Thermodynamics AP Physics Chapter 15 Thermodynamics 13 3











- Slides: 69

Thermodynamics AP Physics Chapter 15

Thermodynamics 13. 3 Zeroth Law of Thermodynamics

13. 3 Zeroth Law of Thermodynamics If two objects of different temperatures are placed in thermal contact, they will eventually reach the same temperature. Thermal Eq Animation They reach Thermal Equilibrium Energy flowing in equals the energy flowing out 13. 3

13. 3 Zeroth Law of Thermodynamics – if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other Allows for a definition of temperature (two objects have the same temperature when they are in thermal equilibirum) 13. 3

Thermodynamics 13. 4 Thermal Expansion

13. 4 Thermal Expansion Objects usually expand when heated and contract when cooled. This can lead to some problems So we include expansion joints 13. 4

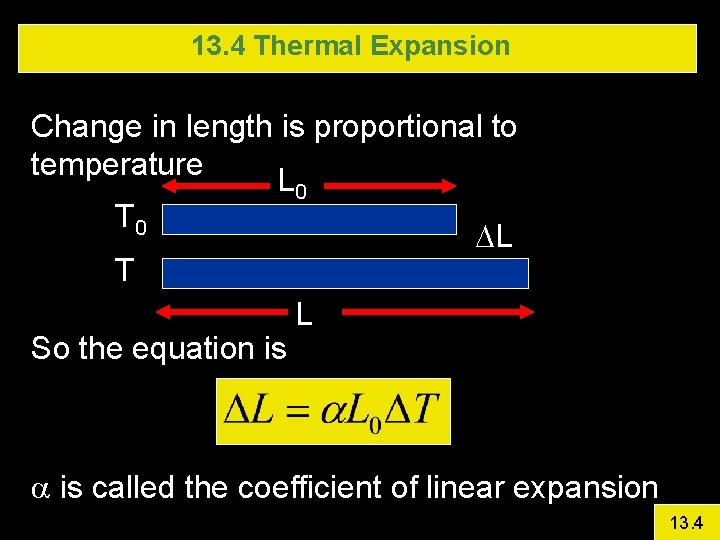
13. 4 Thermal Expansion Change in length is proportional to temperature L T 0 0 DL T So the equation is L a is called the coefficient of linear expansion 13. 4

13. 4 Thermal Expansion Common Misconception When a object with a hole in it is heated, does the hole get larger or smaller? Imagine an infinitely thin ring If it is heated length (circumference) of the ring increase 13. 4

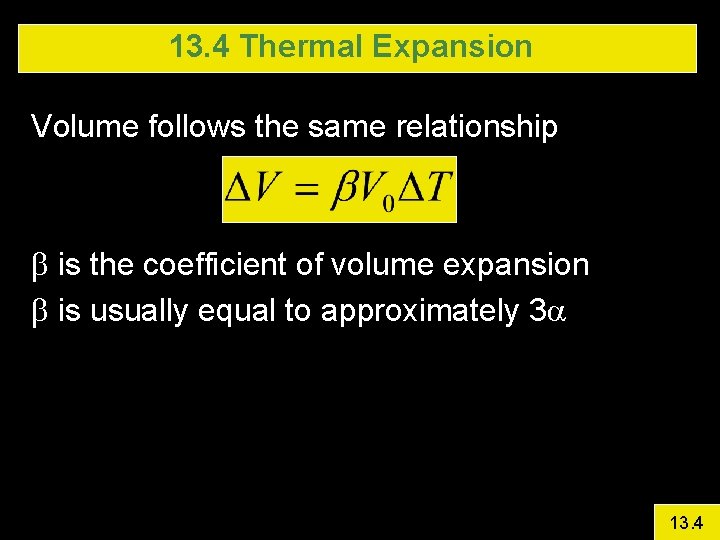
13. 4 Thermal Expansion Volume follows the same relationship b is the coefficient of volume expansion b is usually equal to approximately 3 a 13. 4

Thermodynamics 13. 7 The Ideal Gas Law

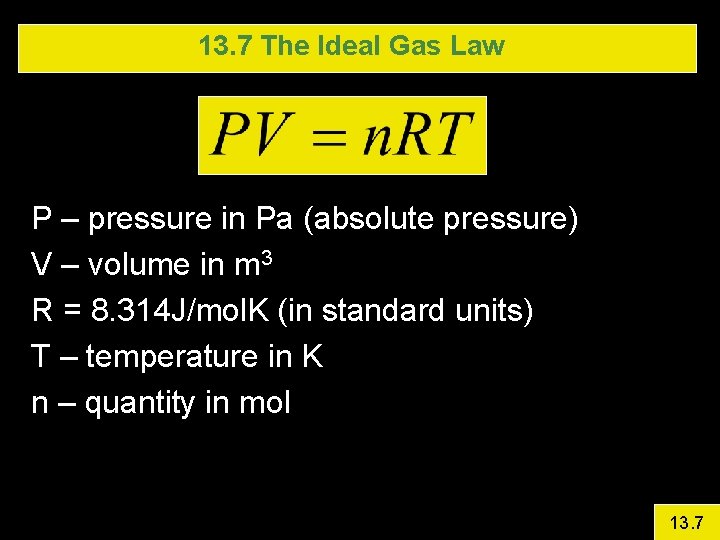
13. 7 The Ideal Gas Law P – pressure in Pa (absolute pressure) V – volume in m 3 R = 8. 314 J/mol. K (in standard units) T – temperature in K n – quantity in mol 13. 7

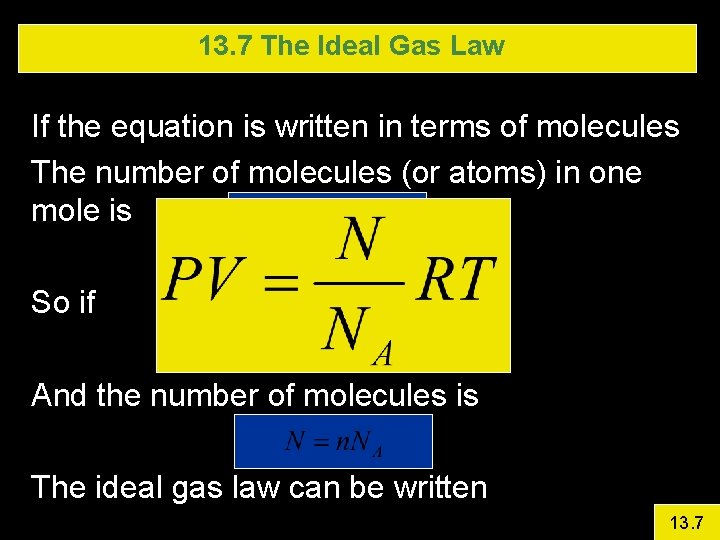
13. 7 The Ideal Gas Law If the equation is written in terms of molecules The number of molecules (or atoms) in one mole is So if And the number of molecules is The ideal gas law can be written 13. 7

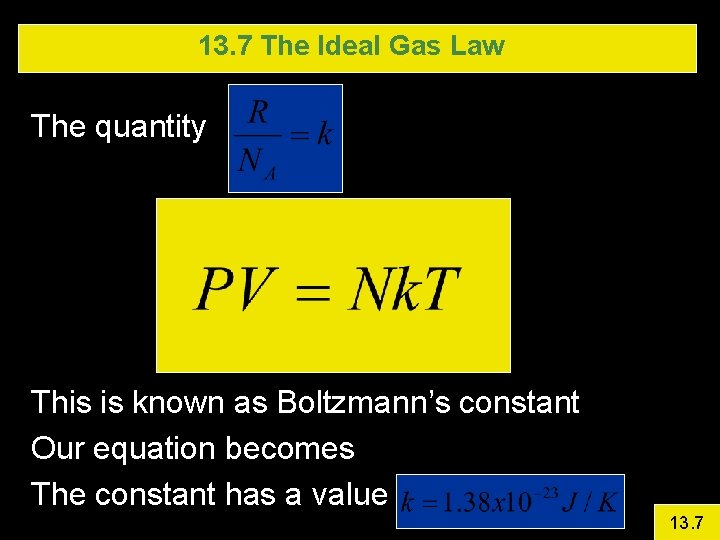
13. 7 The Ideal Gas Law The quantity This is known as Boltzmann’s constant Our equation becomes The constant has a value 13. 7

