Dynamic Causal Modelling DCM Marta I Garrido migarridoucla
- Slides: 29

Dynamic Causal Modelling (DCM) Marta I. Garrido migarrido@ucla. edu Thanks to: Karl J. Friston, Klaas E. Stephan, Andre C. Marreiros, Stefan J. Kiebel, CC Chen, Rosalyn Moran, Lee Harrison, and James M. Kilner

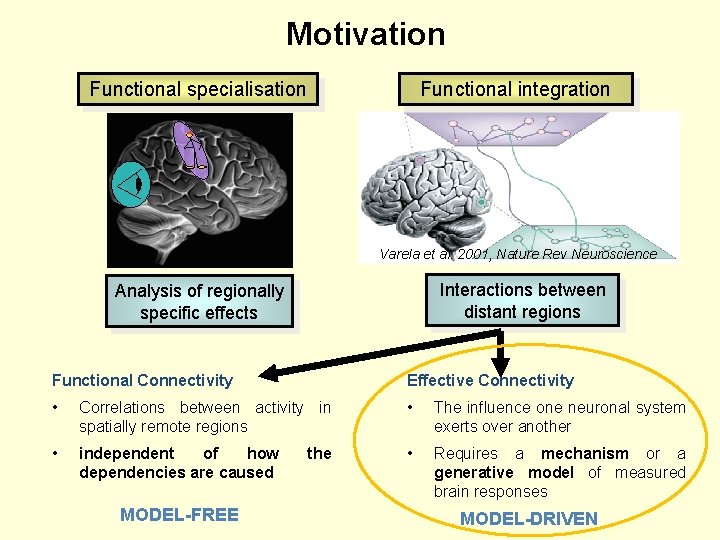
Motivation Functional specialisation Functional integration Varela et al. 2001, Nature Rev Neuroscience Interactions between distant regions Analysis of regionally specific effects Functional Connectivity Effective Connectivity • Correlations between activity in spatially remote regions • The influence one neuronal system exerts over another • independent of how dependencies are caused • Requires a mechanism or a generative model of measured brain responses MODEL-FREE the MODEL-DRIVEN

Outline I. DCM: the neuronal and the hemodynamic models II. Estimation and Bayesian inference III. Application: Attention to motion in the visual system IV. Extensions for f. MRI and EEG data

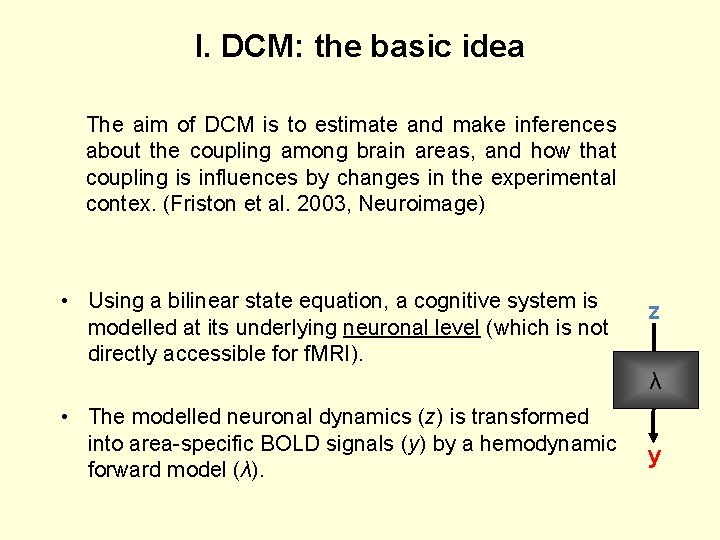
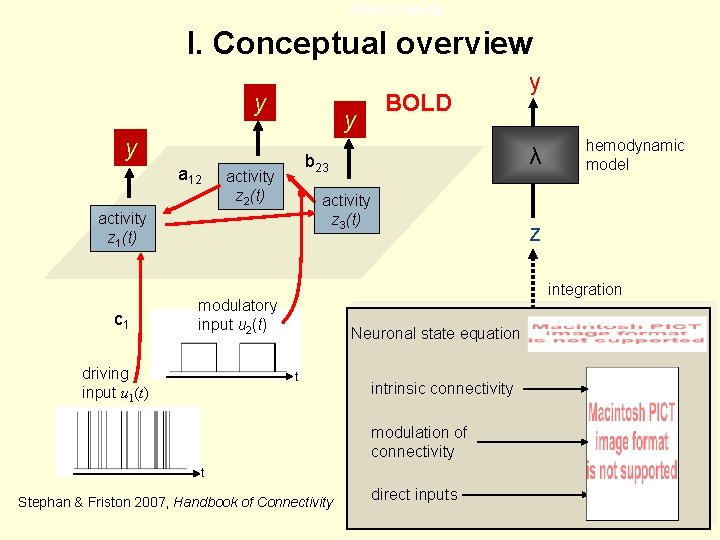
I. DCM: the basic idea The aim of DCM is to estimate and make inferences about the coupling among brain areas, and how that coupling is influences by changes in the experimental contex. (Friston et al. 2003, Neuroimage) • Using a bilinear state equation, a cognitive system is modelled at its underlying neuronal level (which is not directly accessible for f. MRI). z λ • The modelled neuronal dynamics (z) is transformed into area-specific BOLD signals (y) by a hemodynamic forward model (λ). y

direct inputs I. Conceptual overview y y y a 12 activity z 2(t) activity z 3(t) hemodynamic model z integration modulatory input u 2(t) driving input u 1(t) λ b 23 activity z 1(t) c 1 BOLD y Neuronal state equation t intrinsic connectivity modulation of connectivity t Stephan & Friston 2007, Handbook of Connectivity direct inputs

