Part One Physical Properties of Matter Physical Properties





- Slides: 8

Part One Physical Properties of Matter

Physical Properties Physical property is a property that can be observed without changing the identity of the substance. Examples: ü viscosity ü conductivity ü malleability ü hardness ü magnetism ü melting point ü boiling point ü density ü color

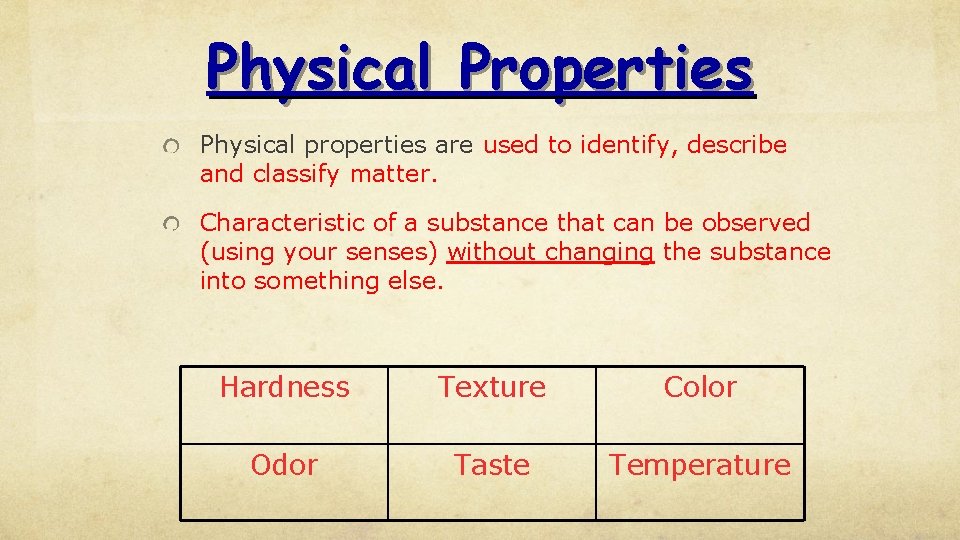
Physical Properties Physical properties are used to identify, describe and classify matter. Characteristic of a substance that can be observed (using your senses) without changing the substance into something else. Hardness Texture Color Odor Taste Temperature

Examples of Physical Properties Viscosity of a substance is its resistance to flow. Examples: water = low viscosity honey = high viscosity Conductivity is a material’s ability to allow heat to flow. Examples: metal = high conductivity wood = poor conductivity

Examples of Physical Properties Malleability of a substance is its ability to be hammered into a thin sheet Melting and Boiling points are the temperatures at which a solid becomes a liquid and a liquid becomes a gas. Density of a substance is the ratio of its mass compared to its volume.

Physical Properties to separate mixtures Two common separation methods: Filtration – process that separates materials based on the size of their particles. Distillation – process that separates the substances in a solution based on their boiling points.

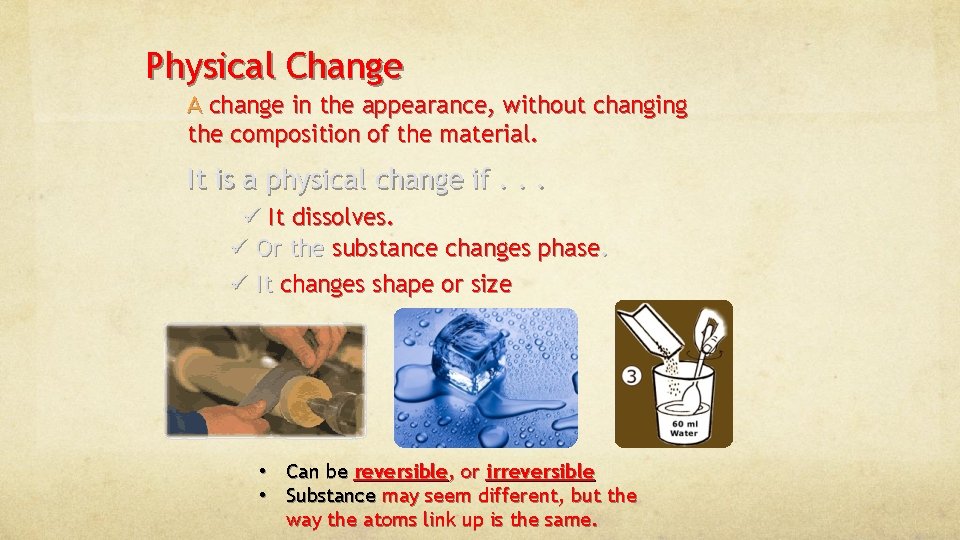
Physical Change A change in the appearance, without changing the composition of the material. It is a physical change if. . . ü It dissolves. ü Or the substance changes phase. ü It changes shape or size • Can be reversible, or irreversible • Substance may seem different, but the way the atoms link up is the same.

Done with notes for the day