Properties of Water Water molecules will stick to























- Slides: 41

Properties of Water

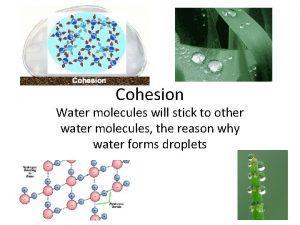
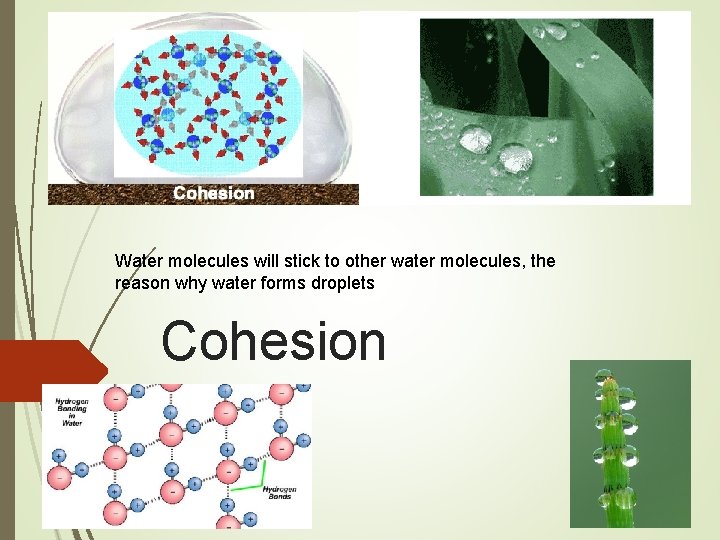
Water molecules will stick to other water molecules, the reason why water forms droplets Cohesion

Question Cohesion How is cohesion different from adhesion?

Water molecules stick to certain surfaces like glass Adhesion

Question Adhesion Give an example of the adhesion of water in your everyday life.

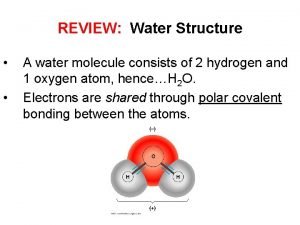
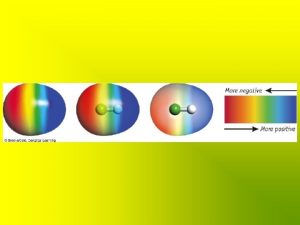
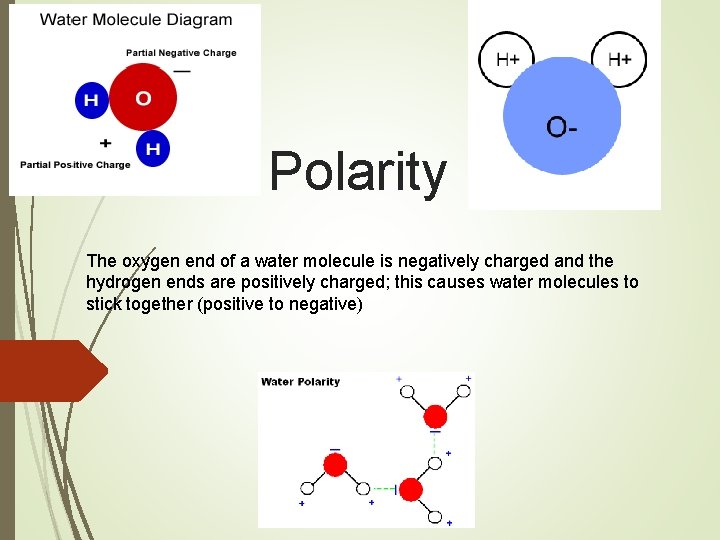
Polarity The oxygen end of a water molecule is negatively charged and the hydrogen ends are positively charged; this causes water molecules to stick together (positive to negative)

Question Polarity How is the polarity of water related to the cohesion of water?