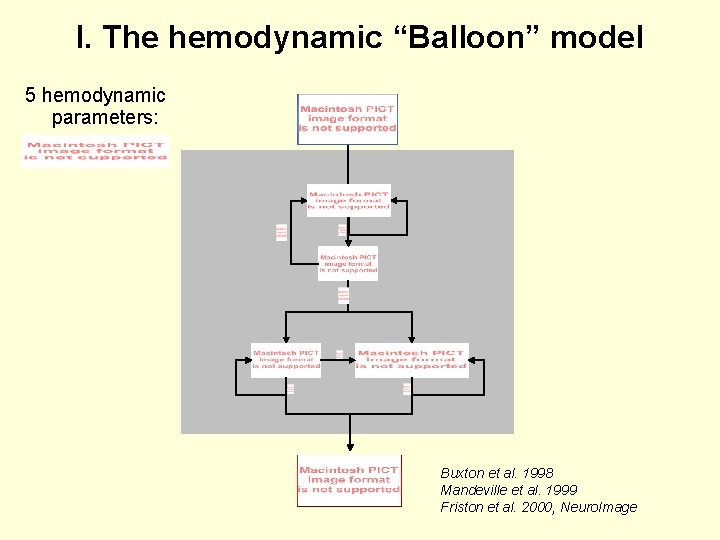
I. The hemodynamic “Balloon” model 5 hemodynamic parameters: Buxton et al. 1998 Mandeville et al. 1999 Friston et al. 2000, Neuro. Image

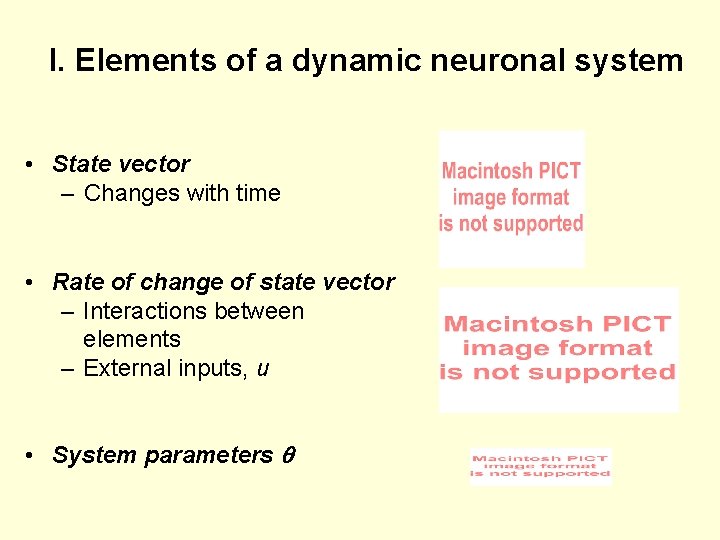
I. Elements of a dynamic neuronal system • State vector – Changes with time • Rate of change of state vector – Interactions between elements – External inputs, u • System parameters

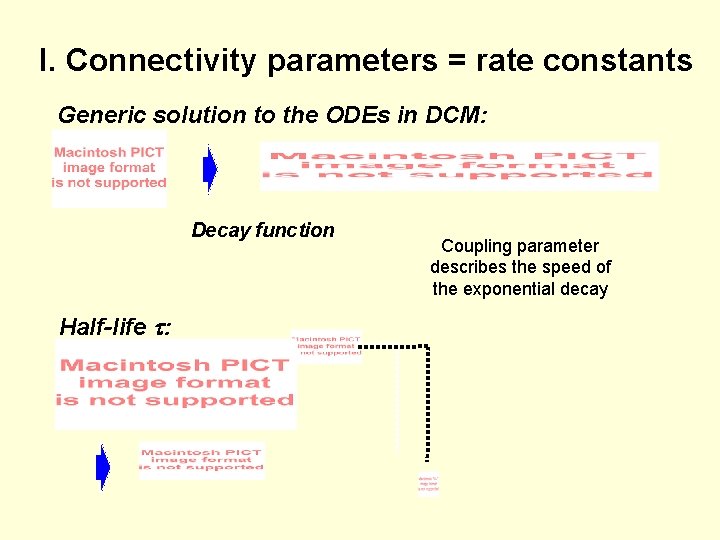
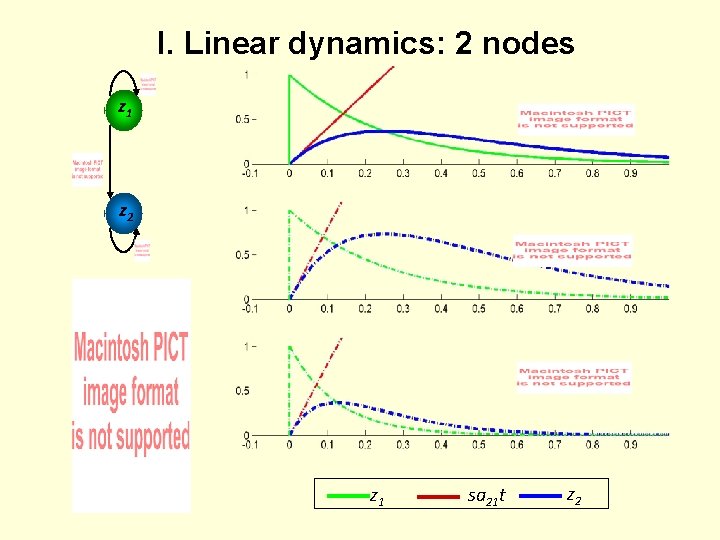
I. Connectivity parameters = rate constants Generic solution to the ODEs in DCM: Decay function Half-life : Coupling parameter describes the speed of the exponential decay