Thermodynamics 14. 1 Heat as Energy Transfer

14. 1 Heat as Energy Transfer Heat – energy transferred from one object to another because of a difference in temperature Unit – joule 14. 1

Thermodynamics 14. 2 Internal Energy

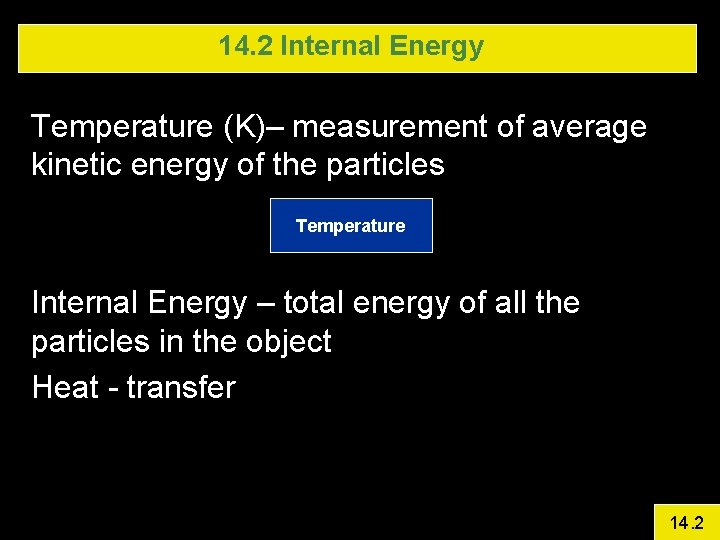
14. 2 Internal Energy Temperature (K)– measurement of average kinetic energy of the particles Temperature Internal Energy – total energy of all the particles in the object Heat - transfer 14. 2

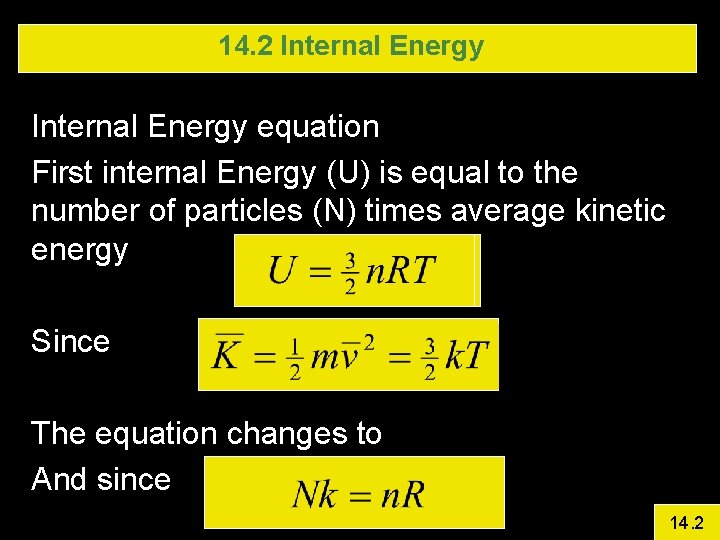
14. 2 Internal Energy equation First internal Energy (U) is equal to the number of particles (N) times average kinetic energy Since The equation changes to And since 14. 2

Thermodynamics 14. 3 Specific Heat and Calorimetry

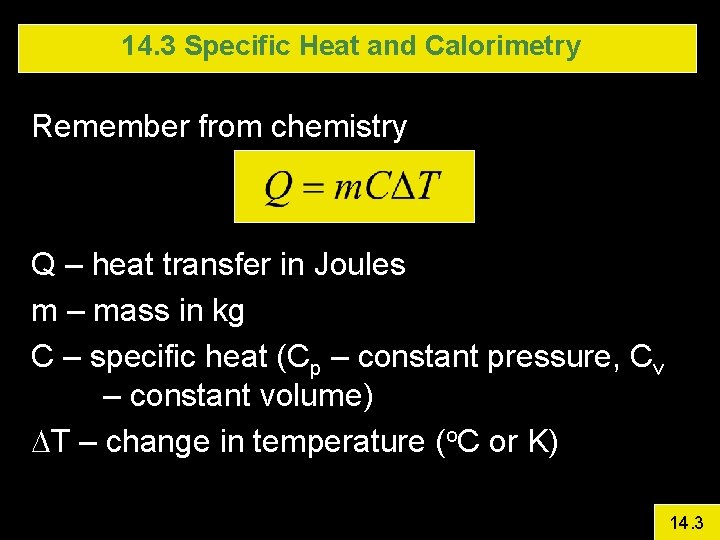
14. 3 Specific Heat and Calorimetry Remember from chemistry Q – heat transfer in Joules m – mass in kg C – specific heat (Cp – constant pressure, Cv – constant volume) DT – change in temperature (o. C or K) 14. 3

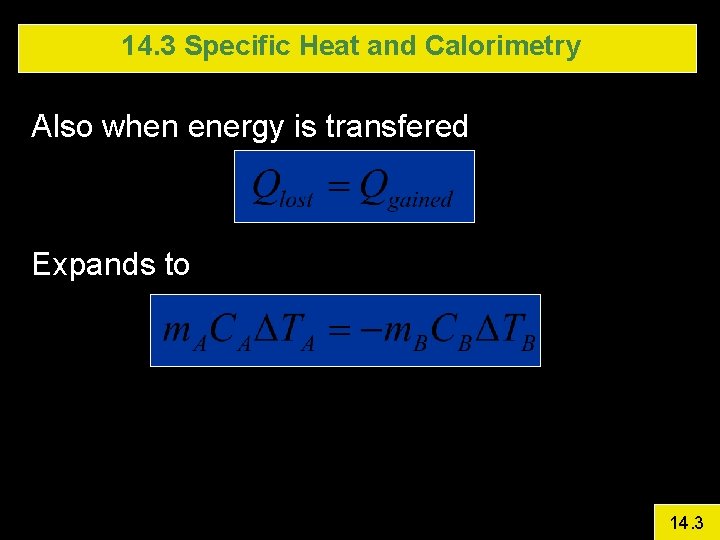
14. 3 Specific Heat and Calorimetry Also when energy is transfered Expands to 14. 3

Thermodynamics 14. 6 Heat Transfer: Conduction

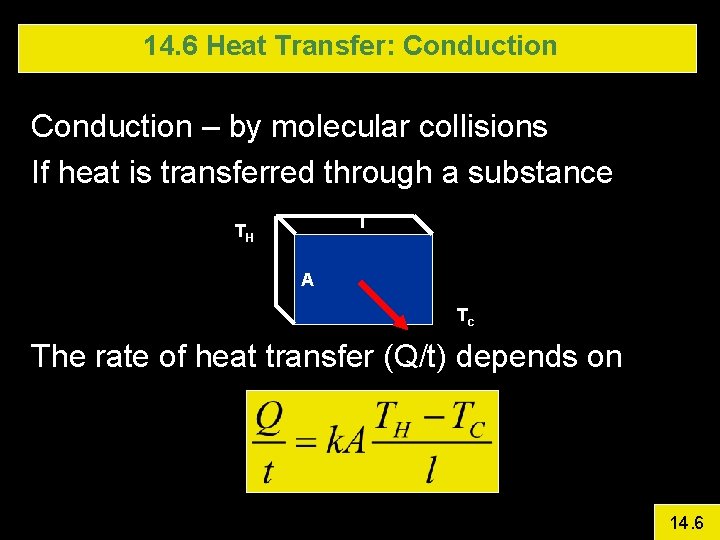
14. 6 Heat Transfer: Conduction – by molecular collisions If heat is transferred through a substance l TH A Tc The rate of heat transfer (Q/t) depends on 14. 6