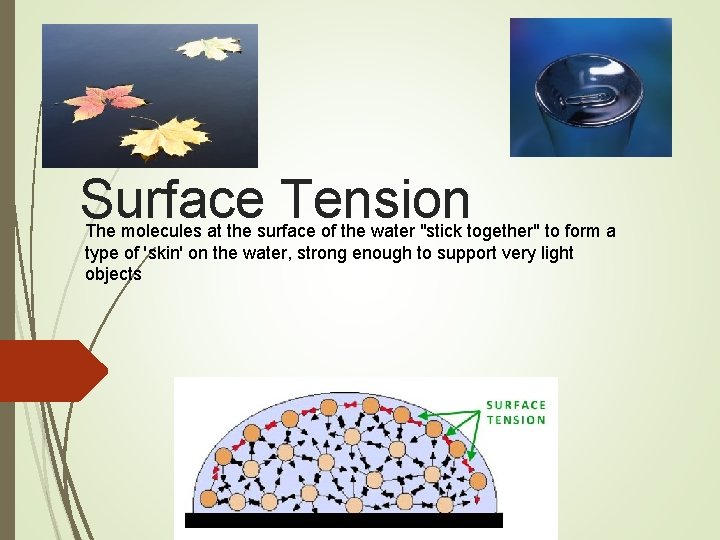
Surface Tension The molecules at the surface of the water "stick together" to form a type of 'skin' on the water, strong enough to support very light objects

Question Surface Tension How is surface tension of water related to the cohesion of water?

The ability of a narrow tube to draw a liquid upwards against the force of gravity Capillary Action

Specific Heat The amount of energy required to raise the temperature of 1 gram of a substance by 1 degree Celsius. Water has a high specific heat. It takes a lot of energy to raise the temperature of water.

Question Specific Heat What does it mean that water has a high specific heat?

Water Quality

Water Quality A measure of the physical, chemical and biological factors that affect a body of water Question: Why should we monitor the quality of our rivers, lakes and streams?

Water Temperature Water quality standard important to monitor because it affects the ability of the water to dissolve gases and the type of organisms that live in it. Question: How does the temperature affect the amount of dissolved oxygen in the water?

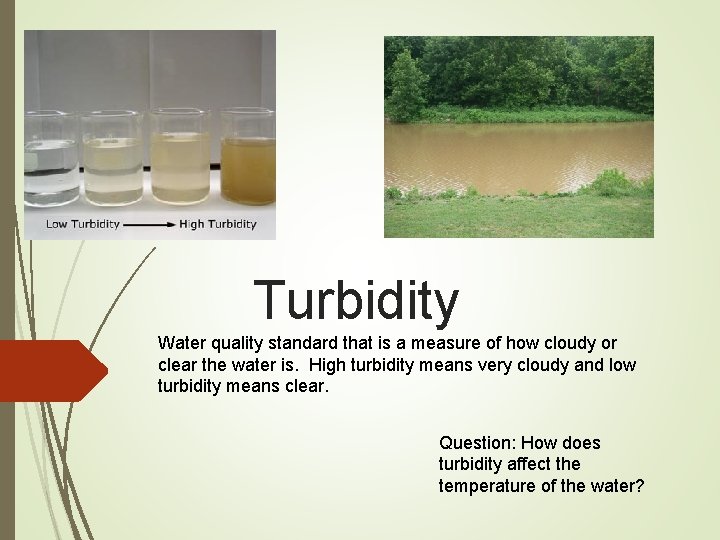
Turbidity Water quality standard that is a measure of how cloudy or clear the water is. High turbidity means very cloudy and low turbidity means clear. Question: How does turbidity affect the temperature of the water?

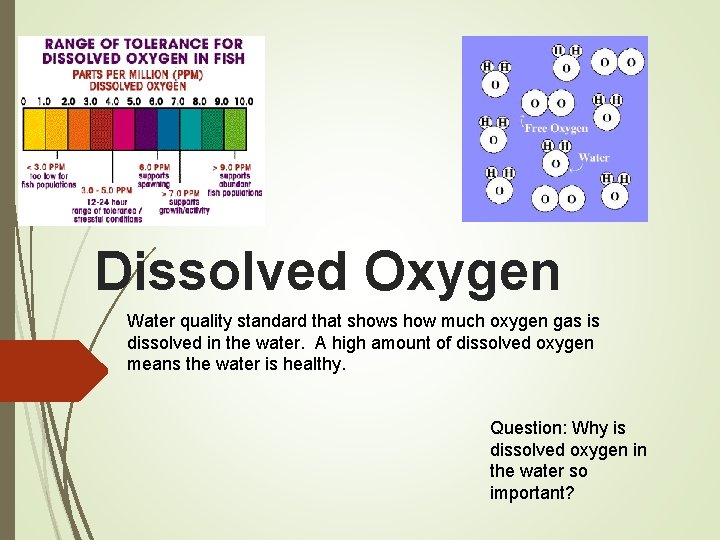
Dissolved Oxygen Water quality standard that shows how much oxygen gas is dissolved in the water. A high amount of dissolved oxygen means the water is healthy. Question: Why is dissolved oxygen in the water so important?

