Physics 220 Lecture 27 Thermodynamics II Lecture 27




- Slides: 20

Physics 220 Lecture 27 Thermodynamics II Lecture 27 Purdue University, Physics 220 1

Overview – First Law of thermodynamics: Energy Conservation • Q = U + W – Heat Engines • Efficiency = 1 -QC/QH – Refrigerators • Coefficient of Performance = QC/(QH – QC) • Today – – Lecture 27 Second law of thermodynamics Carnot Engine (Sadi Carnot 1796 -1832) Entropy Disorder Purdue University, Physics 220 2

Second law of thermodynamics • Heat flow spontaneously from a warm object to a colder one. It is not possible for heat to flow spontaneously from a cold object to a warmer one. Lecture 27 Purdue University, Physics 220 3

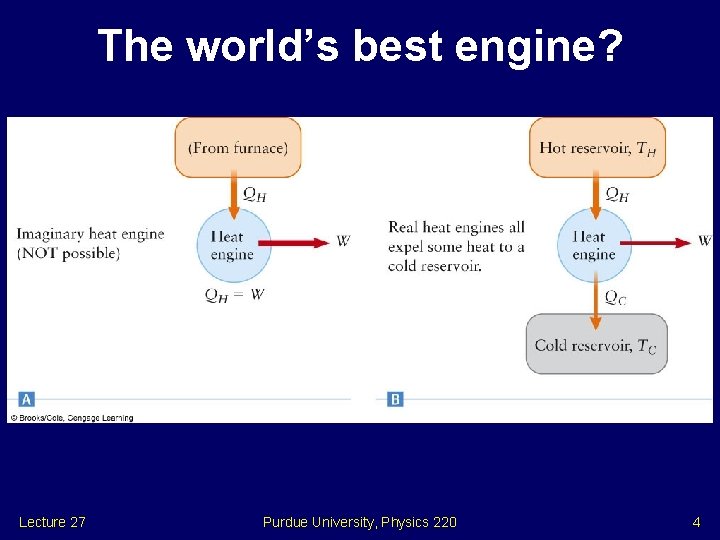
The world’s best engine? Lecture 27 Purdue University, Physics 220 4

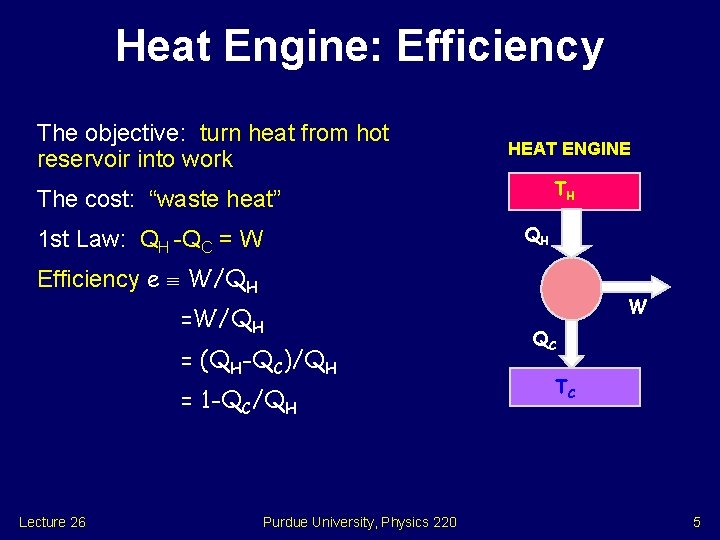
Heat Engine: Efficiency The objective: turn heat from hot reservoir into work HEAT ENGINE TH The cost: “waste heat” QH 1 st Law: QH -QC = W Efficiency e W/QH = (QH-QC)/QH = 1 -QC/QH Lecture 26 Purdue University, Physics 220 W QC TC 5