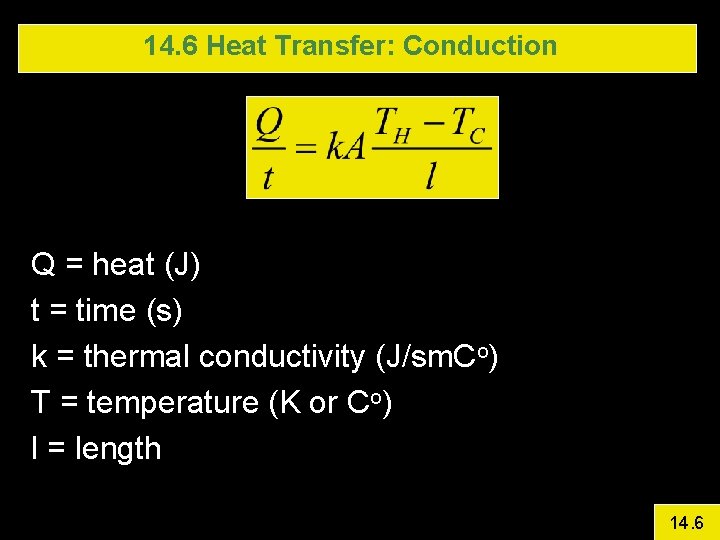
14. 6 Heat Transfer: Conduction Q = heat (J) t = time (s) k = thermal conductivity (J/sm. Co) T = temperature (K or Co) l = length 14. 6

Thermodynamics 15. 1 The First Law of Thermodynamics

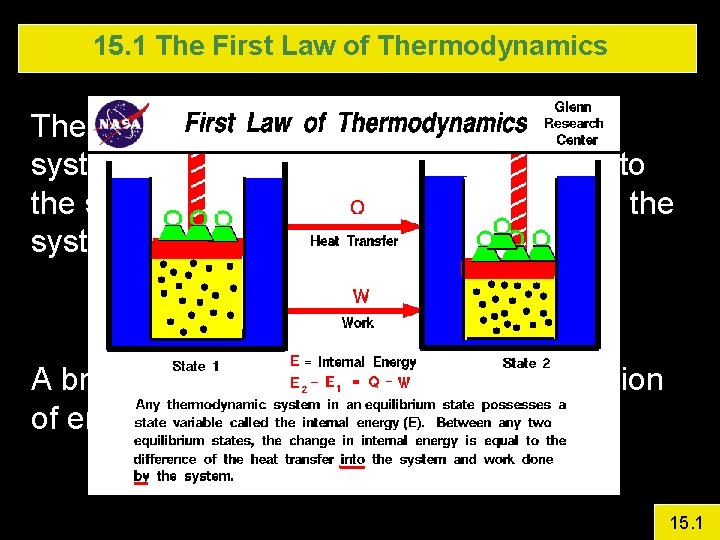
15. 1 The First Law of Thermodynamics The change in internal energy of a closed system will be equal to the energy added to the system by heat plus the work done on the system by the surroundings. A broad statement of the law of conservation of energy 15. 1

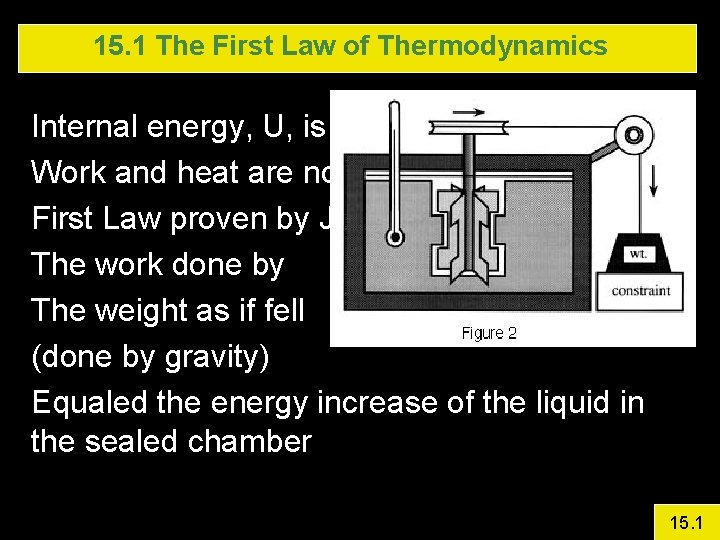
15. 1 The First Law of Thermodynamics Internal energy, U, is a property of the system Work and heat are not First Law proven by Joule in an experiment The work done by The weight as if fell (done by gravity) Equaled the energy increase of the liquid in the sealed chamber 15. 1

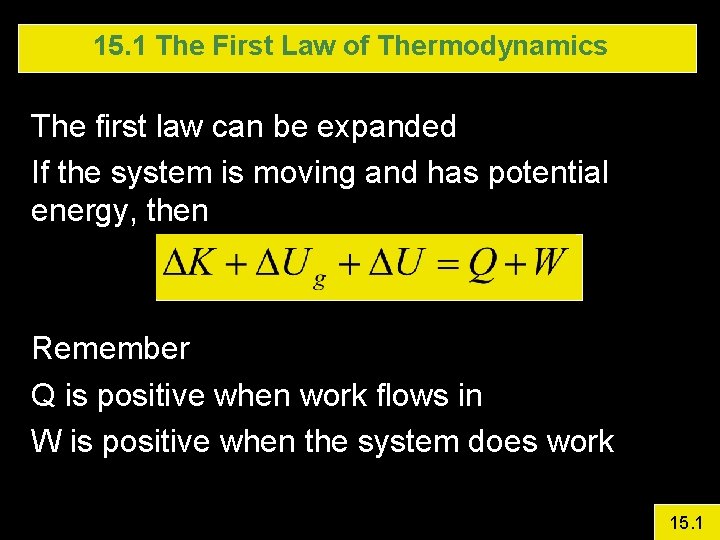
15. 1 The First Law of Thermodynamics The first law can be expanded If the system is moving and has potential energy, then Remember Q is positive when work flows in W is positive when the system does work 15. 1

Thermodynamics 15. 2 Thermodynamic Processes & the First Law

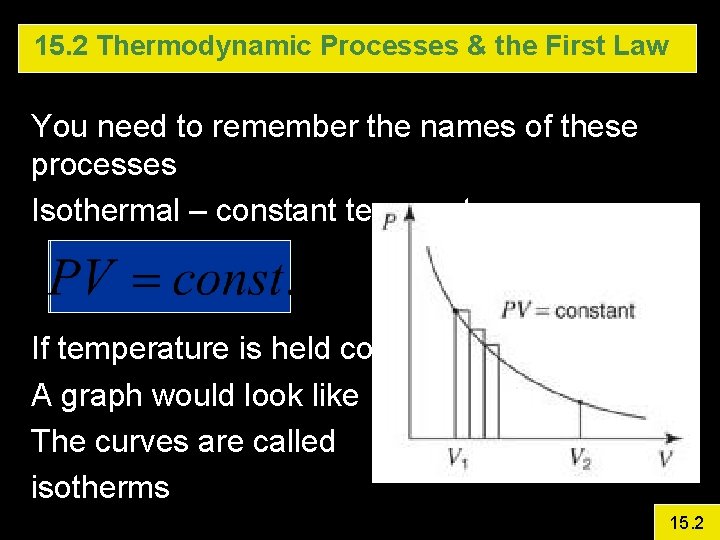
15. 2 Thermodynamic Processes & the First Law You need to remember the names of these processes Isothermal – constant temperature If temperature is held constant, then A graph would look like The curves are called isotherms 15. 2

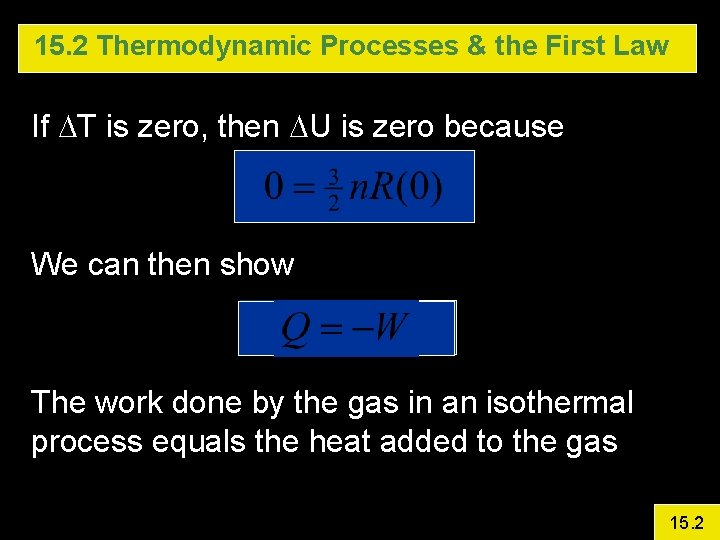
15. 2 Thermodynamic Processes & the First Law If DT is zero, then DU is zero because We can then show The work done by the gas in an isothermal process equals the heat added to the gas 15. 2