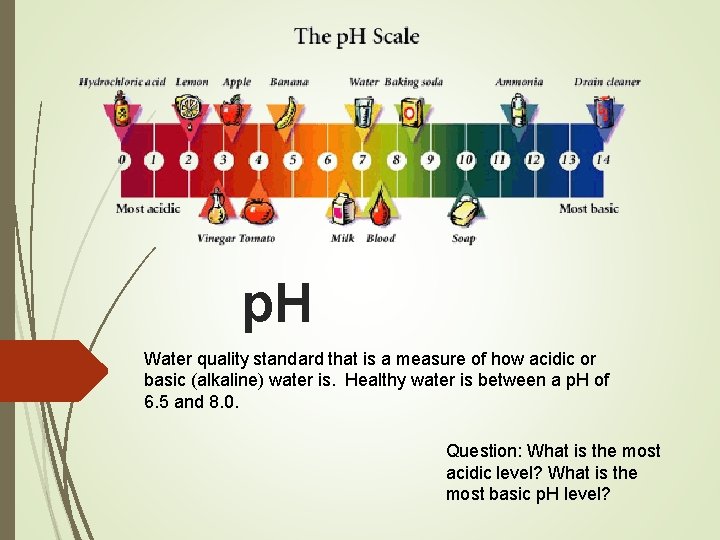
p. H Water quality standard that is a measure of how acidic or basic (alkaline) water is. Healthy water is between a p. H of 6. 5 and 8. 0. Question: What is the most acidic level? What is the most basic p. H level?

Eutrophication A process where water receives too much nutrients (like nitrates) which causes “algal blooms” that use up the dissolved oxygen in the water and animals suffocate. Question: What is an algal bloom?

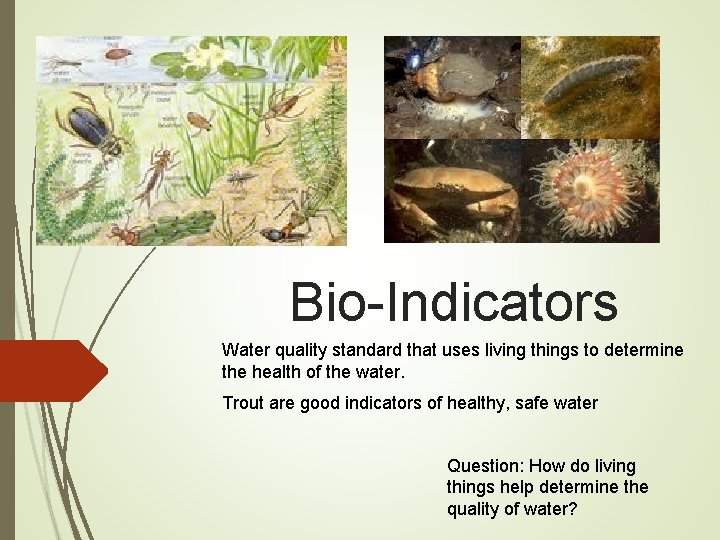
Bio-Indicators Water quality standard that uses living things to determine the health of the water. Trout are good indicators of healthy, safe water Question: How do living things help determine the quality of water?

Point Source Pollution A single identifiable source of pollution such as a pipe or hose dumping chemicals into the water. Question: Why is point source pollution easier to fix than nonpoint source pollution?

Nonpoint Source Pollution An activity that takes place over a large area and results in the release of pollution from many areas such as runoff from pavement and streets. Question: Why is nonpoint source pollution so hard to fix?