I. Linear dynamics: 2 nodes z 1 z 2 z 1 sa 21 t z 2

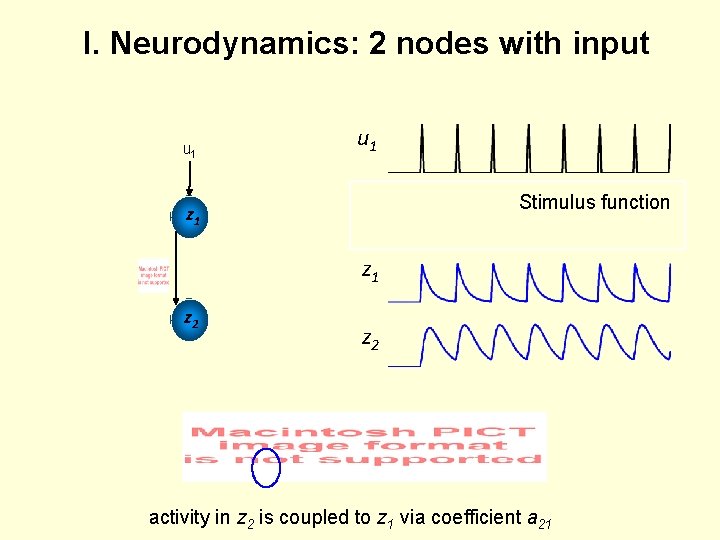
I. Neurodynamics: 2 nodes with input u 1 z 1 u 2 Stimulus function z 1 z 2 activity in z 2 is coupled to z 1 via coefficient a 21

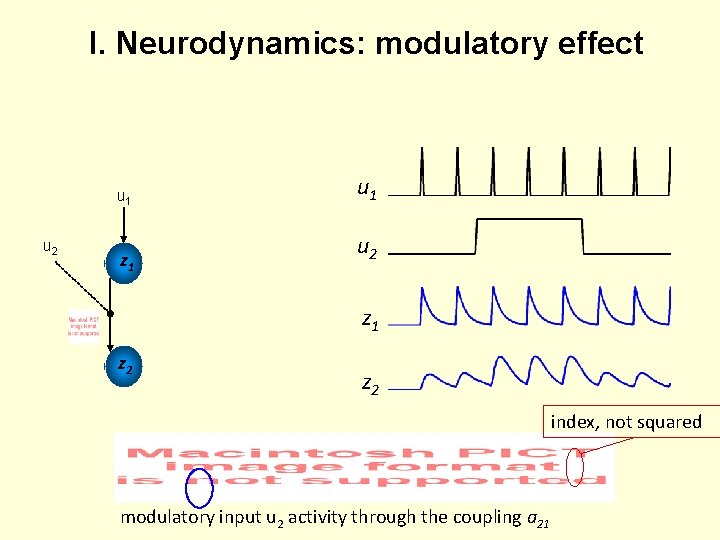
I. Neurodynamics: modulatory effect u 1 u 2 z 1 z 2 index, not squared modulatory input u 2 activity through the coupling a 21

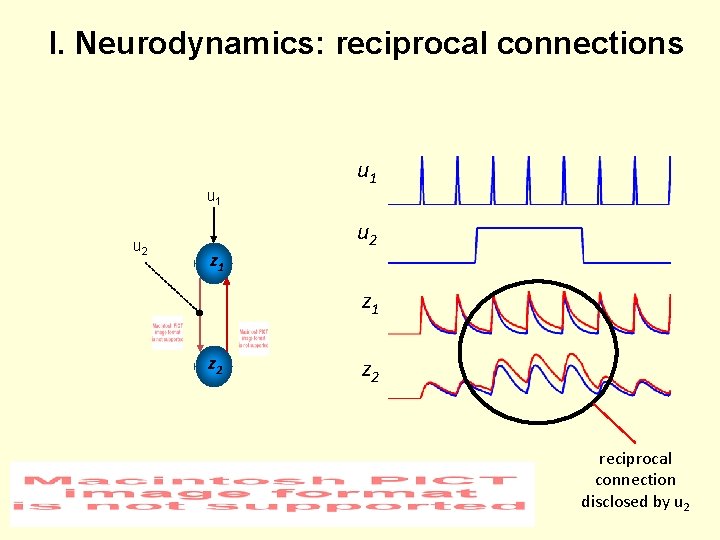
I. Neurodynamics: reciprocal connections u 1 u 2 z 1 z 2 reciprocal connection disclosed by u 2

