Principles of Dynamic Causal Modelling DCM SPM course
![Induced responses in Motor and Occipital areas M O MNI coordinates [34 -28 37] Induced responses in Motor and Occipital areas M O MNI coordinates [34 -28 37]](https://slidetodoc.com/presentation_image_h2/da2f10c4579b263e437bbf1edfdc38d0/image-20.jpg)

- Slides: 36

Principles of Dynamic Causal Modelling (DCM) SPM course for MEG & EEG 2018 Bernadette van Wijk University of Amsterdam Department of Psychology University College London Wellcome Trust Centre for Neuroimaging

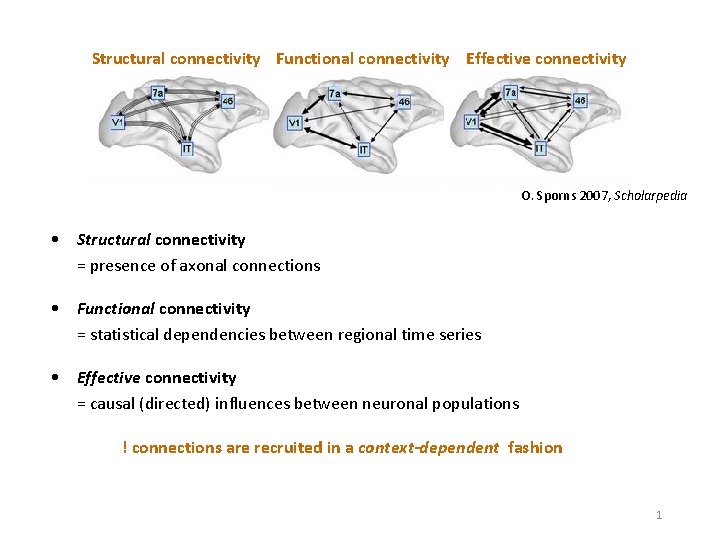
Structural connectivity Functional connectivity Effective connectivity O. Sporns 2007, Scholarpedia • Structural connectivity = presence of axonal connections • Functional connectivity = statistical dependencies between regional time series • Effective connectivity = causal (directed) influences between neuronal populations ! connections are recruited in a context-dependent fashion 1

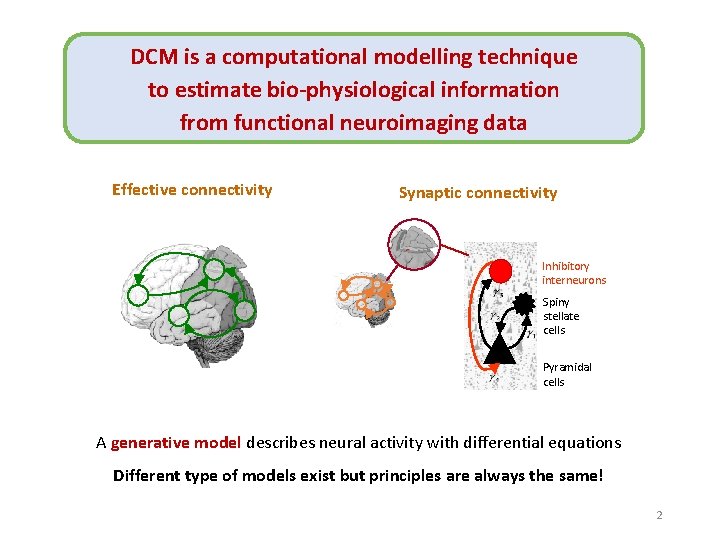
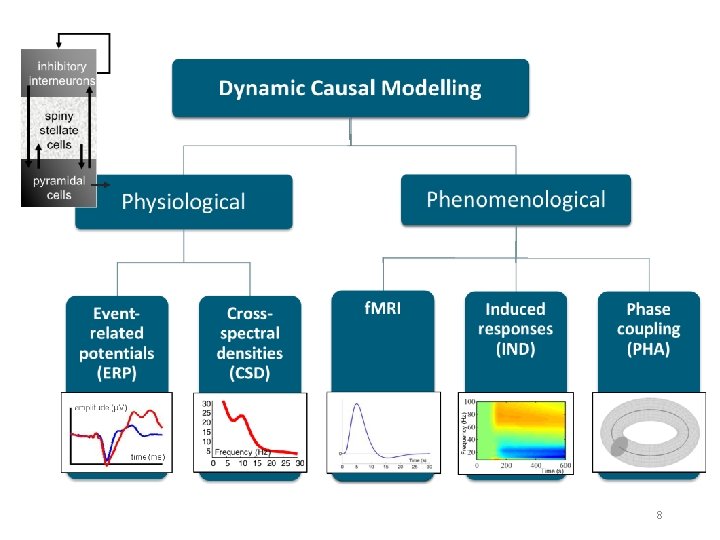
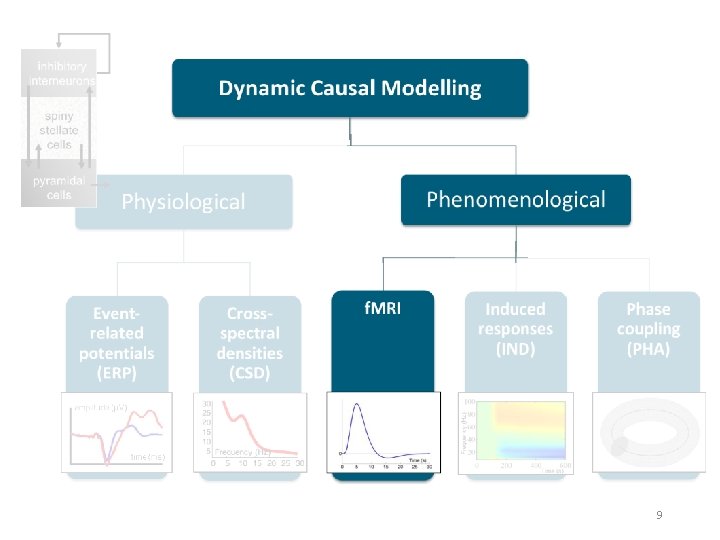
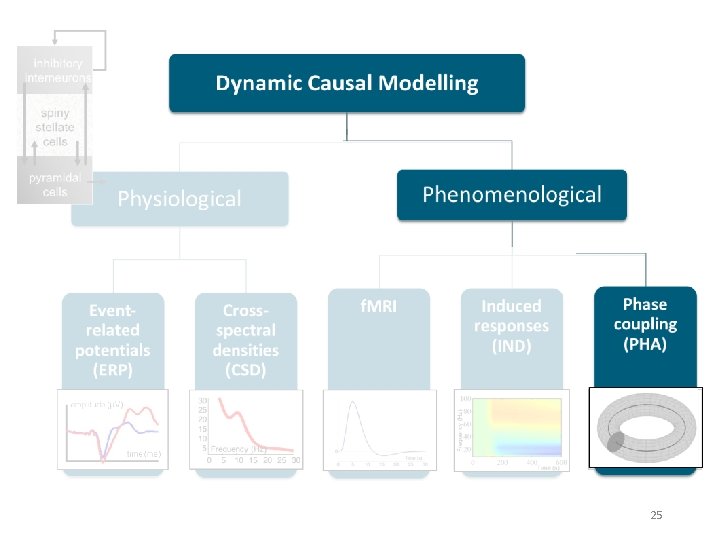
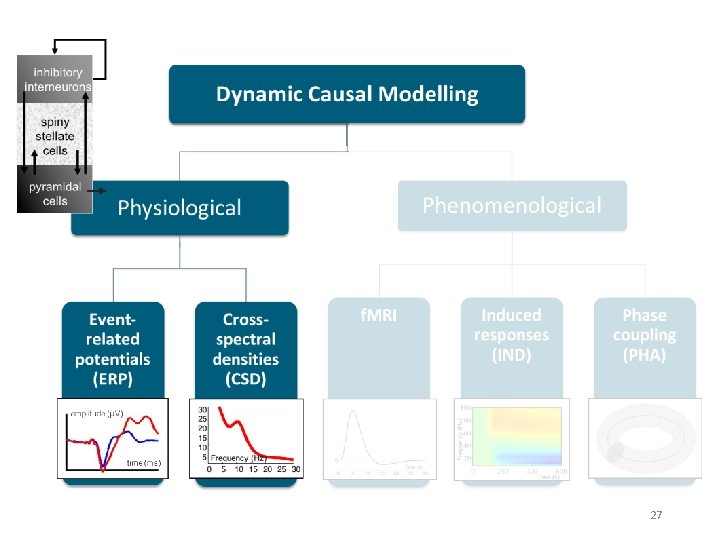
DCM is a computational modelling technique to estimate bio-physiological information from functional neuroimaging data Effective connectivity Synaptic connectivity Inhibitory interneurons Spiny stellate cells Pyramidal cells A generative model describes neural activity with differential equations Different type of models exist but principles are always the same! 2

