Properties of Matter n n n Propertiescharacteristics Physical

















- Slides: 23

Properties of Matter n n n Properties=characteristics Physical properties = can be measured/observed w/o changing the identity of the substance Chemical Properties = substance’s ability to undergo change into different substances

Properties of Matter n n Extensive properties –depend on the amount of matter that is present --volume, mass, amount of energy, length Intensive properties – do NOT depend on the amount of matter present -- color, boiling/melting points, density

Changes in Matter --Physical Changes n n Physical Changes – occur without altering the composition of the substance Examples: crumpling, bending, crushing, splitting, changes in state (boiling, melting, condensing, freezing) The temperature at which substances undergo phase changes are important physical properties – can be used for identification purposes. Phase change-change of state

Changes in Matter – Chemical Changes n n n Chemical changes – changes that involve a substance changing into one or more new substances Examples – burning, fermenting, digestion, rusting, rotting, oxidizing Substances that react= reactants Substances that are formed = products Use arrow (yields) to show direction.

Chemical Reaction Example n n Carbon plus oxygen yields (forms) carbon dioxide C + O 2 CO 2 Whenever chemical & physical changes occur, energy is always involved. Energy can take several forms.

Evidences of Chemical Changes n n Always produces a change in properties. The product(s) have different properties from the reactants. Five things to look for: n n n Evolution of a gas Formation of a precipitate (solid falls out of a liquid solution) Color changes – careful! Giving off heat and/or light Change in odor – careful!

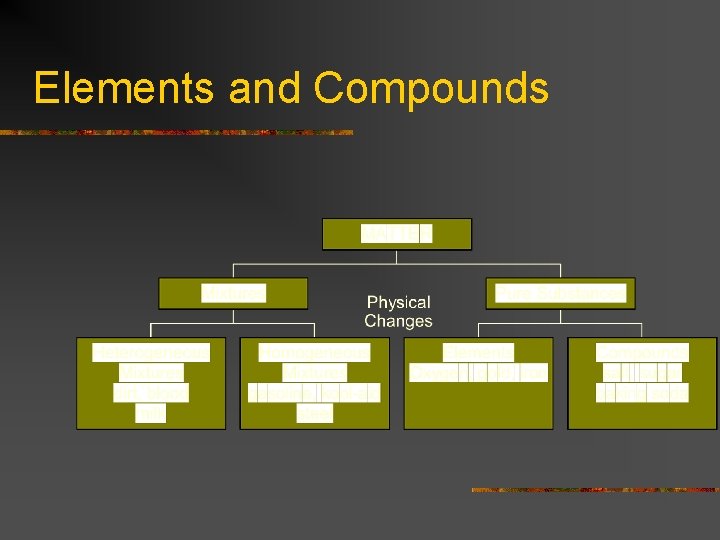
Classifications of Matter n Mixture – combination of 2 or more kinds of matter in which each retains its individual identity & properties n Examples: salt & water, sand & water, Cracker Jacks (peanuts & popcorn), Chex Mix, Kool-Aid

Types of Mixtures n n Heterogeneous Mixtures – one where the substances do not blend smoothly throughout, so the individual substances remain distinct Has distinct phases (a phase is a region where properties are different, such as ice in a glass of water. Ice is one phase, the water another phase). n Examples: pizza, chocolate chip cookies, sand & water, nuts & bolts

Types of Mixtures n n Homogeneous Mixtures – constant composition throughout – has a single phase Also called Solutions n n Solutions can be in any form, not just liquids Examples: a solid-solid solution is brass n n Many solid-solid solutions are called ALLOYS (homogeneous mixture of metals or metal & a non-metal where the metal is the largest part) A gas-liquid solution is the carbon dioxide in a soft drink A gas-gas solution is AIR A liquid-gas solution is water droplets in air

Solutions… n n Solvent – the substance present in the greatest quantity (does the dissolving) Solute – the substance(s) present in the least quantity (what is being dissolved)

Separating Mixtures n n n Heterogeneous Mixtures are easily separated. Some can be done by hand, simply by picking out the different phases. Others can be separated by FILTRATION Filtration uses a porous barrier to separate a solid from a liquid, like a coffee filter. In chemistry, we use a filter paper folded into a funnel.