Water Distribution

Distribution 67% of Earth is made up of water 97% of all water on Earth is saltwater (oceans) 3% of all water is freshwater The majority of freshwater consists of glaciers and ice caps; aquifers also make up a portion of freshwater

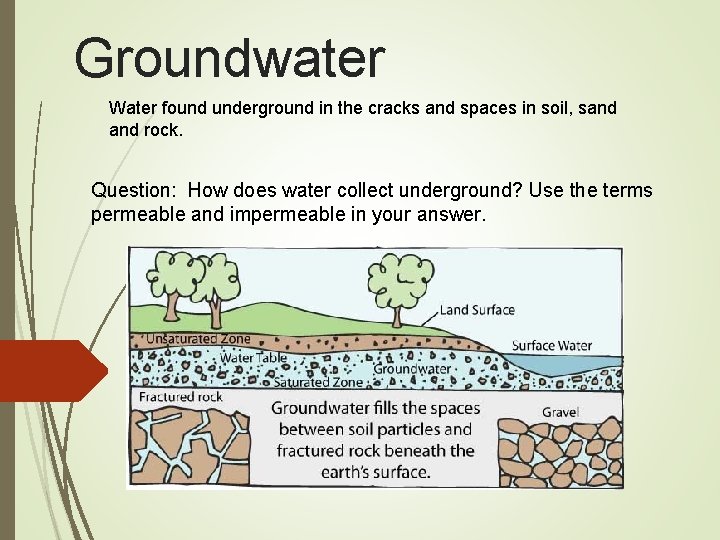
Groundwater Water found underground in the cracks and spaces in soil, sand rock. Question: How does water collect underground? Use the terms permeable and impermeable in your answer.

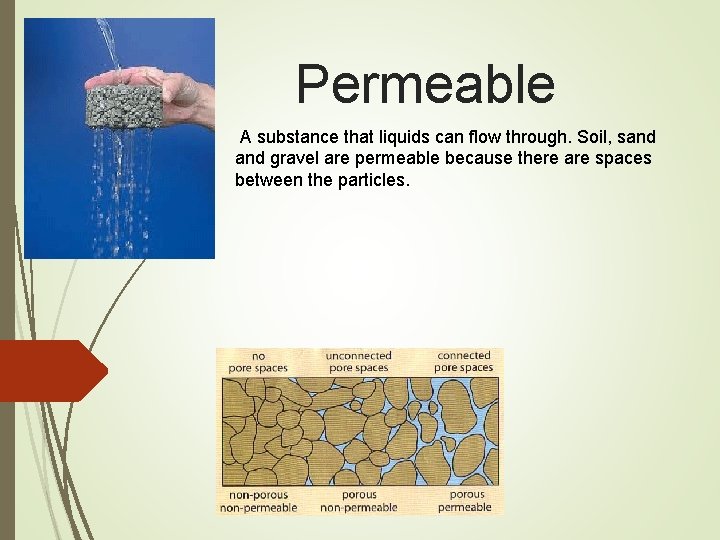
Permeable A substance that liquids can flow through. Soil, sand gravel are permeable because there are spaces between the particles.

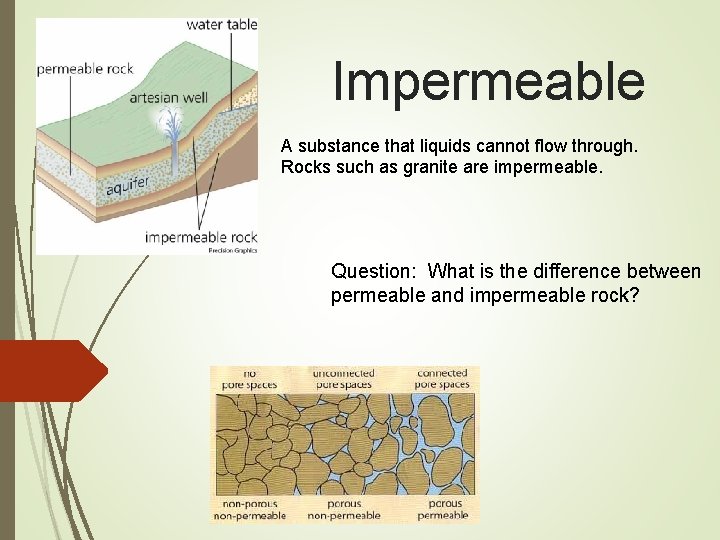
Impermeable A substance that liquids cannot flow through. Rocks such as granite are impermeable. Question: What is the difference between permeable and impermeable rock?