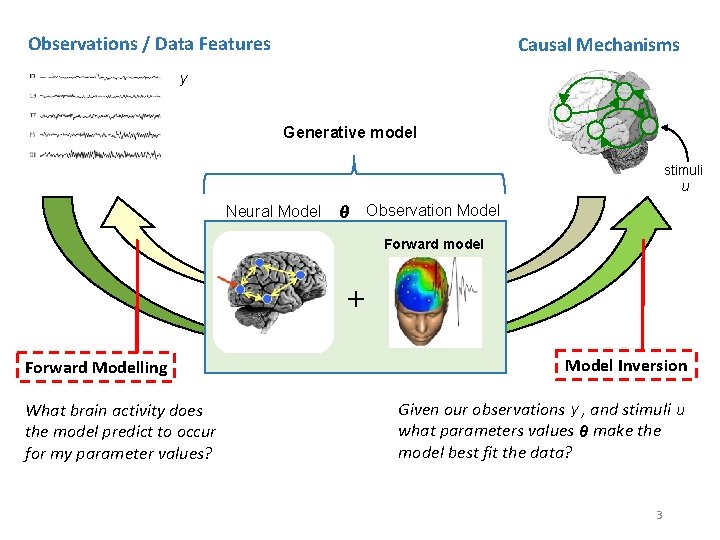
Observations / Data Features Causal Mechanisms y Generative model stimuli u Neural Model θ Observation Model Forward model + Forward Modelling What brain activity does the model predict to occur for my parameter values? Model Inversion Given our observations y , and stimuli u what parameters values θ make the model best fit the data? 3