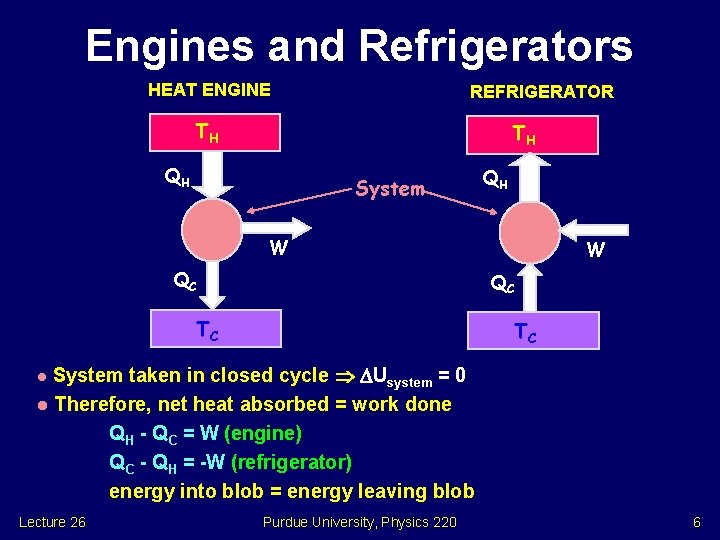
Engines and Refrigerators HEAT ENGINE REFRIGERATOR TH TH QH System QH W QC TC TC System taken in closed cycle Usystem = 0 l Therefore, net heat absorbed = work done QH - QC = W (engine) QC - QH = -W (refrigerator) energy into blob = energy leaving blob l Lecture 26 Purdue University, Physics 220 6

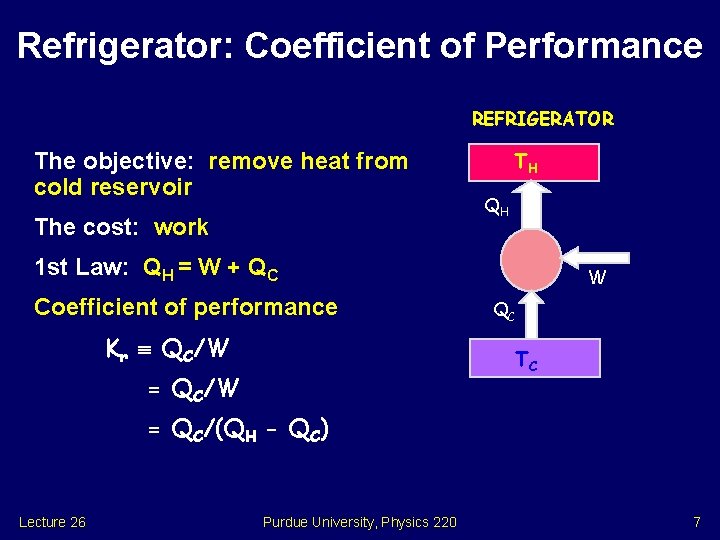
Refrigerator: Coefficient of Performance REFRIGERATOR The objective: remove heat from cold reservoir The cost: work TH QH 1 st Law: QH = W + QC Coefficient of performance Kr QC/W W QC TC = QC/W = QC/(QH - QC) Lecture 26 Purdue University, Physics 220 7

i. Clicker • An ideal heat engine absorbs 36 k. J of heat and exhausts 18 k. J of heat every cycle. What is the efficiency of the engine? A. 1 B. 0. 5 C. 2 D. 0. 25 QH -QC = W Efficiency e W/QH Lecture 26 Purdue University, Physics 220 8

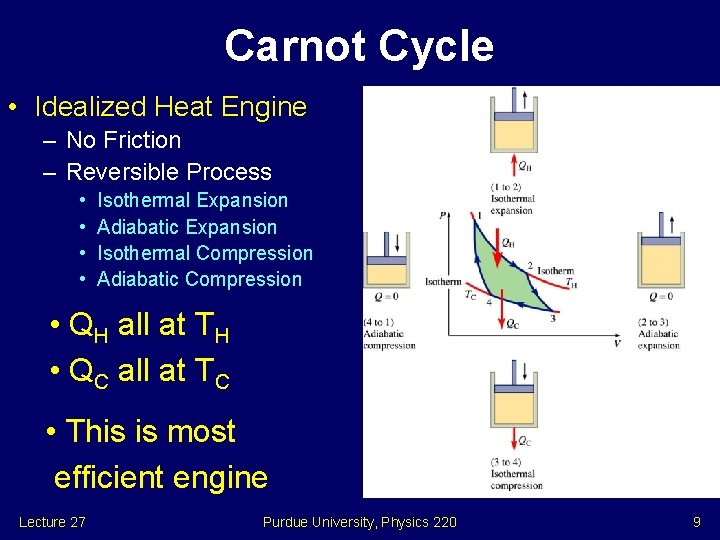
Carnot Cycle • Idealized Heat Engine – No Friction – Reversible Process • • Isothermal Expansion Adiabatic Expansion Isothermal Compression Adiabatic Compression • QH all at TH • QC all at TC • This is most efficient engine Lecture 27 Purdue University, Physics 220 9

