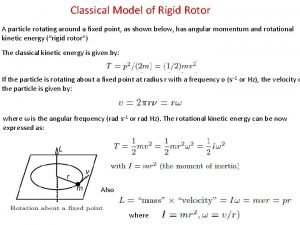
Commutation relation in quantum mechanics Rigid rotor quantum mechanics
Rigid rotor quantum mechanics Time dependent schrodinger wave equation
Time dependent schrodinger wave equation Griffiths
Griffiths Transfer matrix quantum mechanics
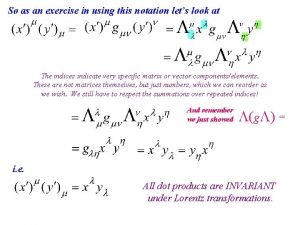
Transfer matrix quantum mechanics Littlejohn quantum mechanics
Littlejohn quantum mechanics Quantum mechanics powerpoint
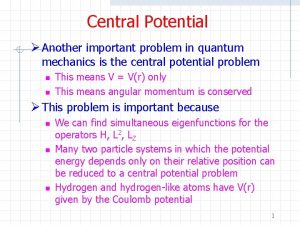
Quantum mechanics powerpoint Central potential in quantum mechanics
Central potential in quantum mechanics Quantum mechanics
Quantum mechanics Quantum mechanics definition
Quantum mechanics definition