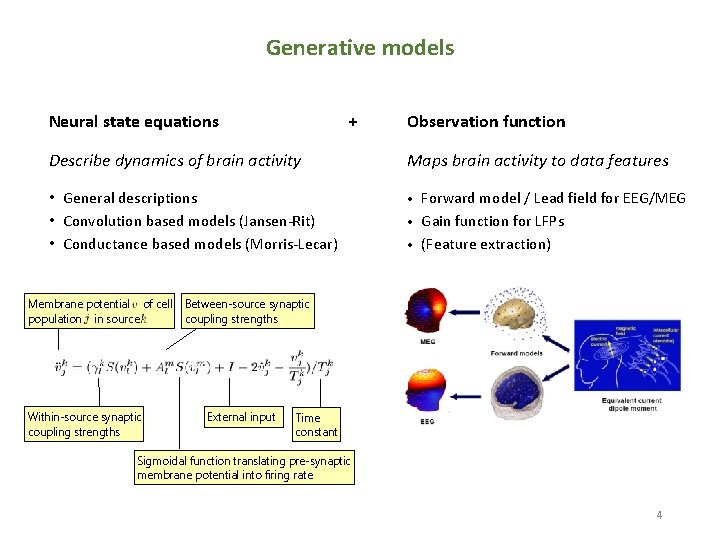
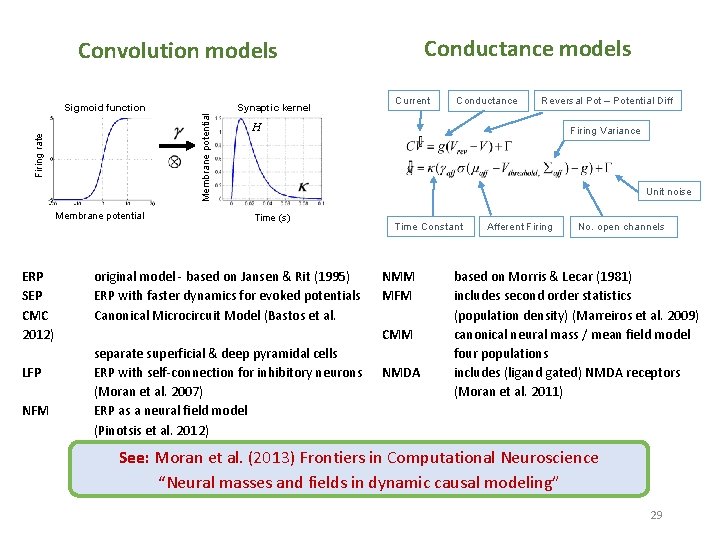
Generative models Neural state equations + Observation function Describe dynamics of brain activity Maps brain activity to data features • General descriptions • Convolution based models (Jansen-Rit) • Conductance based models (Morris-Lecar) • Membrane potential of cell population in source Within-source synaptic coupling strengths Forward model / Lead field for EEG/MEG • Gain function for LFPs • (Feature extraction) Between-source synaptic coupling strengths External input Time constant Sigmoidal function translating pre-synaptic membrane potential into firing rate 4

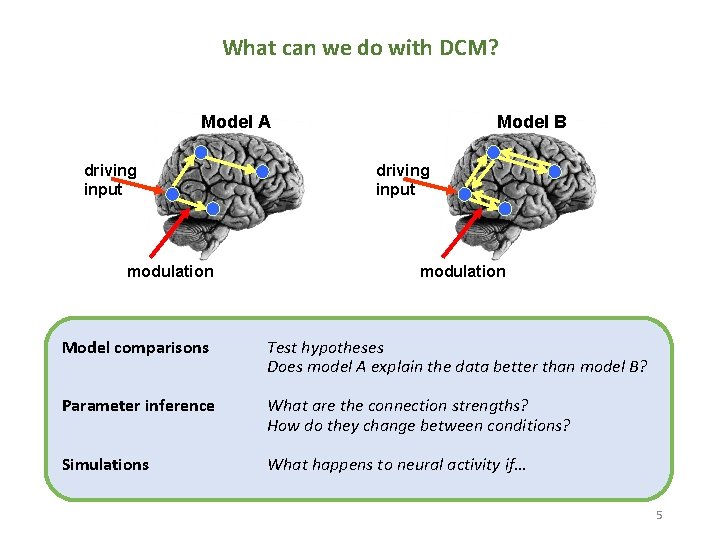
What can we do with DCM? Model A driving input modulation Model B driving input modulation Model comparisons Test hypotheses Does model A explain the data better than model B? Parameter inference What are the connection strengths? How do they change between conditions? Simulations What happens to neural activity if… 5

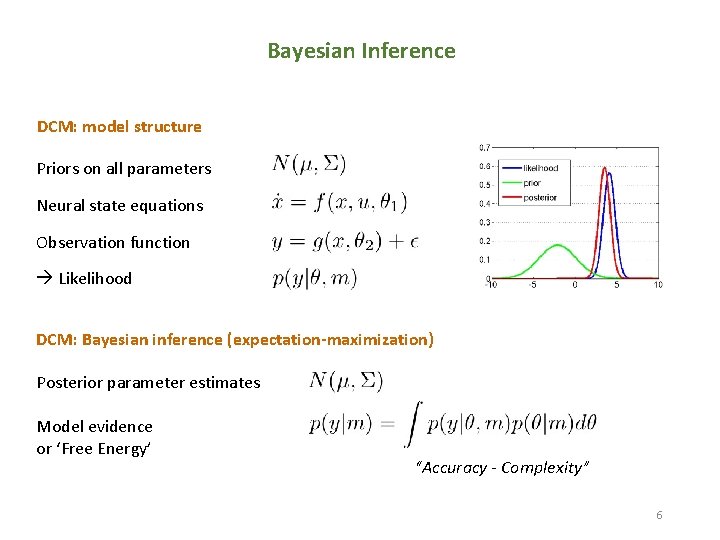
Bayesian Inference DCM: model structure Priors on all parameters Neural state equations Observation function Likelihood DCM: Bayesian inference (expectation-maximization) Posterior parameter estimates Model evidence or ‘Free Energy’ “Accuracy - Complexity” 6