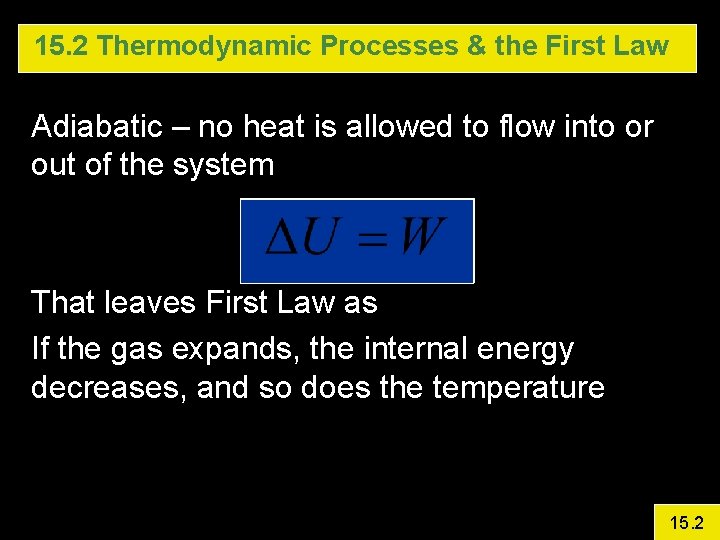
15. 2 Thermodynamic Processes & the First Law Adiabatic – no heat is allowed to flow into or out of the system That leaves First Law as If the gas expands, the internal energy decreases, and so does the temperature 15. 2

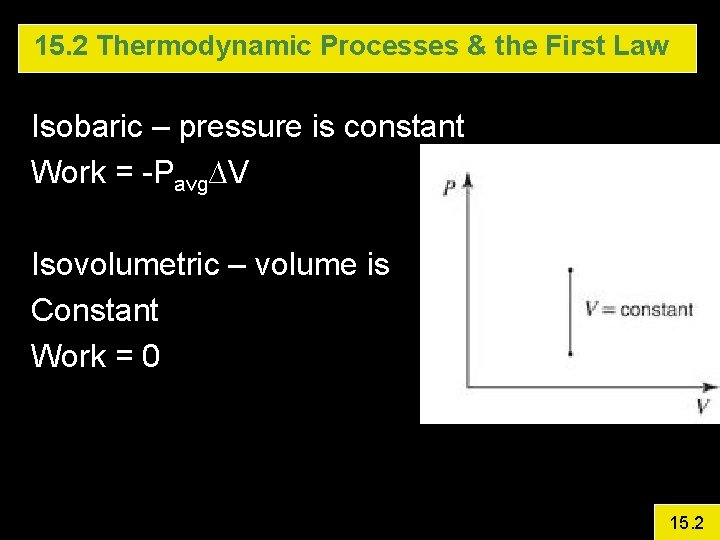
15. 2 Thermodynamic Processes & the First Law Isobaric – pressure is constant Work = -Pavg∆V Isovolumetric – volume is Constant Work = 0 15. 2

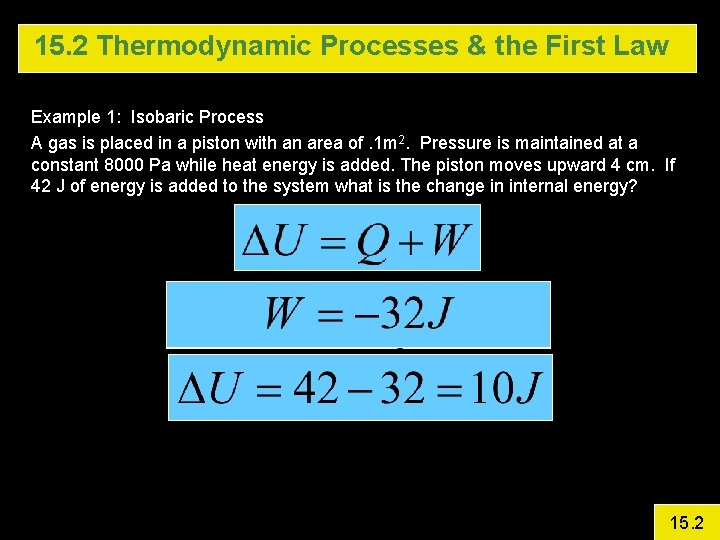
15. 2 Thermodynamic Processes & the First Law Example 1: Isobaric Process A gas is placed in a piston with an area of. 1 m 2. Pressure is maintained at a constant 8000 Pa while heat energy is added. The piston moves upward 4 cm. If 42 J of energy is added to the system what is the change in internal energy? 15. 2

15. 2 Thermodynamic Processes & the First Law Example 1: Isobaric Process A gas is placed in a piston with an area of. 1 m 2. Pressure is maintained at a constant 8000 Pa while heat energy is added. The piston moves upward 4 cm. If 42 J of energy is added to the system what is the change in internal energy? 15. 2

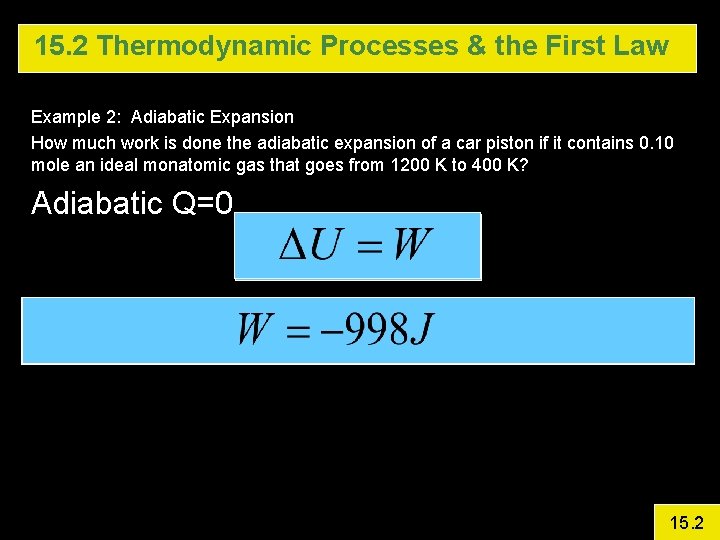
15. 2 Thermodynamic Processes & the First Law Example 2: Adiabatic Expansion How much work is done the adiabatic expansion of a car piston if it contains 0. 10 mole an ideal monatomic gas that goes from 1200 K to 400 K? 15. 2

15. 2 Thermodynamic Processes & the First Law Example 2: Adiabatic Expansion How much work is done the adiabatic expansion of a car piston if it contains 0. 10 mole an ideal monatomic gas that goes from 1200 K to 400 K? Adiabatic Q=0 15. 2

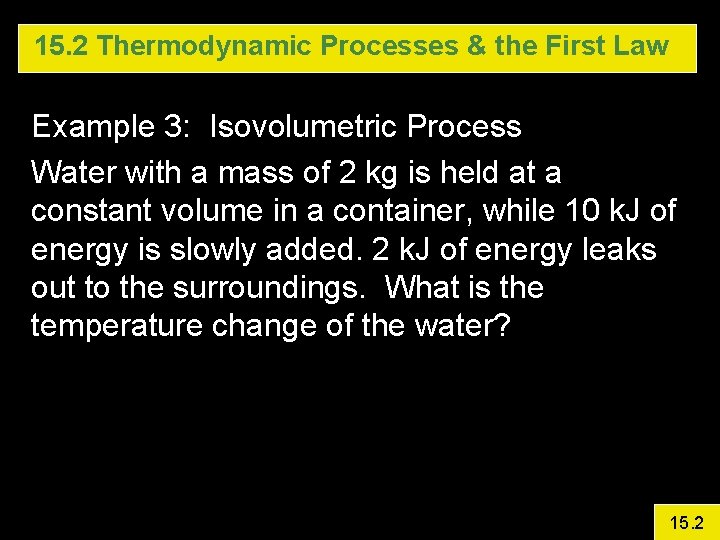
15. 2 Thermodynamic Processes & the First Law Example 3: Isovolumetric Process Water with a mass of 2 kg is held at a constant volume in a container, while 10 k. J of energy is slowly added. 2 k. J of energy leaks out to the surroundings. What is the temperature change of the water? 15. 2