Separating Homogeneous Mixtures n Distillation – separation by a difference in boiling points. Mixture is heated and the substance with the lowest boiling point will vaporize and then condense back into a liquid. n n Crystallization – formation of a solid from a liquid substance n n Example – getting gasoline from crude oil Example – rock candy Chromatography – separates by different travel times of different substances across the surface of another material.

Pure Substances n n n A pure substance has a fixed composition Every sample of a given pure substance has the same exact properties & exactly the same composition. Elements & Compounds are pure substances Element – a pure substance that cannot be separated into simpler substances by either physical or chemical means Examples: carbon, oxygen, iron

Compounds n n Compound – a combination of 2 or more different elements that are combined chemically Approxmately 10 MILLION known compounds, and more added almost daily n Examples: water, table salt, table sugar, aspirin

Compounds n n Compounds can be broken down into simpler forms by chemical means Properties of compounds are different of their component elements.

Elements and Compounds

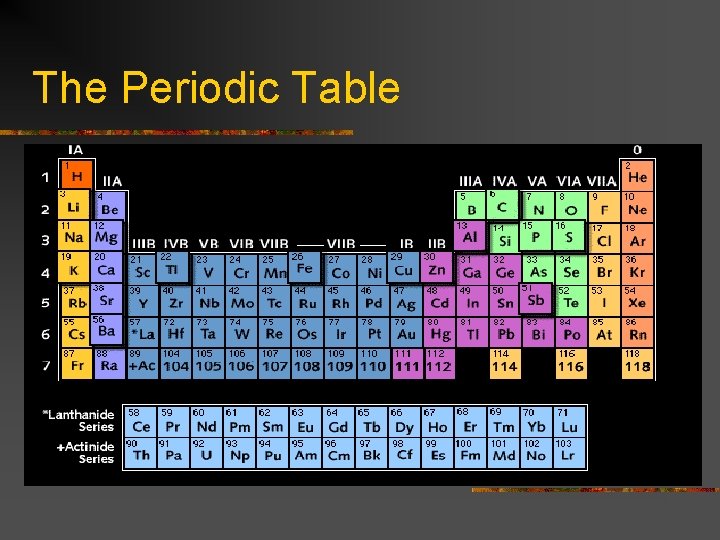
Introduction to the Periodic Table n n Each square shows the element’s: Symbol (C, He, U, Ca) Atomic number (no decimals) Atomic mass (decimals)

The Periodic Table

Periodic Table arrangement n n Vertical columns =“Groups” or “families” Horizontal rows = periods

Types of Elements n n n Metals 1. most are metals 2. on left “side” of table 3. conducts heat, electricity well 4. malleable, ductile, luster (shine) 5. Some soft, some hard

Types of Elements n n Non-metals 1. on right side of table (few elements) 2. solids, liquids, gases 3. do not conduct heat/electricity well

Types of Elements n n Metalloids 1. Found along the “stairstep” of the periodic table 2. Only 8 of them – B, Si, Ge, As, Se, Sb, Te, At 3. Have characteristics of both metals & nonmetals

Types of Elements n n Noble Gases 1. All gases 2. In last group of the table 3. Do not form compounds readily (unreactive)
 Physical properties of matter jeopardy
Physical properties of matter jeopardy Graphic organizer matter classifications
Graphic organizer matter classifications Physical properties of notebook paper
Physical properties of notebook paper Physical and chemical properties
Physical and chemical properties Section 1 composition of matter
Section 1 composition of matter Grey matter nervous system
Grey matter nervous system Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray matter and white matter
Gray matter and white matter Telecephalon
Telecephalon Flow of energy vs flow of matter
Flow of energy vs flow of matter What are the 3 properties of a liquid
What are the 3 properties of a liquid Properties of matter vocabulary
Properties of matter vocabulary Matter concept map
Matter concept map Objectives of properties of matter
Objectives of properties of matter General property of matter
General property of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Is odor observable or measurable
Is odor observable or measurable States of matter jeopardy
States of matter jeopardy Matter jeopardy
Matter jeopardy Properties of matter volume
Properties of matter volume