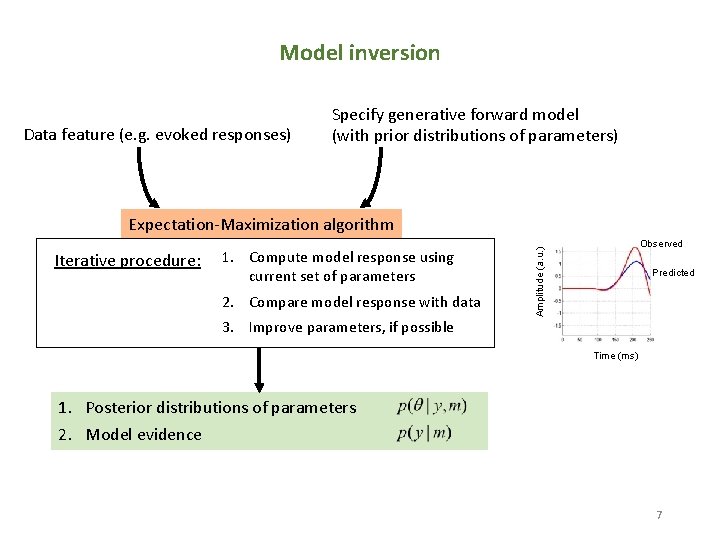
Model inversion Data feature (e. g. evoked responses) Specify generative forward model (with prior distributions of parameters) Expectation-Maximization algorithm 1. Compute model response using current set of parameters 2. Compare model response with data 3. Improve parameters, if possible Amplitude (a. u. ) Iterative procedure: Observed Predicted Time (ms) 1. Posterior distributions of parameters 2. Model evidence 7

8

9

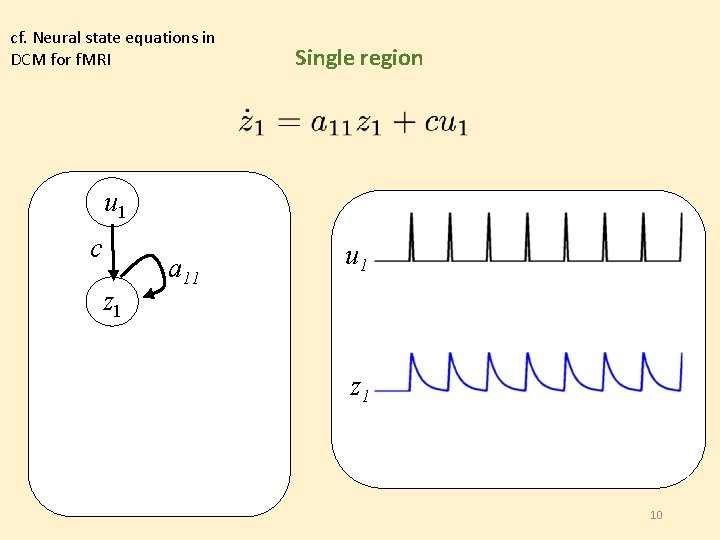
cf. Neural state equations in DCM for f. MRI Single region u 1 c z 1 a 11 u 2 z 1 z 2 10

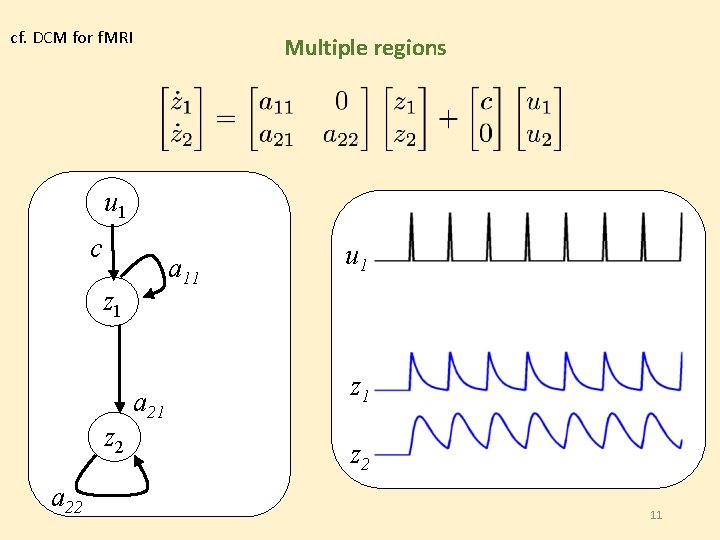
cf. DCM for f. MRI Multiple regions u 1 c a 11 z 2 a 22 u 1 u 2 a 21 z 2 11

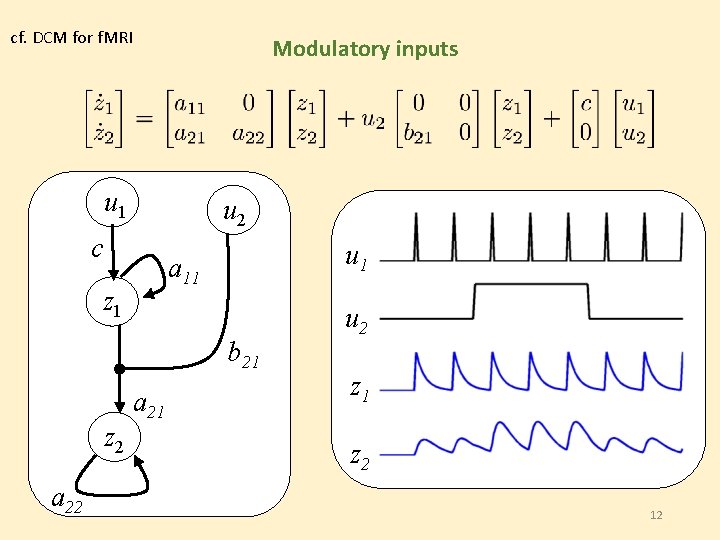
cf. DCM for f. MRI Modulatory inputs u 1 u 2 c u 1 a 11 z 1 b 21 z 2 a 21 u 2 z 1 z 2 12