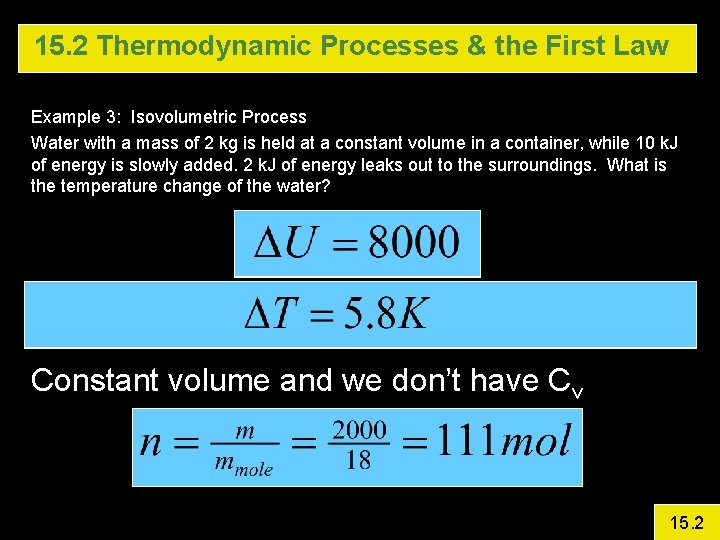
15. 2 Thermodynamic Processes & the First Law Example 3: Isovolumetric Process Water with a mass of 2 kg is held at a constant volume in a container, while 10 k. J of energy is slowly added. 2 k. J of energy leaks out to the surroundings. What is the temperature change of the water? Constant volume and we don’t have Cv 15. 2

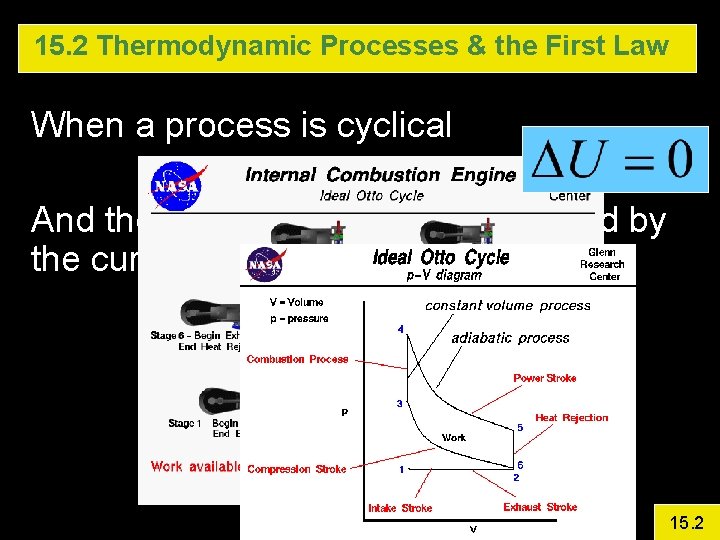
15. 2 Thermodynamic Processes & the First Law When a process is cyclical And the work done is the area bound by the curves 15. 2

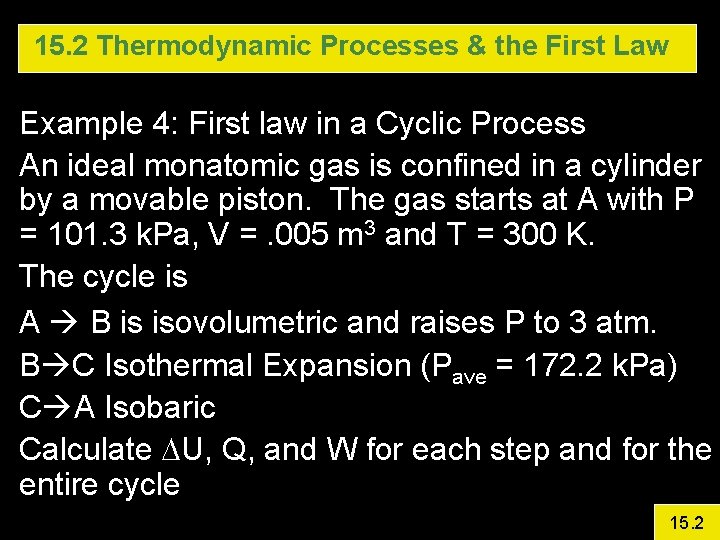
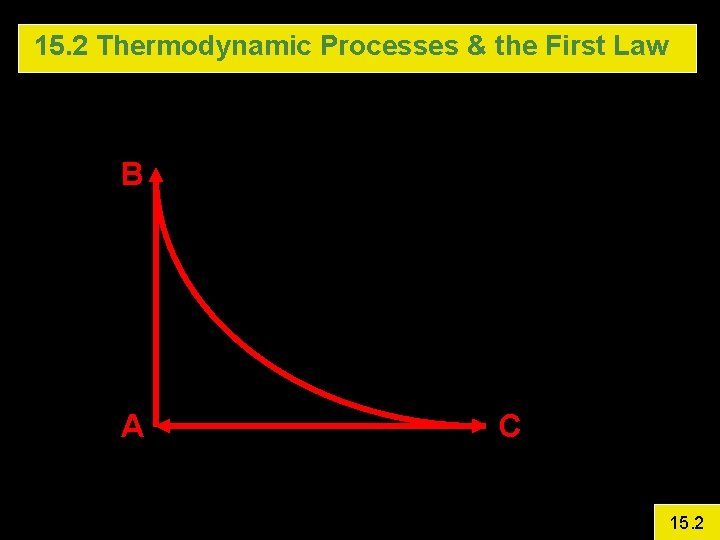
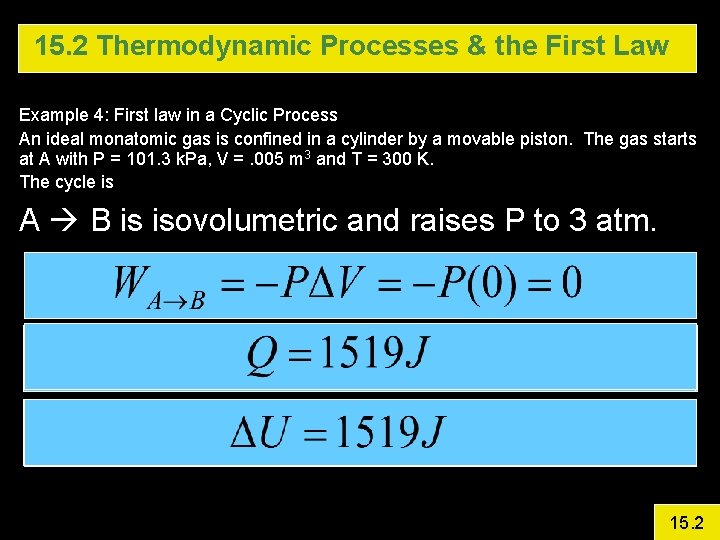
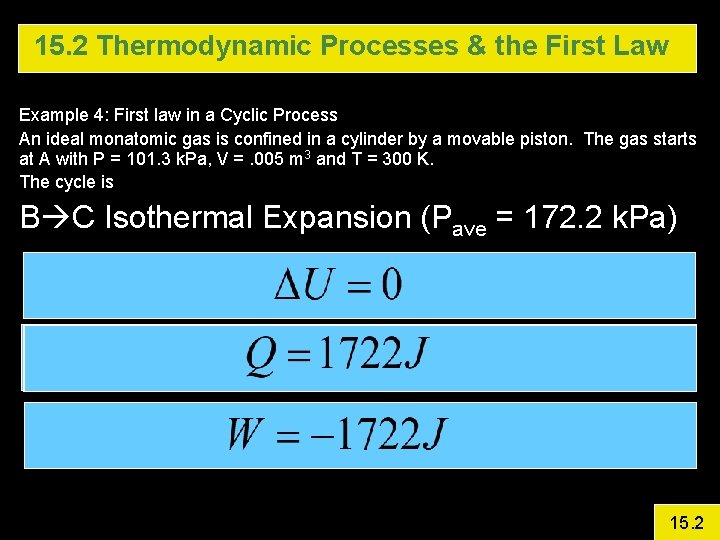
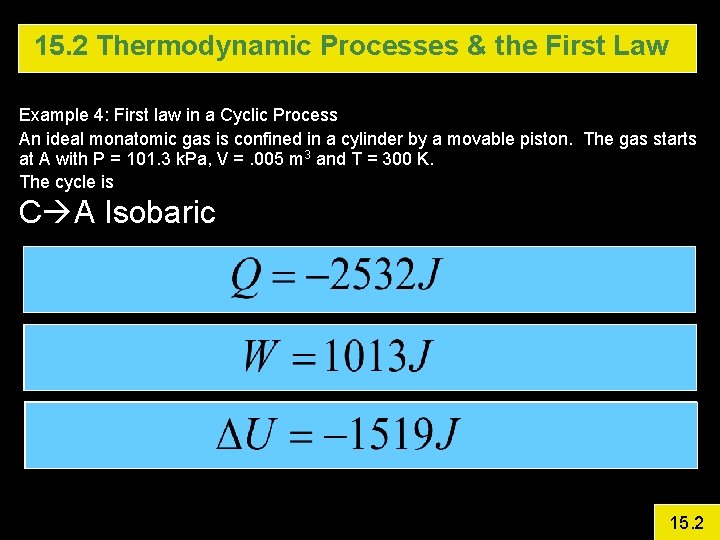
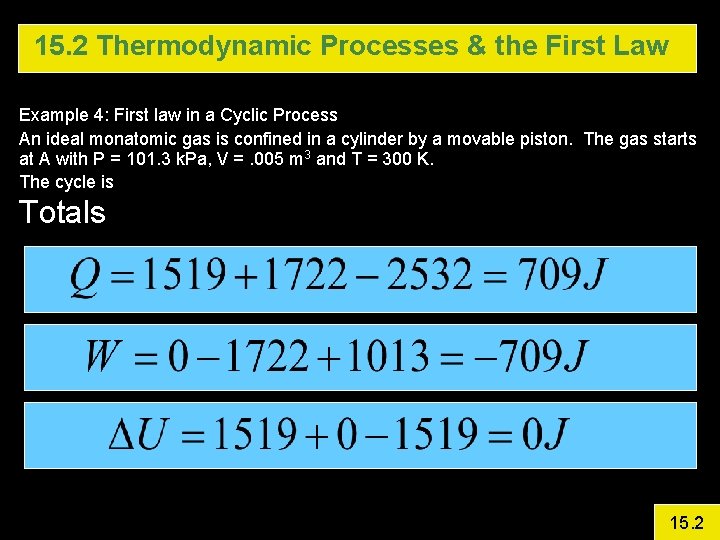
15. 2 Thermodynamic Processes & the First Law Example 4: First law in a Cyclic Process An ideal monatomic gas is confined in a cylinder by a movable piston. The gas starts at A with P = 101. 3 k. Pa, V =. 005 m 3 and T = 300 K. The cycle is A B is isovolumetric and raises P to 3 atm. B C Isothermal Expansion (Pave = 172. 2 k. Pa) C A Isobaric Calculate DU, Q, and W for each step and for the entire cycle 15. 2