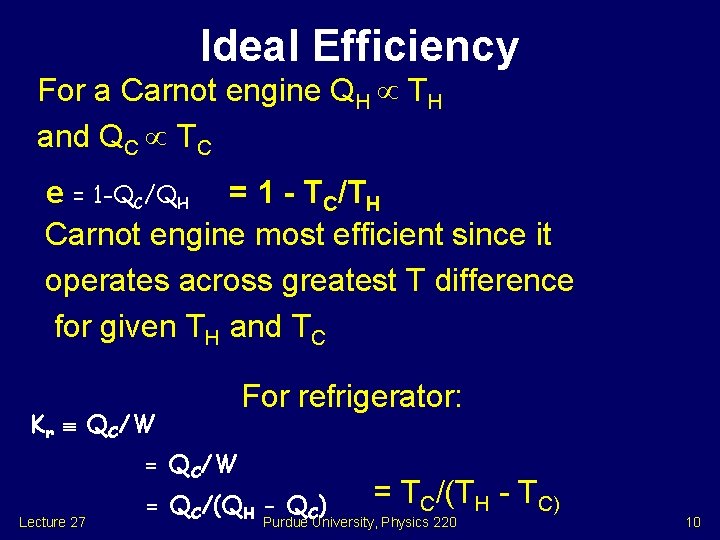
Ideal Efficiency For a Carnot engine QH TH and QC TC e = 1 -QC/QH = 1 - TC/TH Carnot engine most efficient since it operates across greatest T difference for given TH and TC Kr QC/W For refrigerator: = QC/W Lecture 27 = QC/(QH - QC) = TC/(TH - TC) Purdue University, Physics 220 10

Engine Lecture 27 Purdue University, Physics 220 11

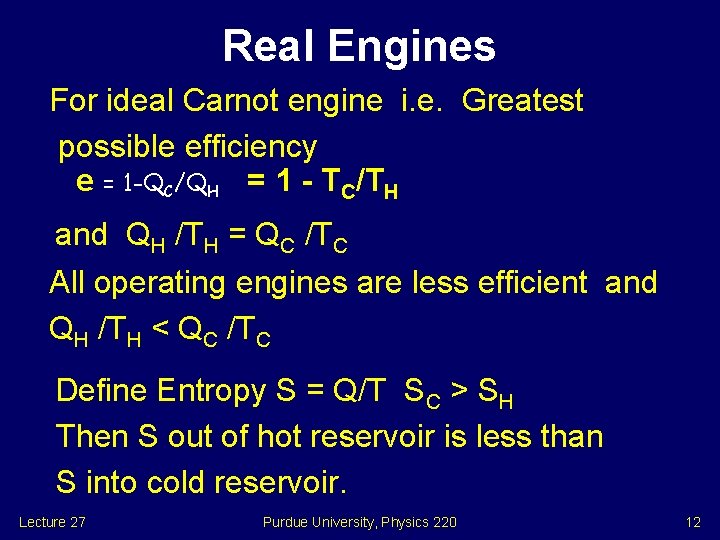
Real Engines For ideal Carnot engine i. e. Greatest possible efficiency e = 1 -QC/QH = 1 - TC/TH and QH /TH = QC /TC All operating engines are less efficient and QH /TH < QC /TC Define Entropy S = Q/T SC > SH Then S out of hot reservoir is less than S into cold reservoir. Lecture 27 Purdue University, Physics 220 12

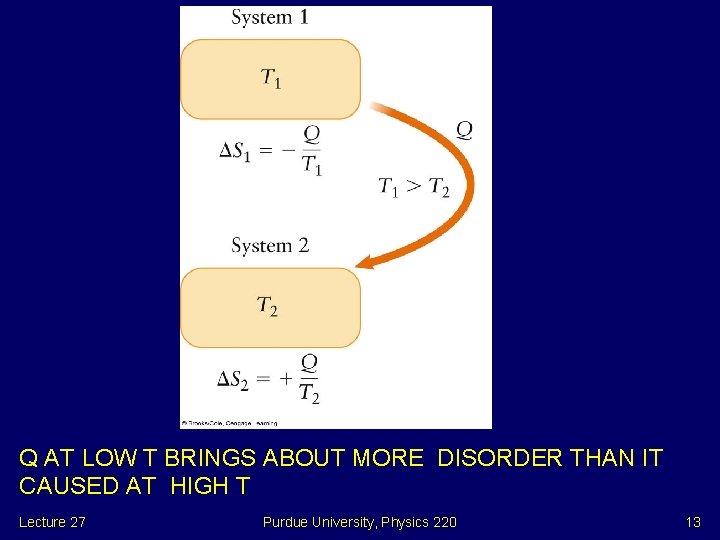
Q AT LOW T BRINGS ABOUT MORE DISORDER THAN IT CAUSED AT HIGH T Lecture 27 Purdue University, Physics 220 13