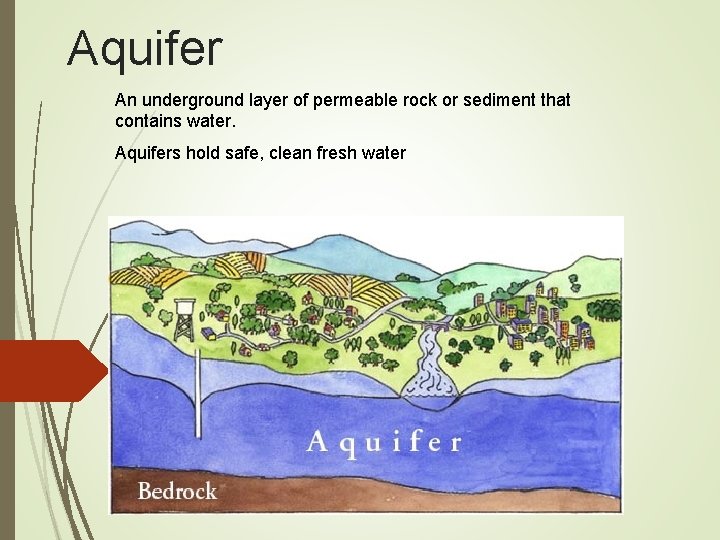
Aquifer An underground layer of permeable rock or sediment that contains water. Aquifers hold safe, clean fresh water

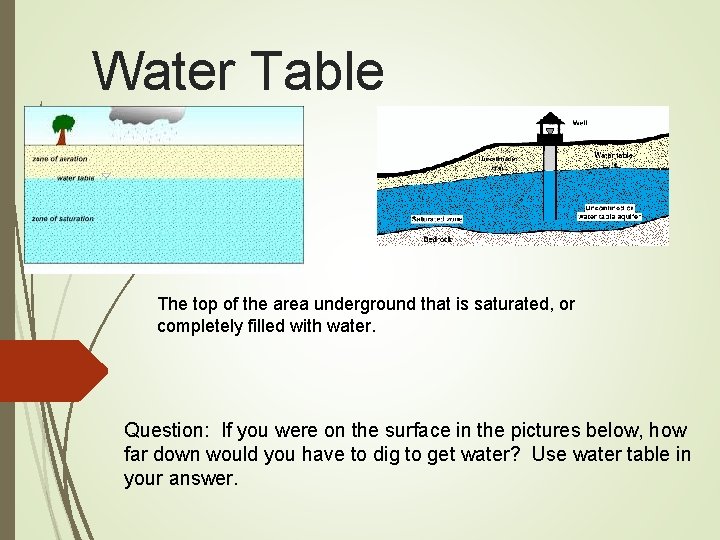
Water Table The top of the area underground that is saturated, or completely filled with water. Question: If you were on the surface in the pictures below, how far down would you have to dig to get water? Use water table in your answer.

Ocean Vocab

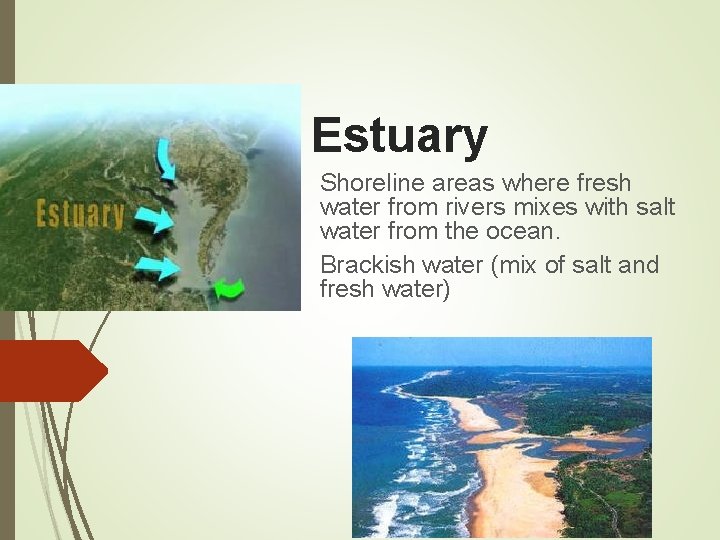
Estuary Shoreline areas where fresh water from rivers mixes with salt water from the ocean. Brackish water (mix of salt and fresh water)