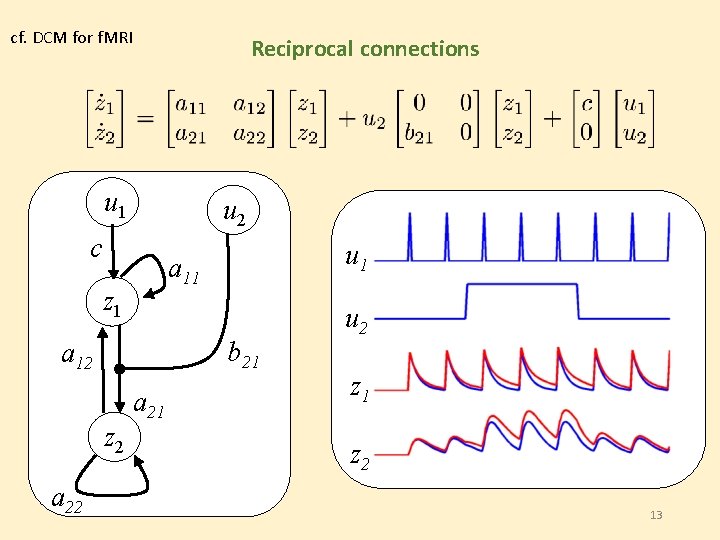
cf. DCM for f. MRI Reciprocal connections u 1 u 2 c z 1 b 21 a 12 z 2 a 22 u 1 a 11 a 21 u 2 z 1 z 2 13

14

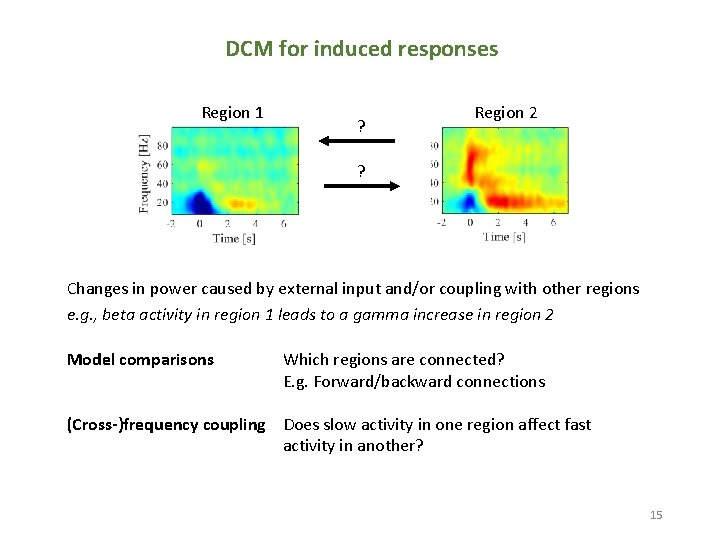
DCM for induced responses Region 1 ? Region 2 ? Changes in power caused by external input and/or coupling with other regions e. g. , beta activity in region 1 leads to a gamma increase in region 2 Model comparisons Which regions are connected? E. g. Forward/backward connections (Cross-)frequency coupling Does slow activity in one region affect fast activity in another? 15

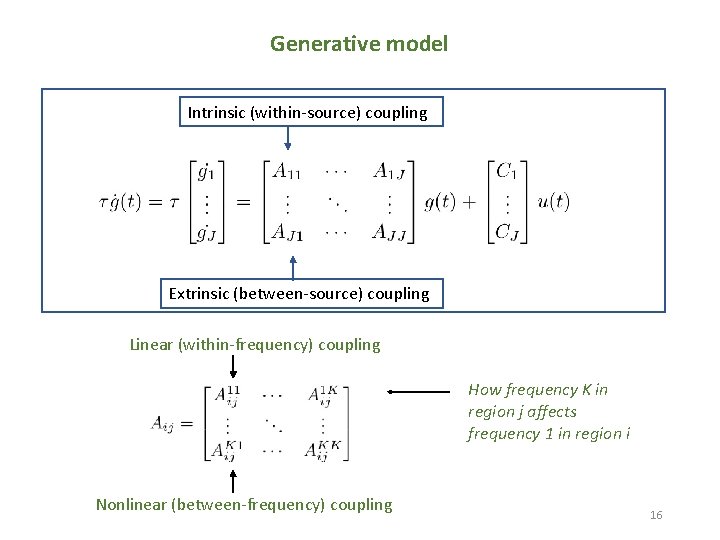
Generative model Intrinsic (within-source) coupling Extrinsic (between-source) coupling Linear (within-frequency) coupling How frequency K in region j affects frequency 1 in region i Nonlinear (between-frequency) coupling 16

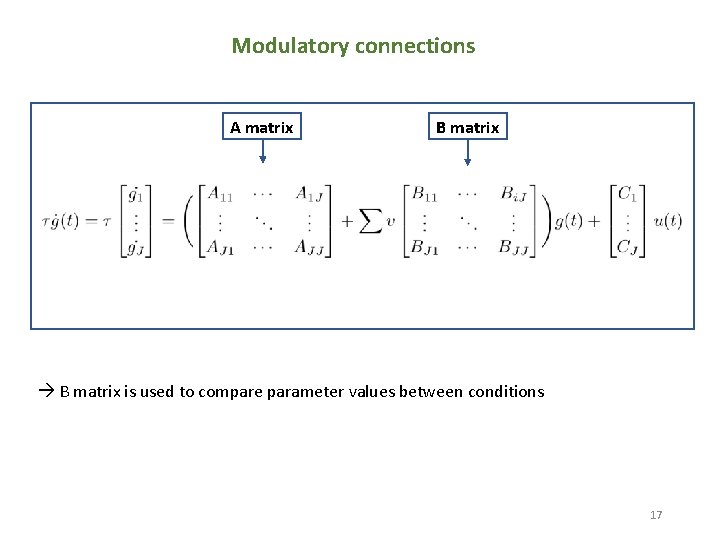
Modulatory connections A matrix B matrix is used to compare parameter values between conditions 17