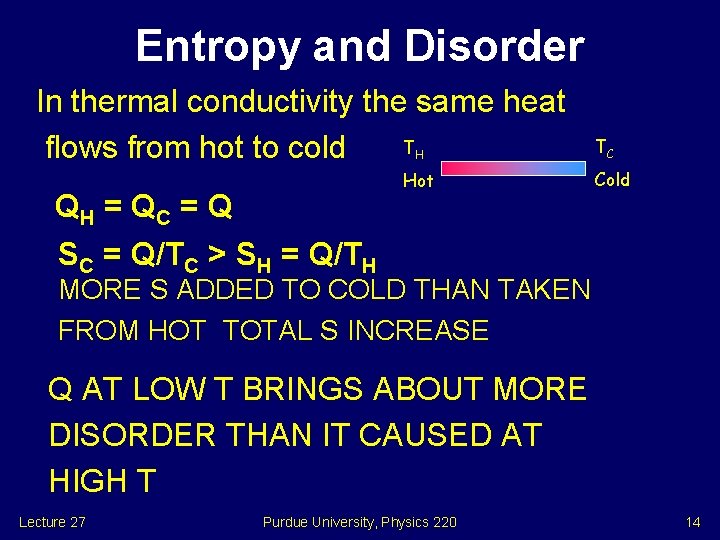
Entropy and Disorder In thermal conductivity the same heat T flows from hot to cold H Hot QH = Q C = Q TC Cold SC = Q/TC > SH = Q/TH MORE S ADDED TO COLD THAN TAKEN FROM HOT TOTAL S INCREASE Q AT LOW T BRINGS ABOUT MORE DISORDER THAN IT CAUSED AT HIGH T Lecture 27 Purdue University, Physics 220 14

New Concept: Entropy (S) • A measure of “disorder” • A property of a system (just like P, V, T, U) – related to number of different “states” of system • Examples of increasing entropy: – ice cube melts – gases expand into vacuum • Change in entropy: S = Q/T • >0 if heat flows into system (Q>0) • <0 if heat flows out of system (Q<0) Lecture 27 Purdue University, Physics 220 15

Entropy Question Some ice (-5 C) is used to cool a cup of water S = Q/T What happens to the entropy of the ice? A) Increase B) Same C) Decreases Heat enters ice: Q>0 What happens to the entropy of the water? A) Increase B) Same C) Decreases Heat Leaves water: Q<0 What happens to the total entropy (water+ice)? A) Increase B) Same C) Decreases Lecture 27 S = Q/Tice – Q/Twater Purdue University, Physics 220 16

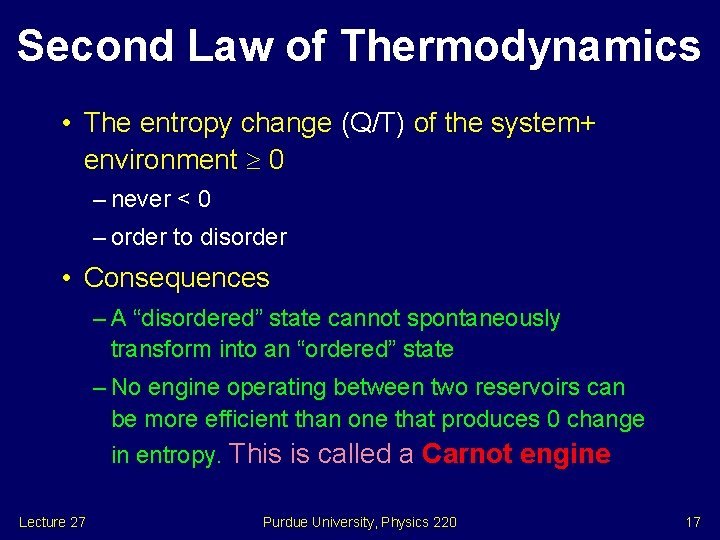
Second Law of Thermodynamics • The entropy change (Q/T) of the system+ environment 0 – never < 0 – order to disorder • Consequences – A “disordered” state cannot spontaneously transform into an “ordered” state – No engine operating between two reservoirs can be more efficient than one that produces 0 change in entropy. This is called a Carnot engine Lecture 27 Purdue University, Physics 220 17