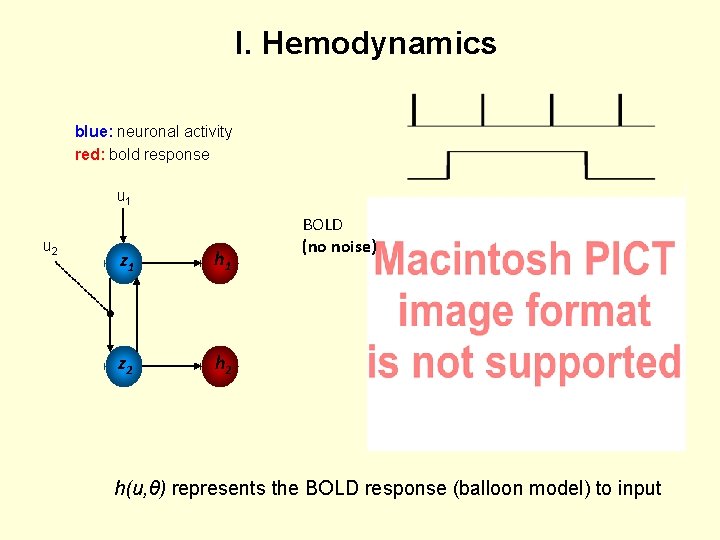
I. Hemodynamics blue: neuronal activity red: bold response u 1 u 2 z 1 h 1 z 2 h 2 BOLD (no noise) h(u, θ) represents the BOLD response (balloon model) to input

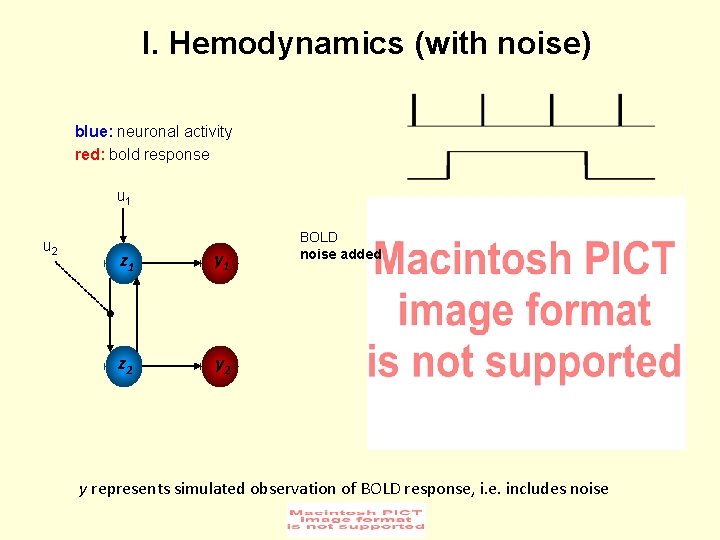
I. Hemodynamics (with noise) blue: neuronal activity red: bold response u 1 u 2 z 1 y 1 z 2 y 2 BOLD noise added y represents simulated observation of BOLD response, i. e. includes noise

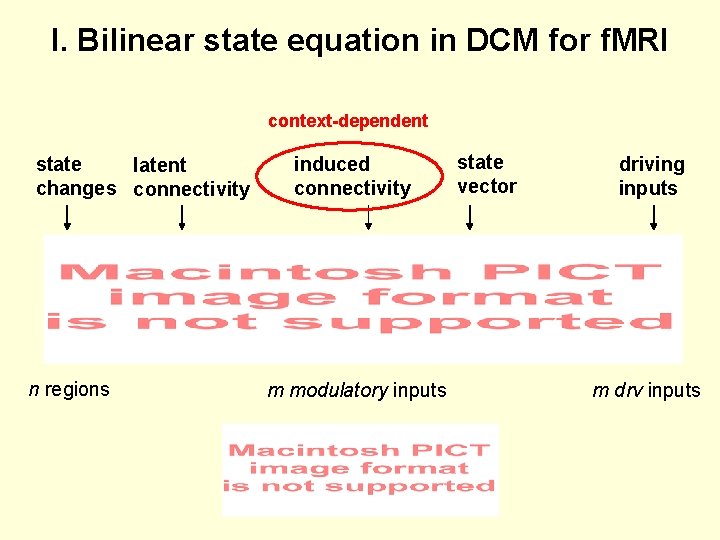
I. Bilinear state equation in DCM for f. MRI context-dependent state latent changes connectivity n regions induced connectivity m modulatory inputs state vector driving inputs m drv inputs