15. 2 Thermodynamic Processes & the First Law B A C 15. 2

15. 2 Thermodynamic Processes & the First Law Example 4: First law in a Cyclic Process An ideal monatomic gas is confined in a cylinder by a movable piston. The gas starts at A with P = 101. 3 k. Pa, V =. 005 m 3 and T = 300 K. The cycle is A B is isovolumetric and raises P to 3 atm. 15. 2

15. 2 Thermodynamic Processes & the First Law Example 4: First law in a Cyclic Process An ideal monatomic gas is confined in a cylinder by a movable piston. The gas starts at A with P = 101. 3 k. Pa, V =. 005 m 3 and T = 300 K. The cycle is B C Isothermal Expansion (Pave = 172. 2 k. Pa) 15. 2

15. 2 Thermodynamic Processes & the First Law Example 4: First law in a Cyclic Process An ideal monatomic gas is confined in a cylinder by a movable piston. The gas starts at A with P = 101. 3 k. Pa, V =. 005 m 3 and T = 300 K. The cycle is C A Isobaric 15. 2

15. 2 Thermodynamic Processes & the First Law Example 4: First law in a Cyclic Process An ideal monatomic gas is confined in a cylinder by a movable piston. The gas starts at A with P = 101. 3 k. Pa, V =. 005 m 3 and T = 300 K. The cycle is Totals 15. 2

Thermodynamics 15. 4 The Second Law of Thermodynamics-Intro

15. 3 The Second Law of Thermodynamics-Intro The first law deals with conservation of energy. However there are situations that would conserve energy, but do not occur. 1. Falling objects convert from Ug to K to Q Falling and Energy Never Q to K to Ug 2. Heat flows from TH to TC Never TC to TH 15. 4

15. 3 The Second Law of Thermodynamics-Intro The second law explains why some processes occur and some don’t In terms of heat, the second law could be stated -Heat can flow spontaneously from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object 15. 4

Thermodynamics 15. 5 Heat Engines

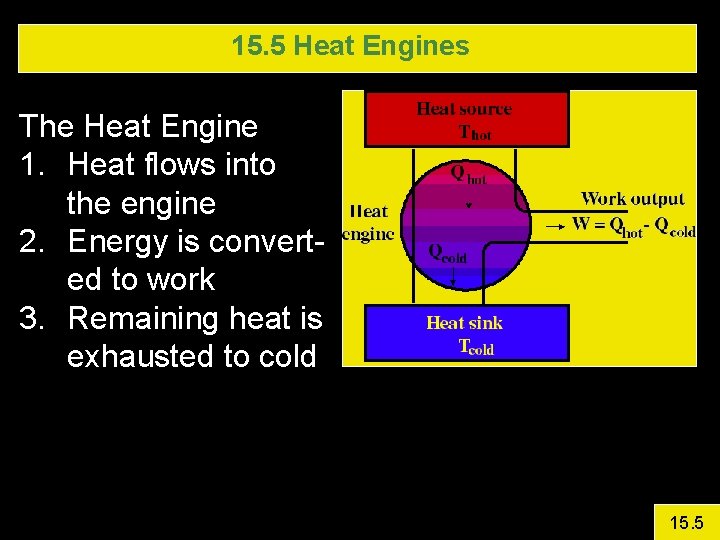
15. 5 Heat Engines The Heat Engine 1. Heat flows into the engine 2. Energy is converted to work 3. Remaining heat is exhausted to cold 15. 5

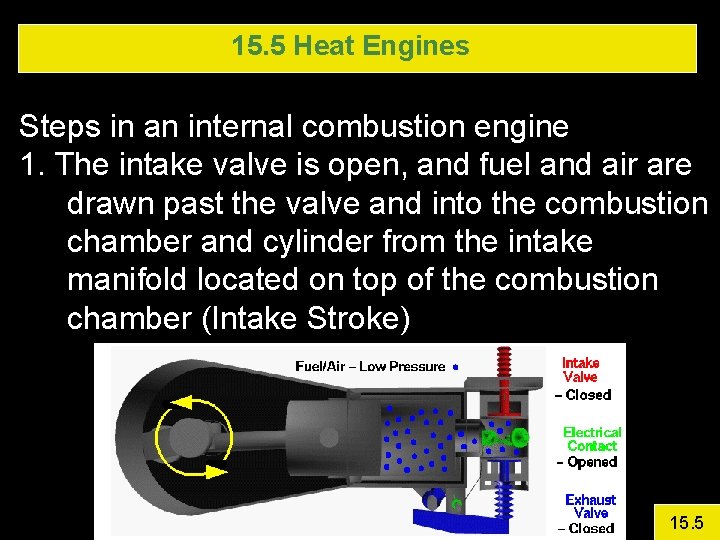
15. 5 Heat Engines Steps in an internal combustion engine 1. The intake valve is open, and fuel and air are drawn past the valve and into the combustion chamber and cylinder from the intake manifold located on top of the combustion chamber (Intake Stroke) 15. 5