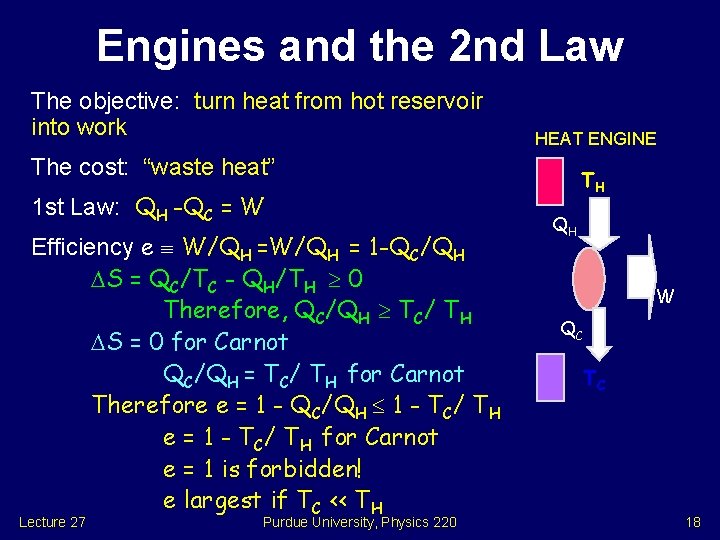
Engines and the 2 nd Law The objective: turn heat from hot reservoir into work HEAT ENGINE The cost: “waste heat” 1 st Law: QH -QC = W Efficiency e W/QH = 1 -QC/QH S = QC/TC - QH/TH 0 Therefore, QC/QH TC/ TH S = 0 for Carnot QC/QH = TC/ TH for Carnot Therefore e = 1 - QC/QH 1 - TC/ TH e = 1 - TC/ TH for Carnot e = 1 is forbidden! e largest if TC << TH Lecture 27 Purdue University, Physics 220 TH QH W QC TC 18

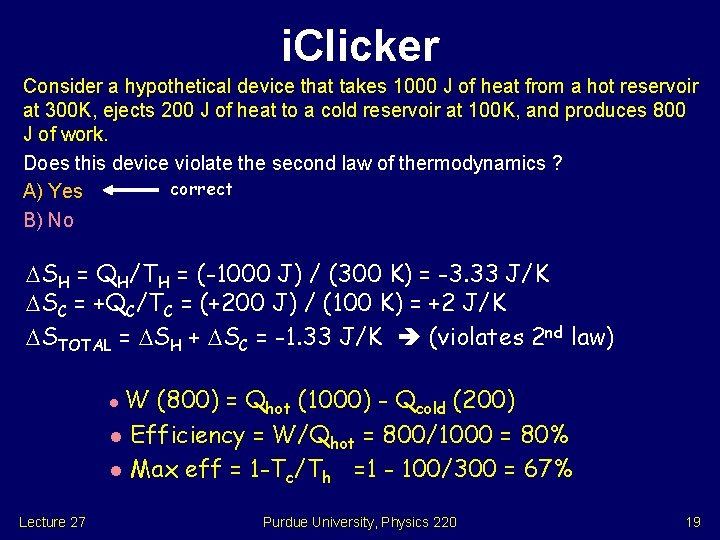
i. Clicker Consider a hypothetical device that takes 1000 J of heat from a hot reservoir at 300 K, ejects 200 J of heat to a cold reservoir at 100 K, and produces 800 J of work. Does this device violate the second law of thermodynamics ? correct A) Yes B) No SH = QH/TH = (-1000 J) / (300 K) = -3. 33 J/K SC = +QC/TC = (+200 J) / (100 K) = +2 J/K STOTAL = SH + SC = -1. 33 J/K (violates 2 nd law) W (800) = Qhot (1000) - Qcold (200) l Efficiency = W/Qhot = 800/1000 = 80% l Max eff = 1 -Tc/Th =1 - 100/300 = 67% l Lecture 27 Purdue University, Physics 220 19

Summary of Concepts • First Law of thermodynamics: Energy Conservation Q = U + W • Heat Engines Efficiency = 1 -QC/QH • Refrigerators Coefficient of Performance = QC/(QH - QC) • Entropy S = Q/T • 2 nd Law: Entropy always increases! • Carnot Cycle: Reversible, Maximum Efficiency e = 1 – Tc/Th Lecture 27 Purdue University, Physics 220 20