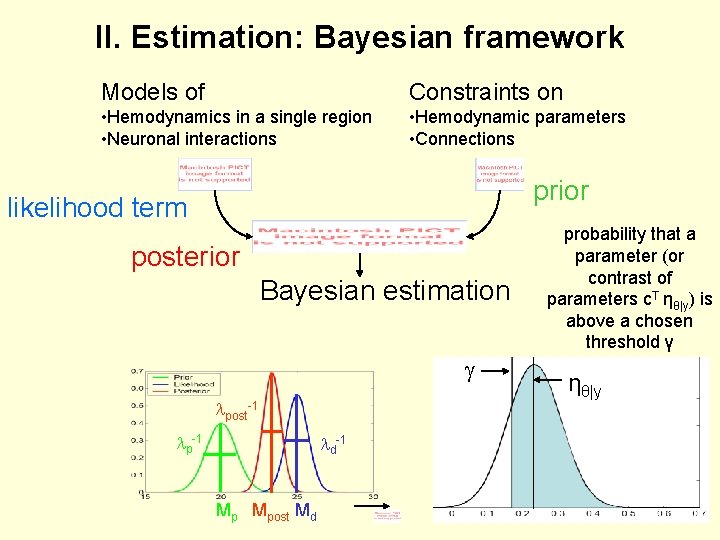
II. Estimation: Bayesian framework Models of Constraints on • Hemodynamics in a single region • Neuronal interactions • Hemodynamic parameters • Connections prior likelihood term posterior Bayesian estimation lpost-1 lp-1 ld-1 Mp Mpost Md probability that a parameter (or contrast of parameters c. T ηθ|y) is above a chosen threshold γ ηθ|y

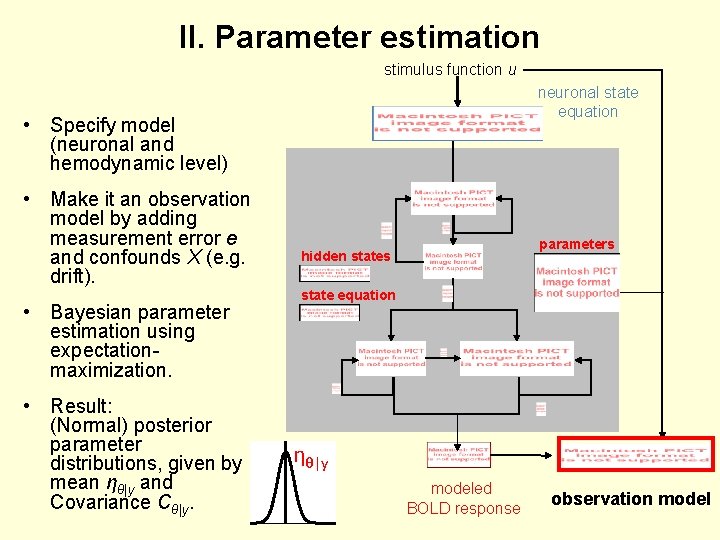
II. Parameter estimation stimulus function u neuronal state equation • Specify model (neuronal and hemodynamic level) • Make it an observation model by adding measurement error e and confounds X (e. g. drift). • Bayesian parameter estimation using expectationmaximization. • Result: (Normal) posterior parameter distributions, given by mean ηθ|y and Covariance Cθ|y. parameters hidden states state equation ηθ|y modeled BOLD response observation model

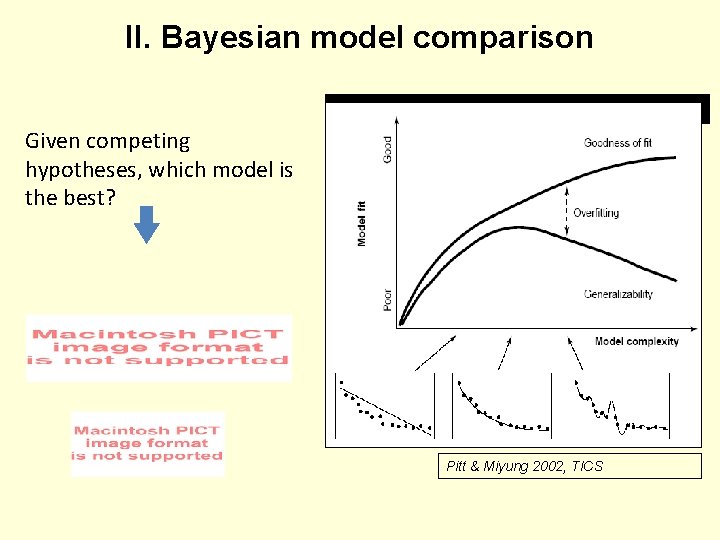
II. Bayesian model comparison Given competing hypotheses, which model is the best? Pitt & Miyung 2002, TICS

