Photoelectric Effect Light hits a metal plate and

![Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] • Note that Planck’s Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] • Note that Planck’s](https://slidetodoc.com/presentation_image/453639b76009729452ff40ef16e7d3ff/image-20.jpg)
![Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] Note that the maximum Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] Note that the maximum](https://slidetodoc.com/presentation_image/453639b76009729452ff40ef16e7d3ff/image-21.jpg)













![The Bohr Theory E = hf = [-13. 6 e. V]*[(1/nf 2) - (1/ni The Bohr Theory E = hf = [-13. 6 e. V]*[(1/nf 2) - (1/ni](https://slidetodoc.com/presentation_image/453639b76009729452ff40ef16e7d3ff/image-54.jpg)
- Slides: 59

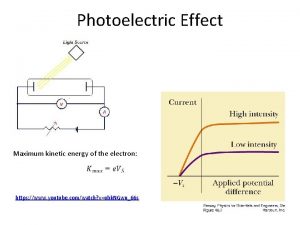
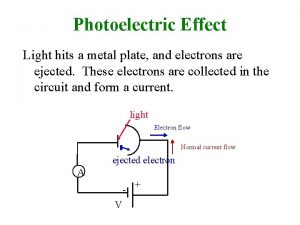
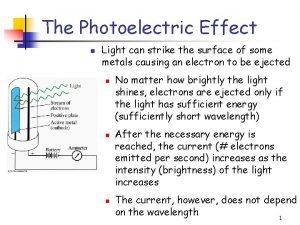
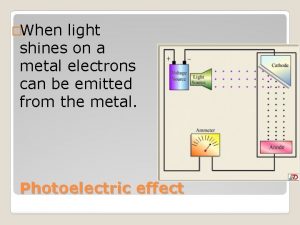
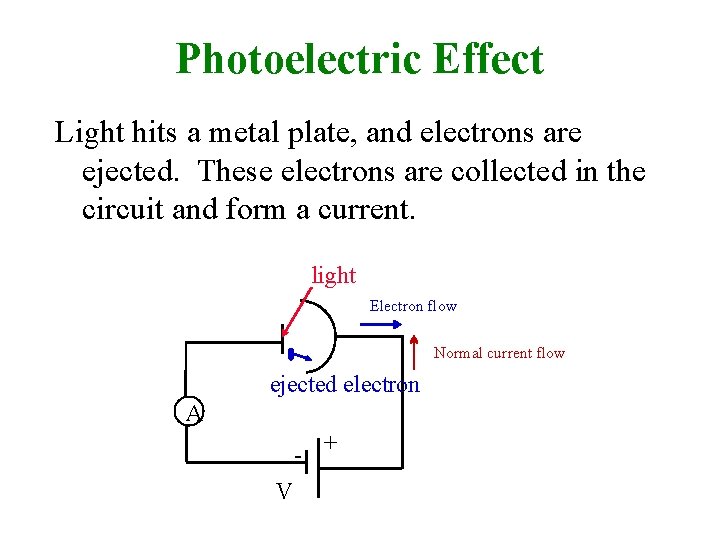
Photoelectric Effect Light hits a metal plate, and electrons are ejected. These electrons are collected in the circuit and form a current. light Electron flow Normal current flow ejected electron A - + V