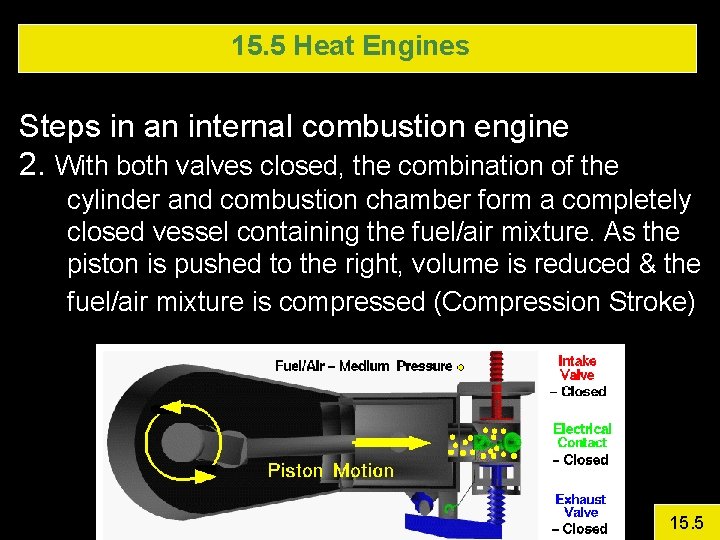
15. 5 Heat Engines Steps in an internal combustion engine 2. With both valves closed, the combination of the cylinder and combustion chamber form a completely closed vessel containing the fuel/air mixture. As the piston is pushed to the right, volume is reduced & the fuel/air mixture is compressed (Compression Stroke) 15. 5

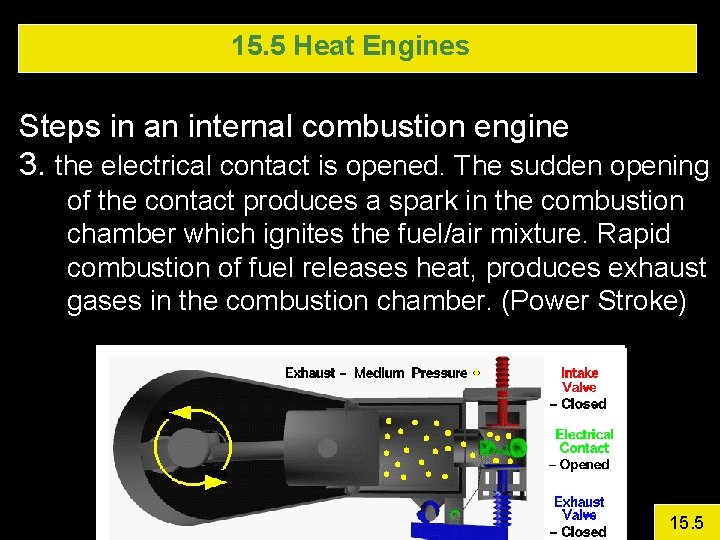
15. 5 Heat Engines Steps in an internal combustion engine 3. the electrical contact is opened. The sudden opening of the contact produces a spark in the combustion chamber which ignites the fuel/air mixture. Rapid combustion of fuel releases heat, produces exhaust gases in the combustion chamber. (Power Stroke) 15. 5

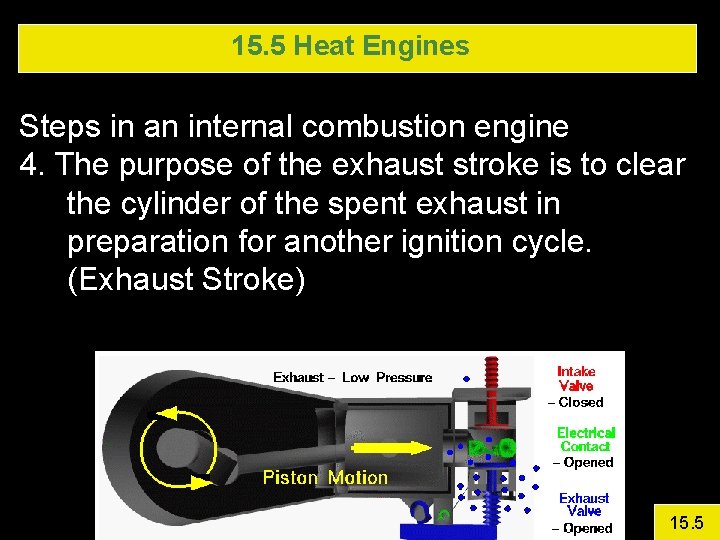
15. 5 Heat Engines Steps in an internal combustion engine 4. The purpose of the exhaust stroke is to clear the cylinder of the spent exhaust in preparation for another ignition cycle. (Exhaust Stroke) 15. 5

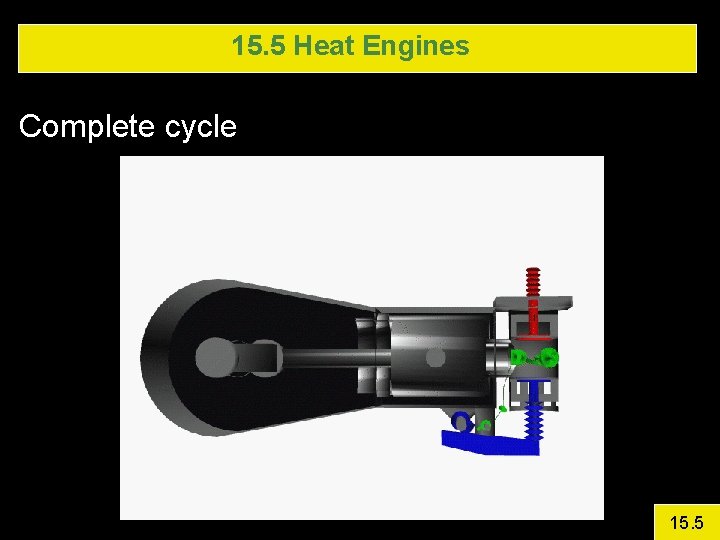
15. 5 Heat Engines Complete cycle 15. 5

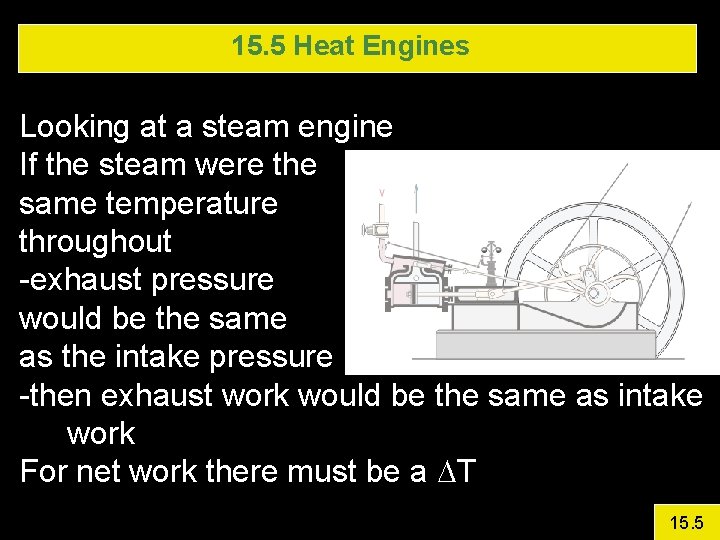
15. 5 Heat Engines Looking at a steam engine If the steam were the same temperature throughout -exhaust pressure would be the same as the intake pressure -then exhaust work would be the same as intake work For net work there must be a DT 15. 5

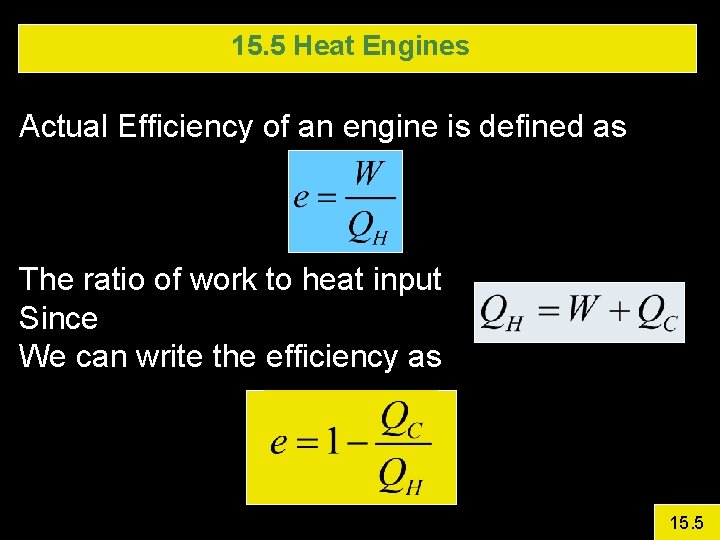
15. 5 Heat Engines Actual Efficiency of an engine is defined as The ratio of work to heat input Since We can write the efficiency as 15. 5