Example with MEG data Motor imagery through mental hand rotation De Lange et al. 2008 Van Wijk et al. 2012, Neuroimage 18
![Induced responses in Motor and Occipital areas M O MNI coordinates 34 28 37 Induced responses in Motor and Occipital areas M O MNI coordinates [34 -28 37]](https://slidetodoc.com/presentation_image_h2/da2f10c4579b263e437bbf1edfdc38d0/image-20.jpg)
Induced responses in Motor and Occipital areas M O MNI coordinates [34 -28 37] [-37 -25 39] [14 -69 -2] [-18 -71 -5] Slow reaction times: Stronger increase in gamma power in O Stronger decrease in beta power in O 19

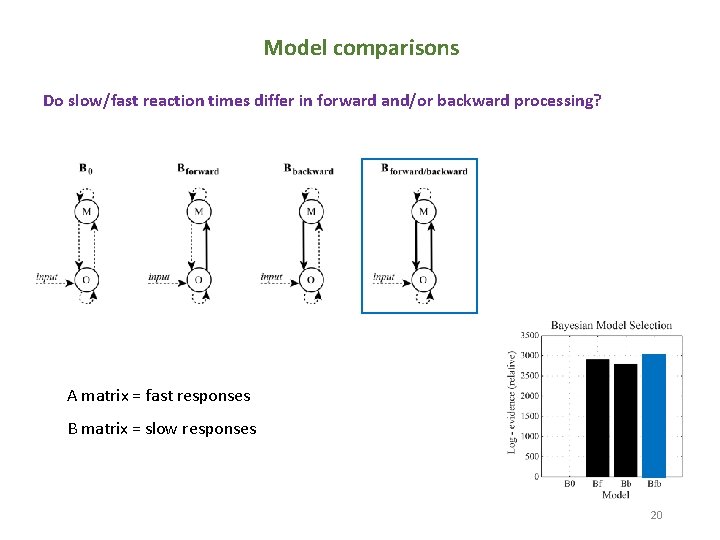
Model comparisons Do slow/fast reaction times differ in forward and/or backward processing? A matrix = fast responses B matrix = slow responses 20

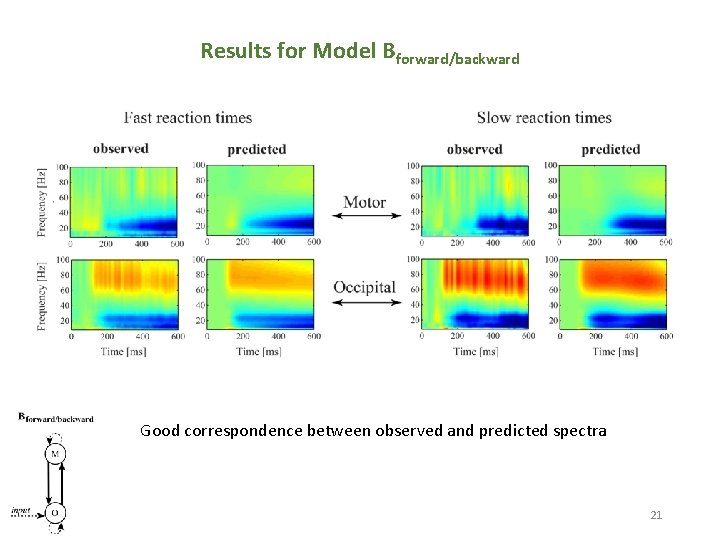
Results for Model Bforward/backward Good correspondence between observed and predicted spectra 21 21

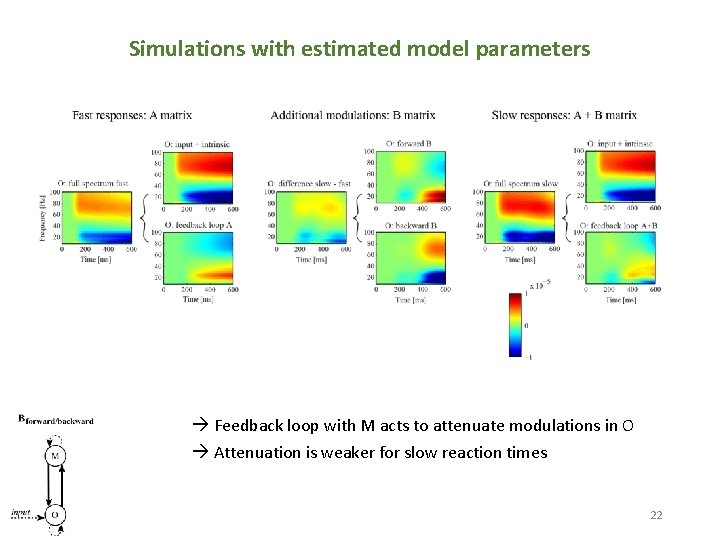
Simulations with estimated model parameters Feedback loop with M acts to attenuate modulations in O Attenuation is weaker for slow reaction times 22 22

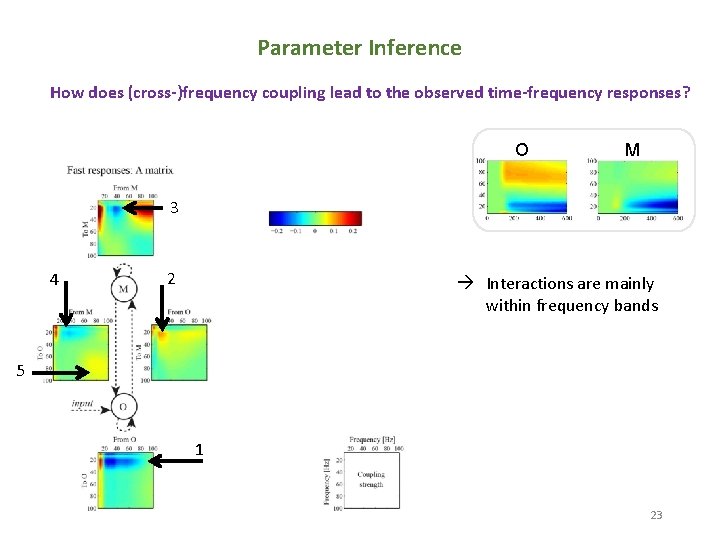
Parameter Inference How does (cross-)frequency coupling lead to the observed time-frequency responses? O M 3 4 2 Interactions are mainly within frequency bands 5 1 23 23