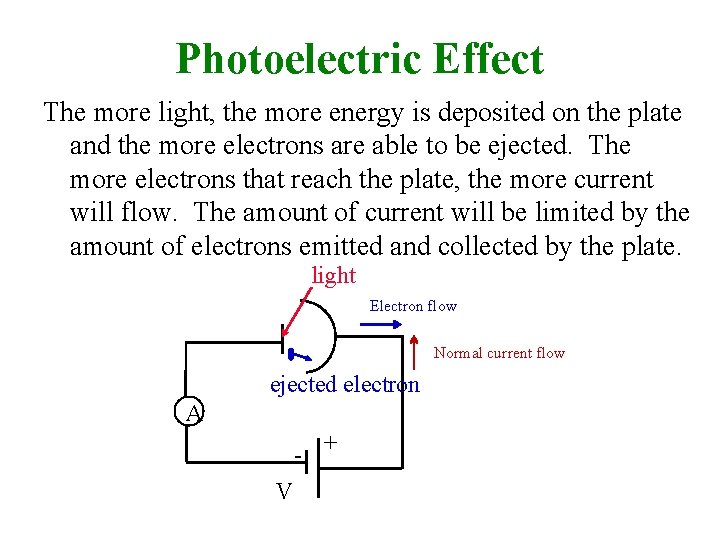
Photoelectric Effect The more light, the more energy is deposited on the plate and the more electrons are able to be ejected. The more electrons that reach the plate, the more current will flow. The amount of current will be limited by the amount of electrons emitted and collected by the plate. light Electron flow Normal current flow ejected electron A - + V

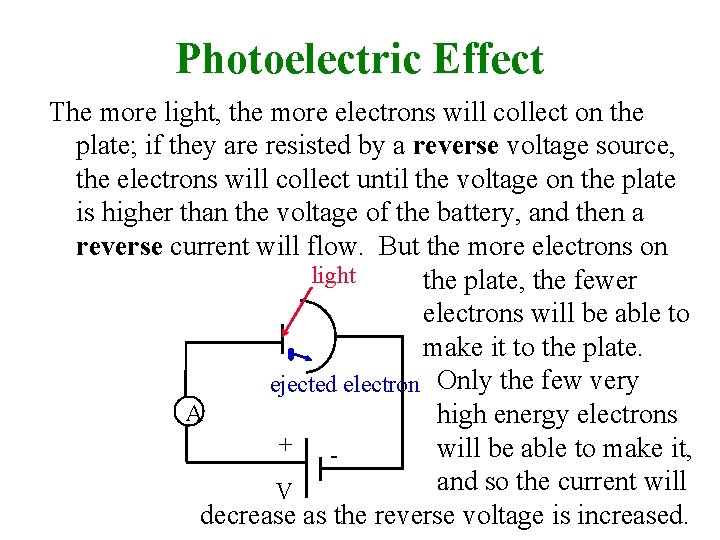
Photoelectric Effect The more light, the more electrons will collect on the plate; if they are resisted by a reverse voltage source, the electrons will collect until the voltage on the plate is higher than the voltage of the battery, and then a reverse current will flow. But the more electrons on light the plate, the fewer electrons will be able to make it to the plate. ejected electron Only the few very A high energy electrons + will be able to make it, and so the current will V decrease as the reverse voltage is increased.

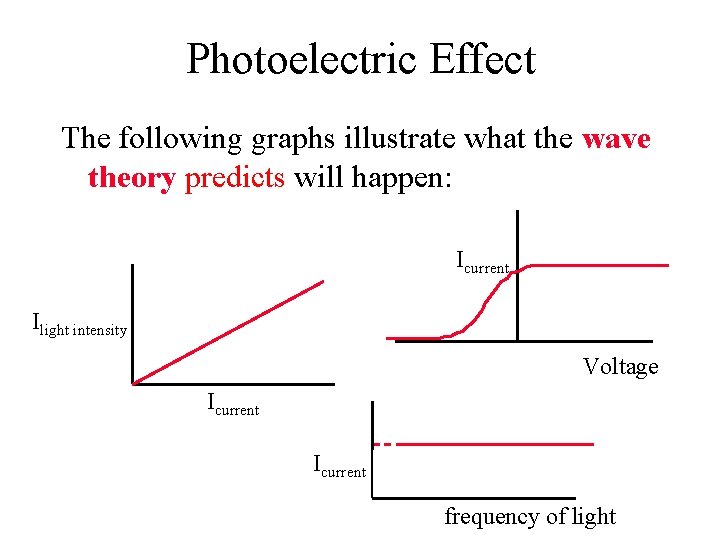
Photoelectric Effect The following graphs illustrate what the wave theory predicts will happen: Icurrent Ilight intensity Voltage Icurrent frequency of light