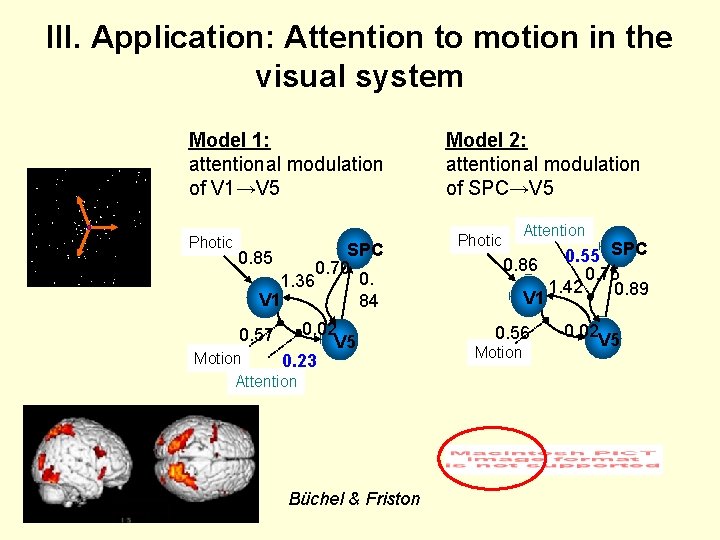
III. Application: Attention to motion in the visual system Model 1: attentional modulation of V 1→V 5 Photic SPC 0. 70 0. 1. 36 V 1 84 0. 85 0. 57 -0. 02 V 5 0. 23 Motion Attention Büchel & Friston Model 2: attentional modulation of SPC→V 5 Photic Attention SPC 0. 55 0. 86 0. 75 1. 42 0. 89 V 1 0. 56 Motion -0. 02 V 5

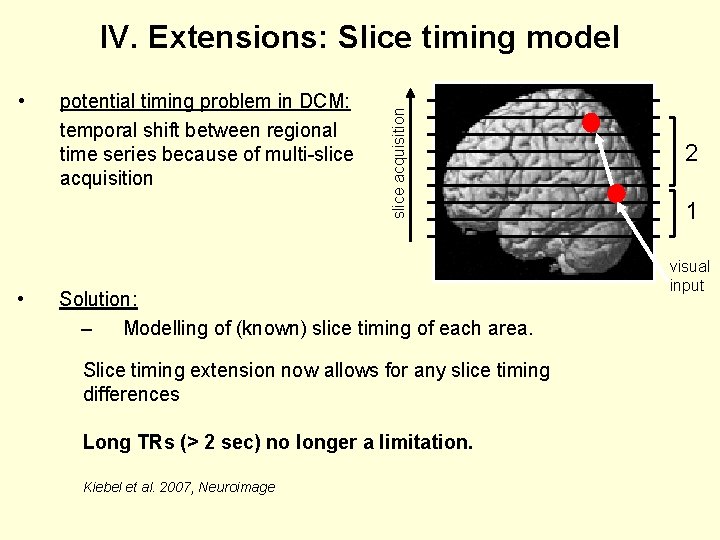
• • potential timing problem in DCM: temporal shift between regional time series because of multi-slice acquisition IV. Extensions: Slice timing model Solution: – Modelling of (known) slice timing of each area. Slice timing extension now allows for any slice timing differences Long TRs (> 2 sec) no longer a limitation. Kiebel et al. 2007, Neuroimage 2 1 visual input

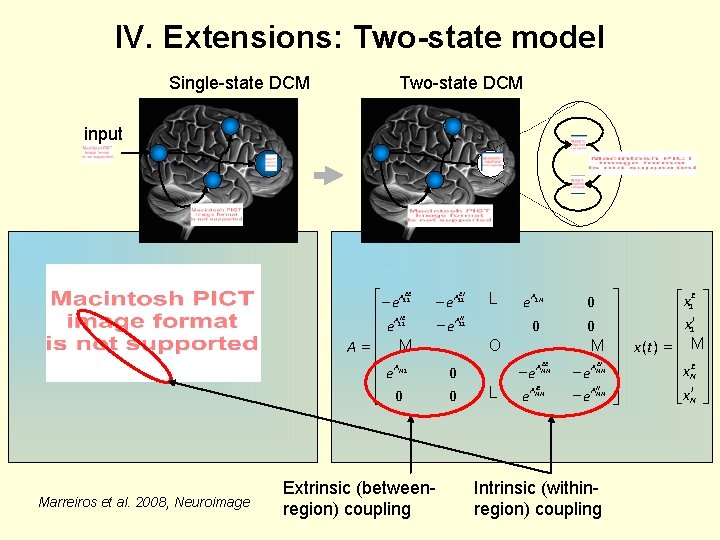
IV. Extensions: Two-state model Single-state DCM Two-state DCM input é - e. A 11 EE ê AIE ê e 11 A =ê M ê A ê e N 1 ê ë 0 Marreiros et al. 2008, Neuroimage Extrinsic (betweenregion) coupling EI - e. A 11 II - e. A 11 L A e 1 N 0 O 0 0 EE - e. ANN L IE e. ANN 0 0 M EI - e. ANN Intrinsic (withinregion) coupling ù éx 1 E ù ú ê I ú ú ê x 1 ú ú x ( t) = ê Mú ú ê E ú ú êx. N ú ú êx. I ú ë N û û