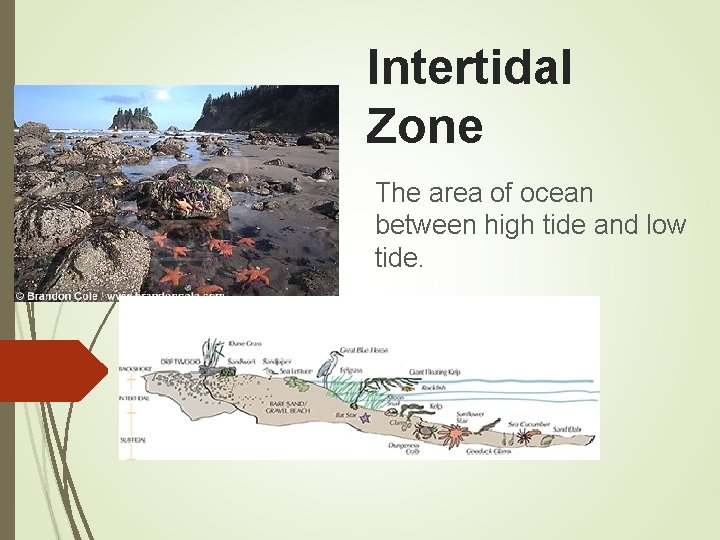
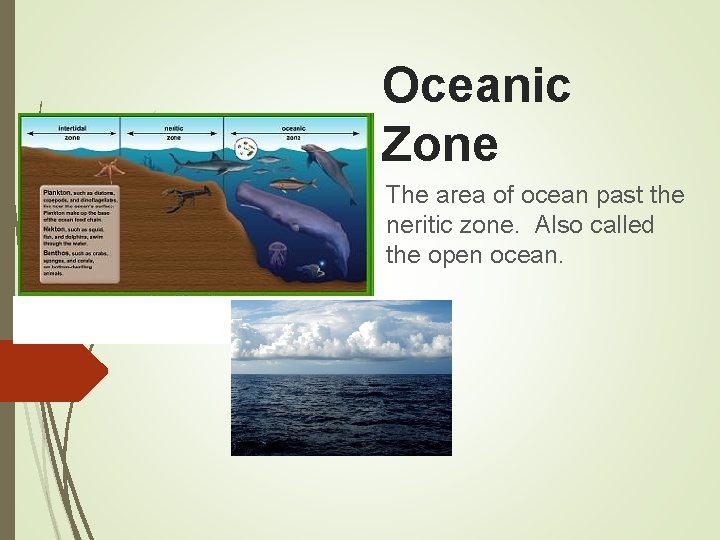
Intertidal Zone The area of ocean between high tide and low tide.

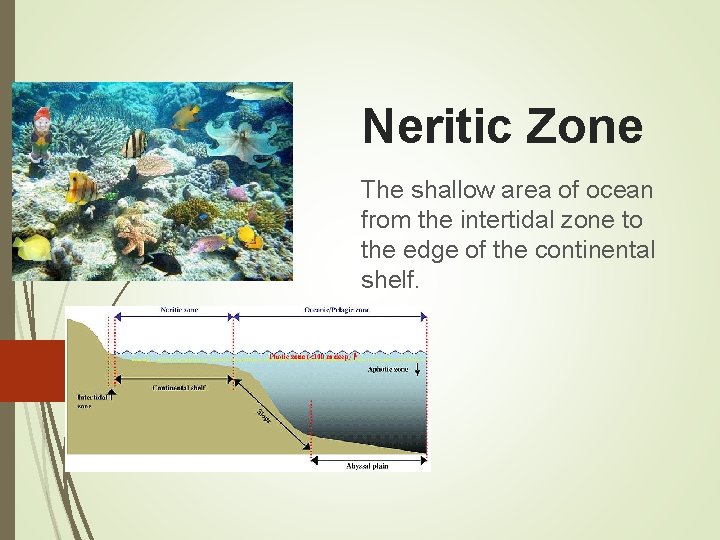
Neritic Zone The shallow area of ocean from the intertidal zone to the edge of the continental shelf.