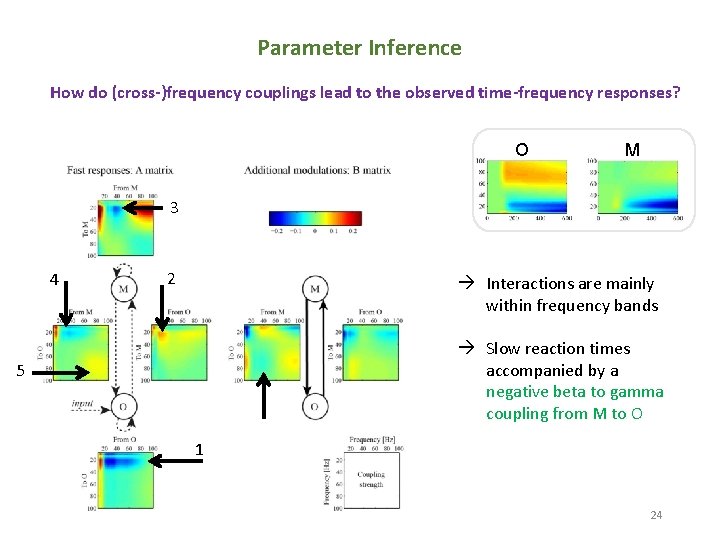
Parameter Inference How do (cross-)frequency couplings lead to the observed time-frequency responses? O M 3 4 2 Interactions are mainly within frequency bands Slow reaction times accompanied by a negative beta to gamma coupling from M to O 5 1 24 24

25

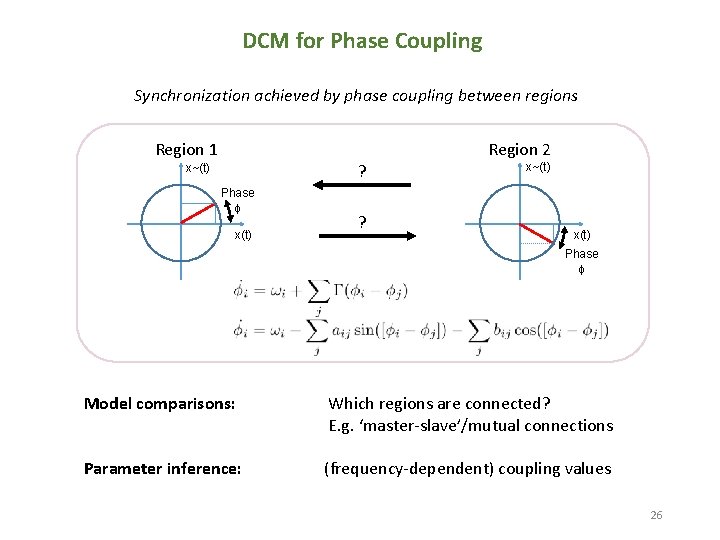
DCM for Phase Coupling Synchronization achieved by phase coupling between regions Region 1 Region 2 ? x~(t) Phase x(t) ? x~(t) x(t) Phase Model comparisons: Which regions are connected? E. g. ‘master-slave’/mutual connections Parameter inference: (frequency-dependent) coupling values 26

27

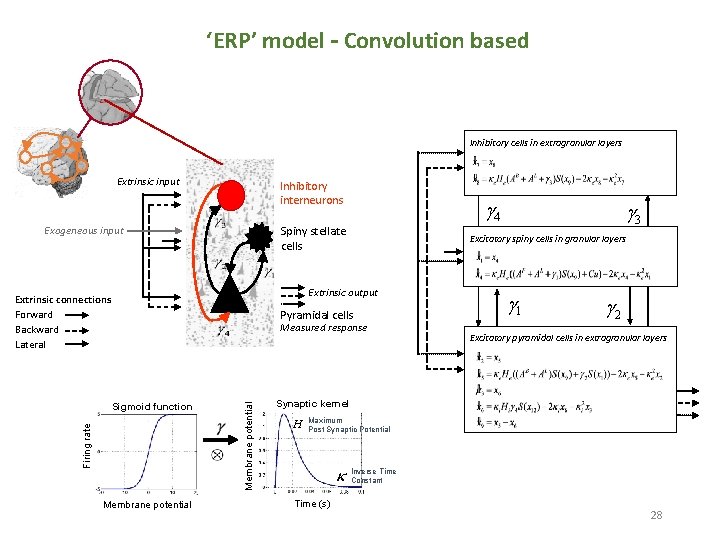
‘ERP’ model – Convolution based Inhibitory cells in extragranular layers Extrinsic input Inhibitory interneurons Exogeneous input g 4 Spiny stellate cells Excitatory spiny cells in granular layers Extrinsic output Extrinsic connections Forward Backward Lateral Pyramidal cells Firing rate Membrane potential Measured response Sigmoid function g 3 g 1 g 2 Excitatory pyramidal cells in extragranular layers Synaptic kernel H Maximum Post Synaptic Potential Inverse Time Constant Time (s) 28