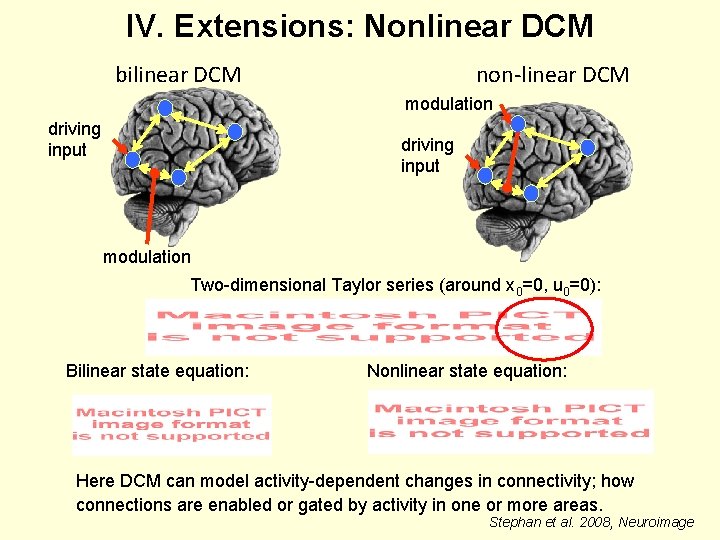
IV. Extensions: Nonlinear DCM bilinear DCM non-linear DCM modulation driving input modulation Two-dimensional Taylor series (around x 0=0, u 0=0): Bilinear state equation: Nonlinear state equation: Here DCM can model activity-dependent changes in connectivity; how connections are enabled or gated by activity in one or more areas. Stephan et al. 2008, Neuroimage

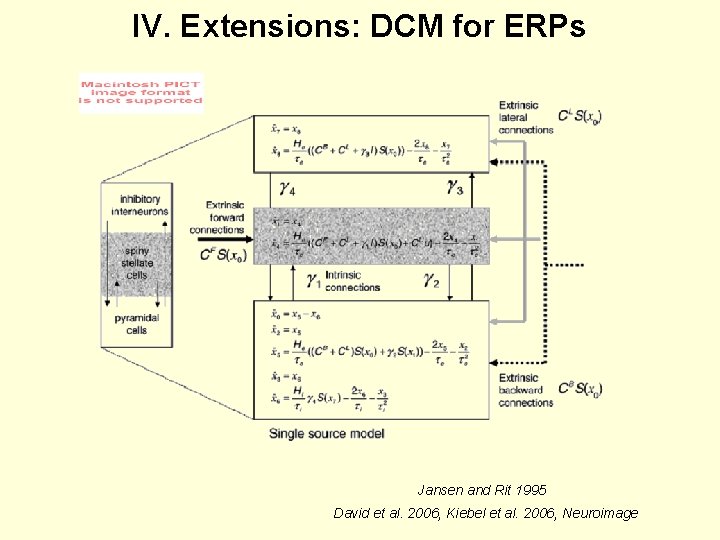
IV. Extensions: DCM for ERPs Jansen and Rit 1995 David et al. 2006, Kiebel et al. 2006, Neuroimage

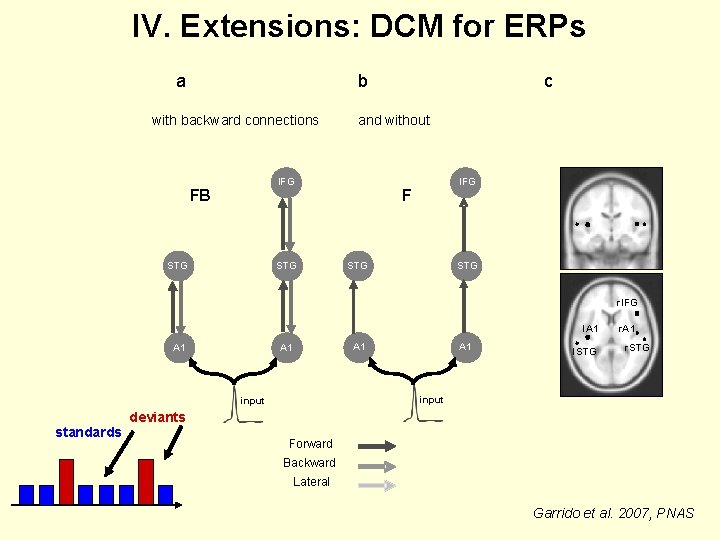
IV. Extensions: DCM for ERPs a b with backward connections and without IFG FB STG c IFG F STG r. IFG l. A 1 A 1 l. STG r. STG input standards A 1 r. A 1 deviants Forward Backward Lateral Garrido et al. 2007, PNAS

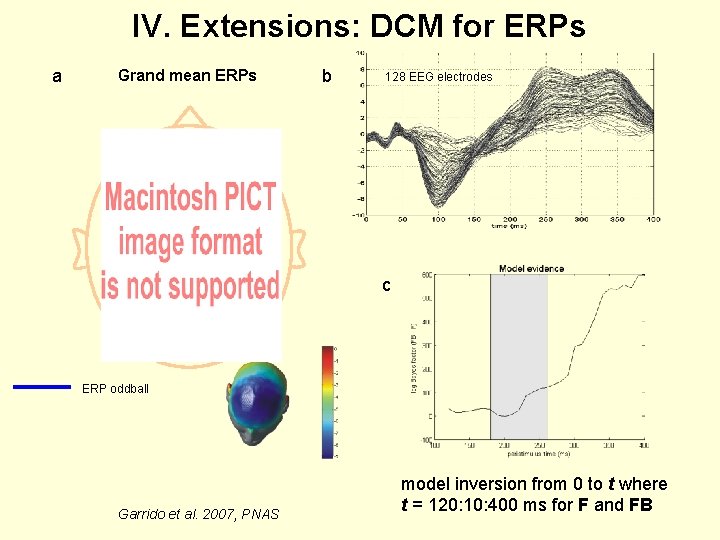
IV. Extensions: DCM for ERPs a Grand mean ERPs b 128 EEG electrodes c ERP oddball Garrido et al. 2007, PNAS model inversion from 0 to t where t = 120: 10: 400 ms for F and FB