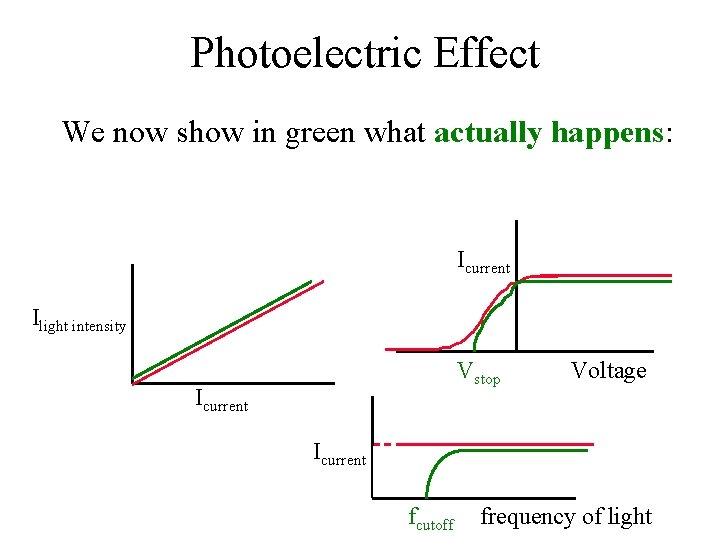
Photoelectric Effect We now show in green what actually happens: Icurrent Ilight intensity Vstop Icurrent Voltage Icurrent fcutoff frequency of light

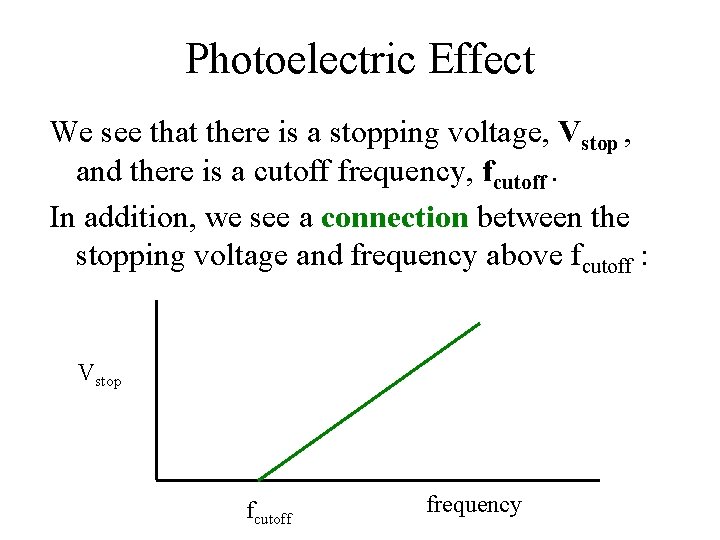
Photoelectric Effect We see that there is a stopping voltage, Vstop , and there is a cutoff frequency, fcutoff. In addition, we see a connection between the stopping voltage and frequency above fcutoff : Vstop fcutoff frequency

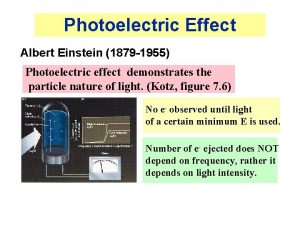
Photoelectric Effect • Einstein received the Nobel Prize for his explanation of this. (He did NOT receive the prize for his theory of relativity. )

Photoelectric Effect • Einstein suggested that light consisted of discrete units of energy, E = hf. Electrons could either get hit with and absorb a whole photon, or they could not. There was no in-between (getting part of a photon). • If the energy of the unit of light (photon) was not large enough to let the electron escape from the metal, no electrons would be ejected. Hence, the existence of fcutoff.

Photoelectric Effect If the photon energy, hf, were large enough to eject the electron from the metal (here, W is the energy necessary to eject the electron called the Work Function), then the following conservation of energy equation would apply: hf = W + KE The energy of the photon absorbed (hf) goes into ejecting the electron (W) plus any extra energy left over which would show up as kinetic energy (KE).

