Physical Properties of Matter Types of Physical Properties








- Slides: 13

Physical Properties of Matter

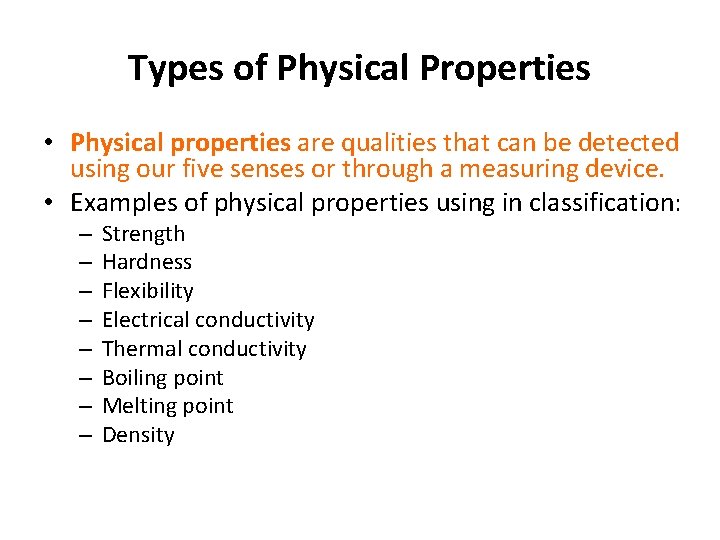
Types of Physical Properties • Physical properties are qualities that can be detected using our five senses or through a measuring device. • Examples of physical properties using in classification: – – – – Strength Hardness Flexibility Electrical conductivity Thermal conductivity Boiling point Melting point Density

Strength • The strength of a material refers to its ability to support a heavy load without breaking or tearing. • Examples of strong materials: concrete and steel. These are used in making buildings, roads and bridges. • Examples of weak materials: paper and cloth.

Hardness • The hardness of a material refers to its ability to withstand scratches. • A material will be able to cut or scratch a material softer than itself, but it cannot cut or scratch something that is harder.

Hardness • Diamond is the hardest substance that we know of. • It is used to cut glass, ceramic tiles and concrete. • Drills studded with diamonds can cut through rocks. This cutter is encrusted with diamonds and can be used to cut diamonds.

Flexibility • The flexibility of a material refers to its ability to bend without breaking and to return to its original shape and size. • A material that is not flexible is rigid. • E. g. of flexible objects: rubber bands, electrical wires and papers. • E. g. of rigid objects: tables, mirrors and computers.

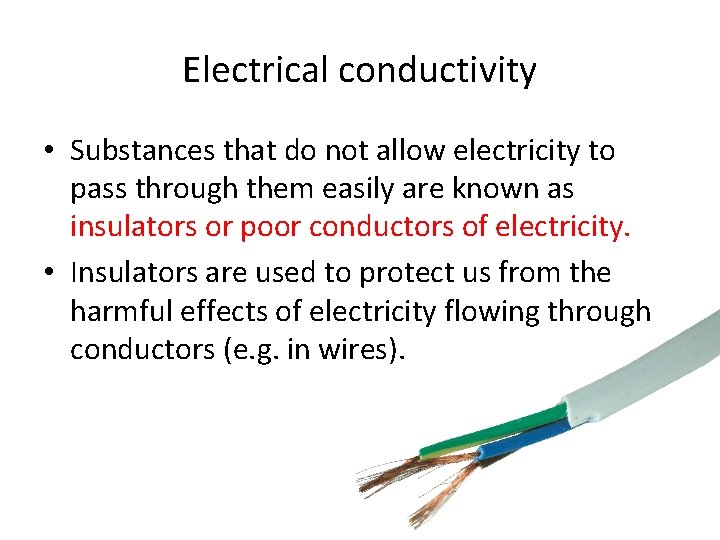
Electrical conductivity • The electrical conductivity of a material is a measure of how readily electricity passes through it. • Substances that let electricity to pass through them easily are known as conductors. • Metals are good electrical conductors. • Materials that do not allow electricity to pass through them easily are called insulators or poor electrical conductors.

Electrical conductivity • Substances that do not allow electricity to pass through them easily are known as insulators or poor conductors of electricity. • Insulators are used to protect us from the harmful effects of electricity flowing through conductors (e. g. in wires).

Thermal conductivity • The thermal conductivity or heat conductivity of a material is a measure of how readily heat passes through it. • Materials that allow heat to pass through them easily are called heat conductors (e. g. metals). • Materials that do not allow heat to pass through them easily are called heat insulators or poor conductors of heat (e. g. plastic, ice).

Melting point • The melting point of a material refers to the temperature at which it changes in state from a solid to a liquid. • For example, the melting point of water is 0 0 C. • Some substances have a very high melting point (e. g. iron melts at 1535 0 C). • Some substances have a very low melting point (e. g. oxygen melts at – 218 0 C).

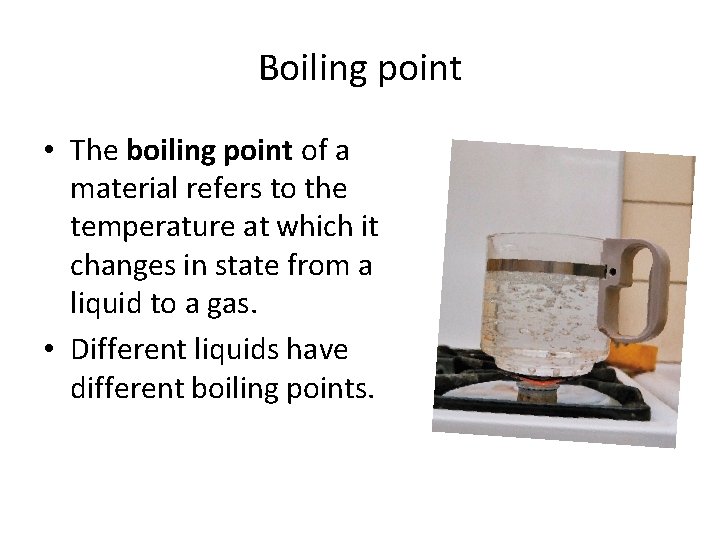
Boiling point • The boiling point of a material refers to the temperature at which it changes in state from a liquid to a gas. • Different liquids have different boiling points.

Boiling point • The boiling point of water is 100 0 C, while that of ethanol is 78 0 C. • Some substances have a very low boiling point (e. g. liquid oxygen boils at – 183 0 C). • Some substances have a very high boiling point (e. g. iron boils at 2750 0 C).

Density • The density of a material refers to the ratio of its mass to its volume. • The same volume of a denser substance will have a greater mass than a less dense substance.
 Physical properties of matter jeopardy
Physical properties of matter jeopardy Classifying matter graphic organizer
Classifying matter graphic organizer Physical properties of notebook paper
Physical properties of notebook paper Physical properties of ice cube
Physical properties of ice cube Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is the difference between gray and grey
What is the difference between gray and grey Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Gray matter and white matter
Gray matter and white matter Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Gray matter vs white matter
Gray matter vs white matter Flow energy review
Flow energy review Properties of liquid matter
Properties of liquid matter