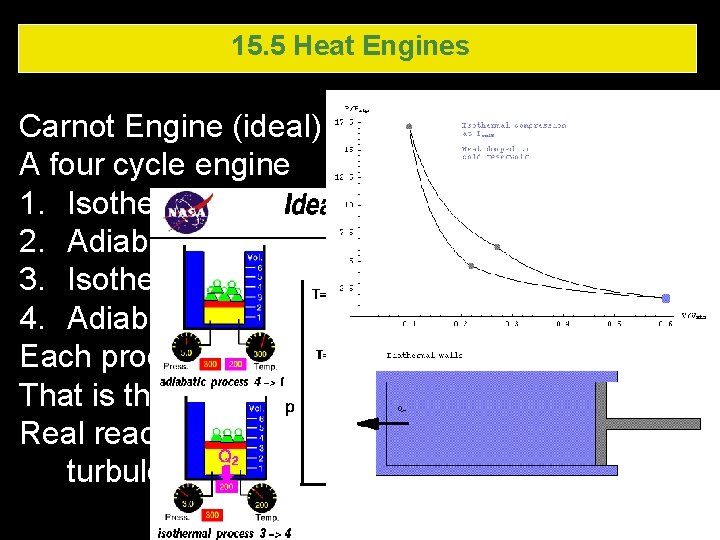
15. 5 Heat Engines Carnot Engine (ideal) – no actual Carnot engine A four cycle engine 1. Isothermal expansion (DT=0, Q=W) 2. Adiabatic expansion (Q=0, DU=-W) 3. Isothermal compression 4. Adiabatic compression Each process was considered reversible That is that each step is done very slowly Real reactions occur quickly – there would be turbulence, friction - irreversible 15. 5

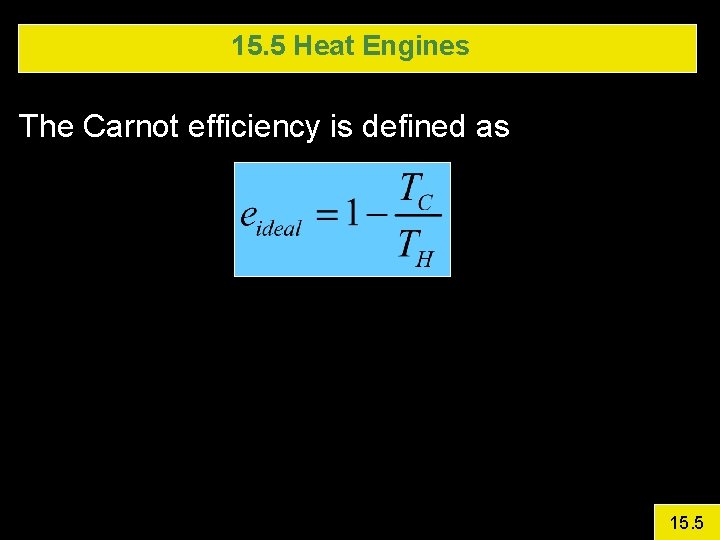
15. 5 Heat Engines The Carnot efficiency is defined as 15. 5

Thermodynamics 15. 6 Refrigerators, Air Conditioners

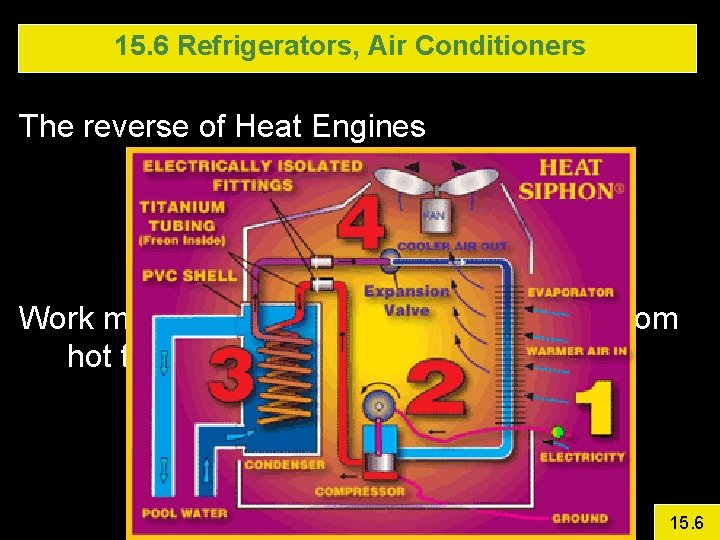
15. 6 Refrigerators, Air Conditioners The reverse of Heat Engines Work must be done – because heat flows from hot to cold 15. 6

Thermodynamics 15. 7 Entropy and the 2 nd Law of Thermodynamics

15. 7 Entropy and the 2 nd Law of Thermodynamics Entropy – a measure of the order or disorder of a system Change in entropy is defined as Q must be added as a reversible process at a constant temperature 15. 7

15. 7 Entropy and the 2 nd Law of Thermodynamics The Second Law of Thermodynamics – the entropy of an isolated system never decreases. It can only stay the same or increase. Only idealized processes have a DS=0 Or – the total entropy of any system plus that of its environment increases as a result of any natural process 15. 7

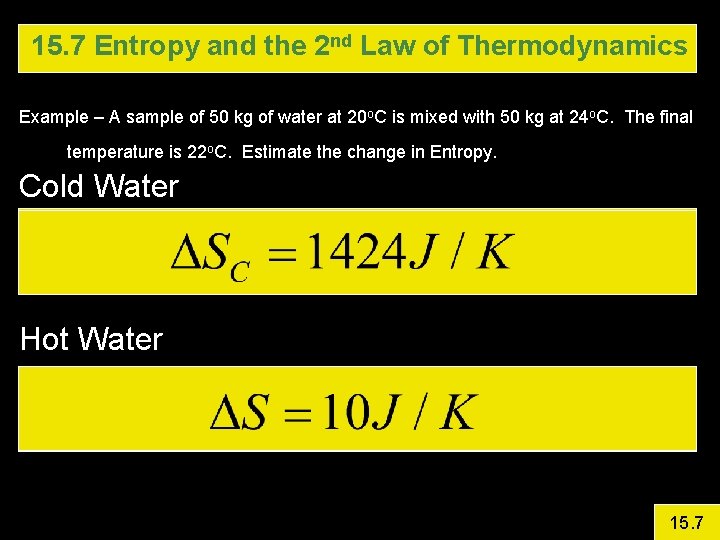
15. 7 Entropy and the 2 nd Law of Thermodynamics Example – A sample of 50 kg of water at 20 o. C is mixed with 50 kg at 24 o. C. The final temperature is 22 o. C. Estimate the change in Entropy. The reaction does not occur at constant temperature, so use the average temperature to estimate the entropy change. 15. 7

15. 7 Entropy and the 2 nd Law of Thermodynamics Example – A sample of 50 kg of water at 20 o. C is mixed with 50 kg at 24 o. C. The final temperature is 22 o. C. Estimate the change in Entropy. Cold Water Hot Water 15. 7

Thermodynamics 15. 8 Order to Disorder

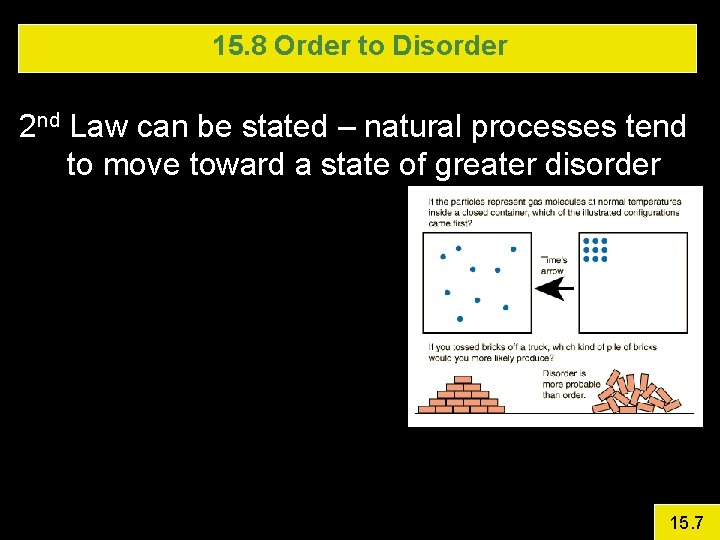
15. 8 Order to Disorder 2 nd Law can be stated – natural processes tend to move toward a state of greater disorder 15. 7