Photoelectric Effect This extra kinetic energy (KE) would allow the electron to climb up a “hill”, but the size of the hill that the electron could climb up would be limited to the extra kinetic energy the electron had. By measuring the steepest hill, we could arrive at the extra energy of the electron. Hill sizes are in electrical terms, not gravitational, and are in VOLTS: KE = PE = q. Vstop.

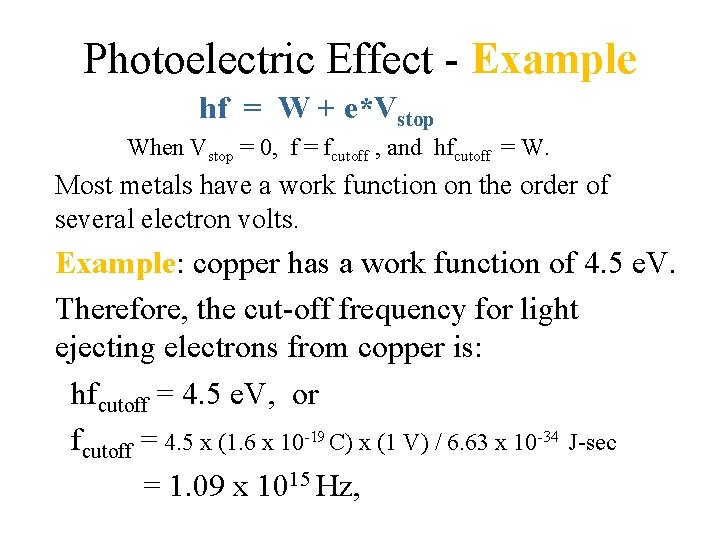
Photoelectric Effect Put into a nice equation: hf = W + e*Vstop – where f is the frequency of the light – W is the “WORK FUNCTION”, or the amount of energy needed to get the electron out of the metal – Vstop is the voltage necessary to stop the photoelectric current. When Vstop = 0, f = fcutoff , and hfcutoff = W.

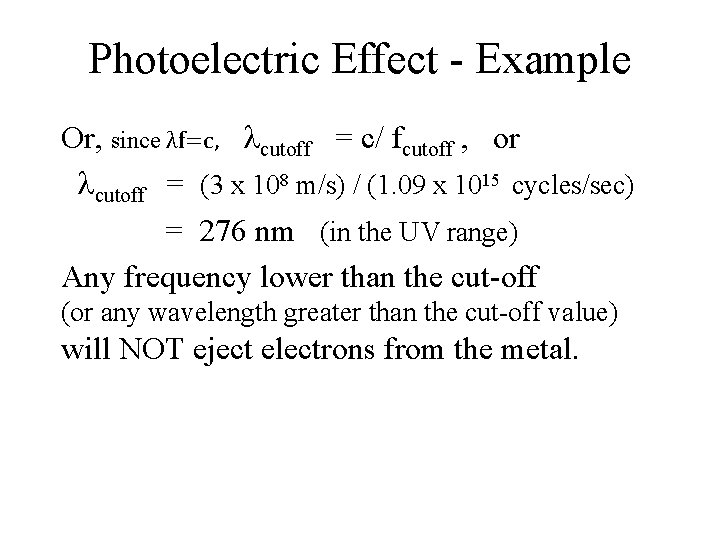
Photoelectric Effect - Example hf = W + e*Vstop When Vstop = 0, f = fcutoff , and hfcutoff = W. Most metals have a work function on the order of several electron volts. Example: copper has a work function of 4. 5 e. V. Therefore, the cut-off frequency for light ejecting electrons from copper is: hfcutoff = 4. 5 e. V, or fcutoff = 4. 5 x (1. 6 x 10 -19 C) x (1 V) / 6. 63 x 10 -34 J-sec = 1. 09 x 1015 Hz,

Photoelectric Effect - Example Or, since λf=c, cutoff = c/ fcutoff , or cutoff = (3 x 108 m/s) / (1. 09 x 1015 cycles/sec) = 276 nm (in the UV range) Any frequency lower than the cut-off (or any wavelength greater than the cut-off value) will NOT eject electrons from the metal.