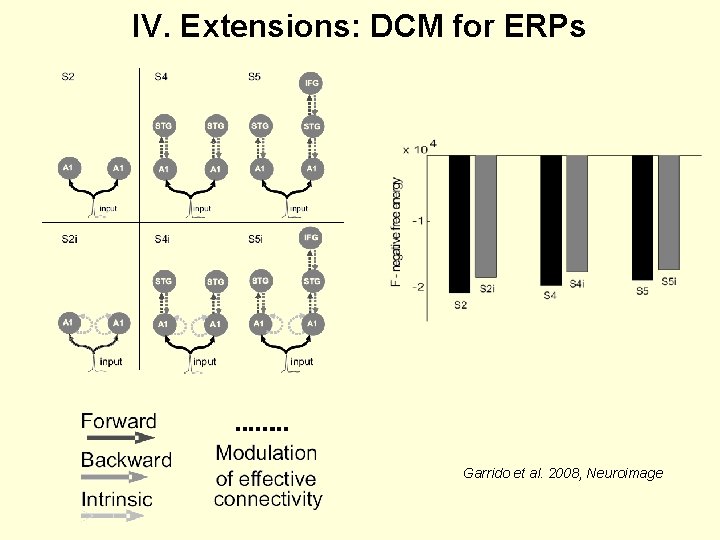
IV. Extensions: DCM for ERPs Garrido et al. 2008, Neuroimage

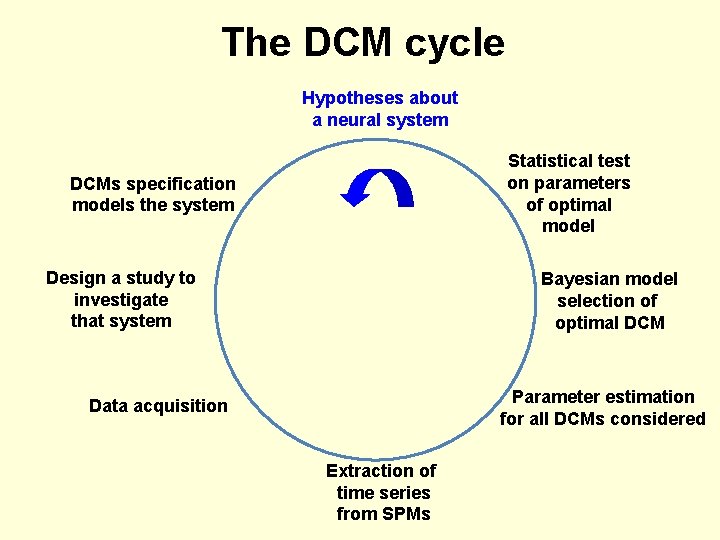
The DCM cycle Hypotheses about a neural system Statistical test on parameters of optimal model DCMs specification models the system Design a study to investigate that system Bayesian model selection of optimal DCM Parameter estimation for all DCMs considered Data acquisition Extraction of time series from SPMs

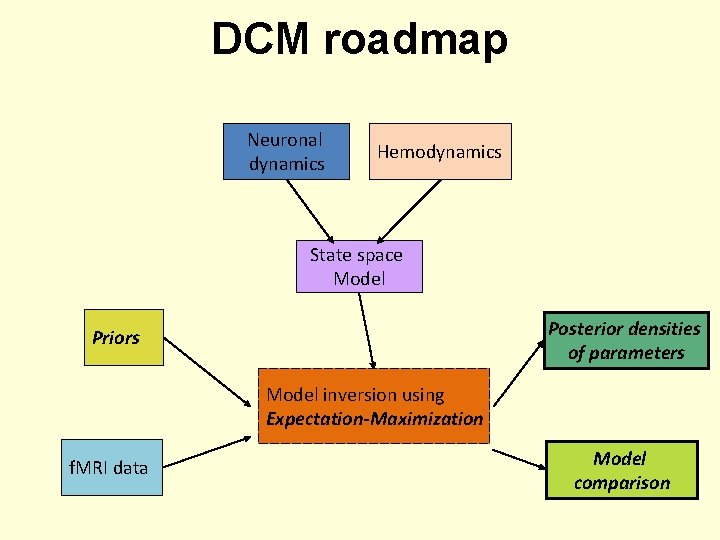
DCM roadmap Neuronal dynamics Hemodynamics State space Model Posterior densities of parameters Priors Model inversion using Expectation-Maximization f. MRI data Model comparison

Dynamic Causal Modelling (DCM) Marta I. Garrido migarrido@ucla. edu Thanks to: Karl J. Friston, Klaas E. Stephan, Andre C. Marreiros, Stefan J. Kiebel, CC Chen, Rosalyn Moran, Lee Harrison, and James M. Kilner