Oceanic Zone The area of ocean past the neritic zone. Also called the open ocean.

Sunlight or Photic Zone The top layer of the ocean down to about 200 meters. Lots of sunlight for photosynthesis. Most organisms in the ocean live here.

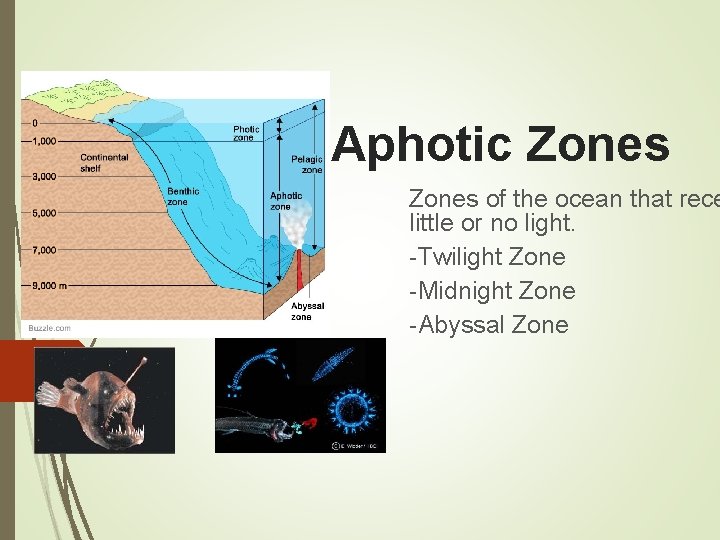
Aphotic Zones of the ocean that rece little or no light. -Twilight Zone -Midnight Zone -Abyssal Zone

Phytoplankton Producers and photosynthesizes of the ocean. Microscopic

Plankton Organisms that are floaters or very poo swimmers

Zooplankton Animals that eat phytoplankton. Most are microscopic.

Benthos Organisms that live on the ocean floor.

Hydrothermal Vents Since sunlight cannot reach deeper parts of the ocean, photosynthesis cannot occur in the deeper parts of the ocean Hydrothermal vents, a relatively recent discovery, are found near volcano active places, areas where tectonic plates are moving apart Provide energy source for deep sea creatures and life
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Water and water and water water
Water and water and water water Is h2o a polar molecule
Is h2o a polar molecule Intermolecular forces present in hbr
Intermolecular forces present in hbr A solid compound that contains water molecules
A solid compound that contains water molecules Properties of water
Properties of water Cohesion bond
Cohesion bond Molecul
Molecul Extensive vs intensive
Extensive vs intensive Physical properties and chemical properties
Physical properties and chemical properties Concept map properties of water
Concept map properties of water Properties of water ap biology
Properties of water ap biology Physical properties of sea water
Physical properties of sea water Properties of water clipart
Properties of water clipart Properties of water lab report
Properties of water lab report Ocean water properties
Ocean water properties Propeties of water
Propeties of water Whats capillary action
Whats capillary action Honors biology properties of water lab
Honors biology properties of water lab The extraordinary properties of water
The extraordinary properties of water The extraordinary properties of water
The extraordinary properties of water Properties of water foldable
Properties of water foldable Unique facts about water
Unique facts about water Properties of water key
Properties of water key Hydrogen bonding properties of water
Hydrogen bonding properties of water Properties of water summary
Properties of water summary Properties of water polarity
Properties of water polarity Water chemical and physical properties
Water chemical and physical properties What are the life supporting properties of water
What are the life supporting properties of water Why ice sticks to skin
Why ice sticks to skin African rainstick
African rainstick Lambda based design rules
Lambda based design rules Unity or sticking together
Unity or sticking together What stick
What stick Egyptian throwing stick
Egyptian throwing stick Biltmore stick
Biltmore stick A short tube or stick carried in a relay race
A short tube or stick carried in a relay race Ftb stick team
Ftb stick team Stick diagrams
Stick diagrams Dollar diplomacy
Dollar diplomacy Smaw unit
Smaw unit Cross chek
Cross chek