Conductance models Convolution models Firing rate Membrane potential ERP SEP CMC 2012) LFP NFM Synaptic kernel Membrane potential Sigmoid function Current Conductance Reversal Pot – Potential Diff H Firing Variance Unit noise Time (s) original model - based on Jansen & Rit (1995) ERP with faster dynamics for evoked potentials Canonical Microcircuit Model (Bastos et al. Time Constant NMM MFM CMM separate superficial & deep pyramidal cells ERP with self-connection for inhibitory neurons (Moran et al. 2007) ERP as a neural field model (Pinotsis et al. 2012) NMDA Afferent Firing No. open channels based on Morris & Lecar (1981) includes second order statistics (population density) (Marreiros et al. 2009) canonical neural mass / mean field model four populations includes (ligand gated) NMDA receptors (Moran et al. 2011) See: Moran et al. (2013) Frontiers in Computational Neuroscience “Neural masses and fields in dynamic causal modeling” 29

Generic DCM ‘Mix-and-Match’ of generative models More flexible than standard implementation Adding a new model is easy Van Wijk et al. Under review 30

Which DCM should I use? 0) What is your research question? ? 1) Select type of generative model • Physiological: convolution or conductance, several options • Phenomenological: fixed choice 2) Select data feature of interest • Event-related design: event-related potentials, induced responses • Steady state activity: cross-spectral densities, phase coupling 3) Specify networks - what do you want to test? • What is the hypothesis? • Which regions? • Which connections? 4) Think about condition-specific effects (B matrix) • Do you have more than 1 experimental condition? • Which connections may show a difference between conditions? (A matrix) 31

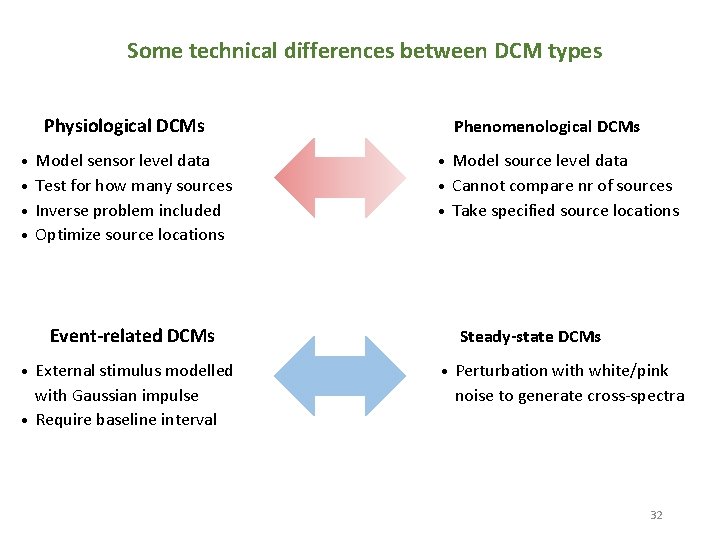
Some technical differences between DCM types Physiological DCMs Model sensor level data • Test for how many sources • Inverse problem included • Optimize source locations • Phenomenological DCMs Model source level data • Cannot compare nr of sources • Take specified source locations • Event-related DCMs External stimulus modelled with Gaussian impulse • Require baseline interval • Steady-state DCMs • Perturbation with white/pink noise to generate cross-spectra 32

In SPM manual Online videos http: //www. fil. ion. ucl. ac. uk/spm/course/ 33

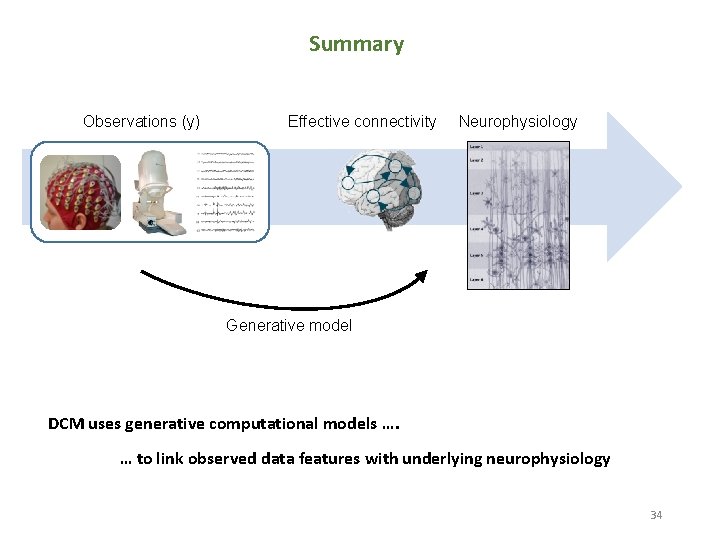
Summary Observations (y) Effective connectivity Neurophysiology Generative model DCM uses generative computational models …. … to link observed data features with underlying neurophysiology 34

Further reading The original DCM paper Friston et al. 2003, Neuro. Image Guide to MEG/EEG analysis in SPM Litvak et al. (2011) Comput Intell & Neurosci Descriptive / Tutorial papers Ten Simple Rules for DCM Stephan et al. 2010, Neuro. Image Overview of generative models Moran et al. 2013, Front Comput Neurosci Model selection for group studies Stephan et al. 2009, Neuroimage Comparing families of DCMs Penny et al. 2010, PLo. S One DCM applications Event-related potentials David et al. 2006, Neuroimage Garrido et al. 2007, PNAS Boly et al. 2011, Science Auksztulewicz & Friston, 2015, Cerebral Cortex Cross-spectral densities Moran et al. 2009, Neuroimage Moran et al. 2011, PLo. S One Friston et al. 2012, Neuroimage Induced responses Chen et al. 2008, 2009, Neuroimage Van Wijk et al. 2012, Neuroimage Phase coupling Penny et al. 2009, J Neurosci Methods 35