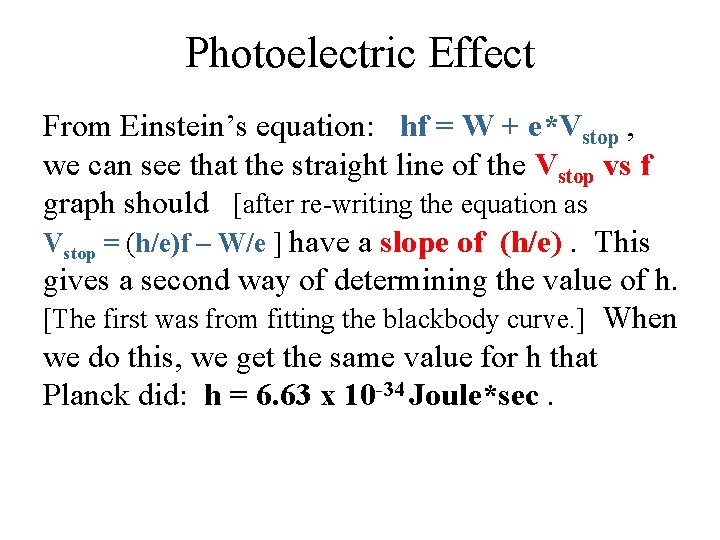
Photoelectric Effect From Einstein’s equation: hf = W + e*Vstop , we can see that the straight line of the Vstop vs f graph should [after re-writing the equation as Vstop = (h/e)f – W/e ] have a slope of (h/e). This gives a second way of determining the value of h. [The first was from fitting the blackbody curve. ] When we do this, we get the same value for h that Planck did: h = 6. 63 x 10 -34 Joule*sec.

Computer Homework The computer homework program on Photons (Vol 5, #5) deals with both Blackbody Radiation and the Photoelectric Effect.

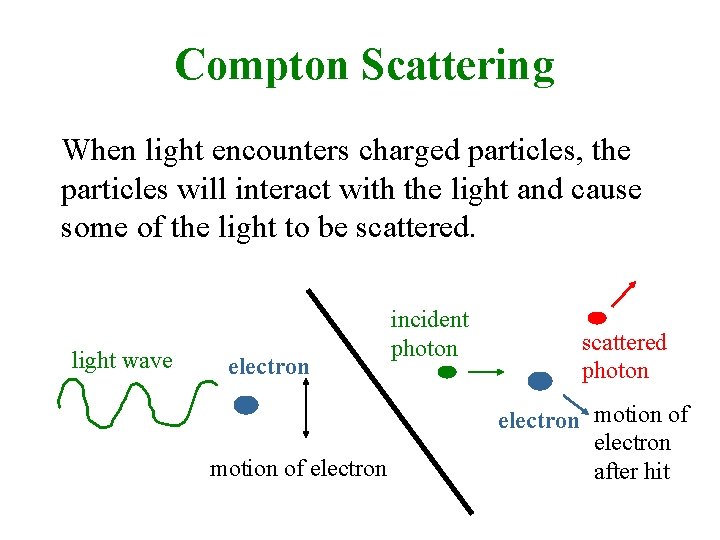
Compton Scattering When light encounters charged particles, the particles will interact with the light and cause some of the light to be scattered. light wave electron motion of electron incident photon scattered photon electron motion of electron after hit

Compton Scattering From the wave theory, we can understand that charged particles would interact with the light since the light is an electromagnetic wave! But the actual predictions of how the light should scatter from the charged particles by our simple wave model does not fully match experiment. It is good for long wavelengths, and leads to an understanding of why the sky is blue – Rayleigh scattering considered in PHYS 415 Optics. It fails, though, for short wavelength scattering.

Compton Scattering If we consider the photon idea of light, some of the photons would “hit” the charged particles and “bounce off”. The laws of conservation of energy and momentum should then predict the scattering.

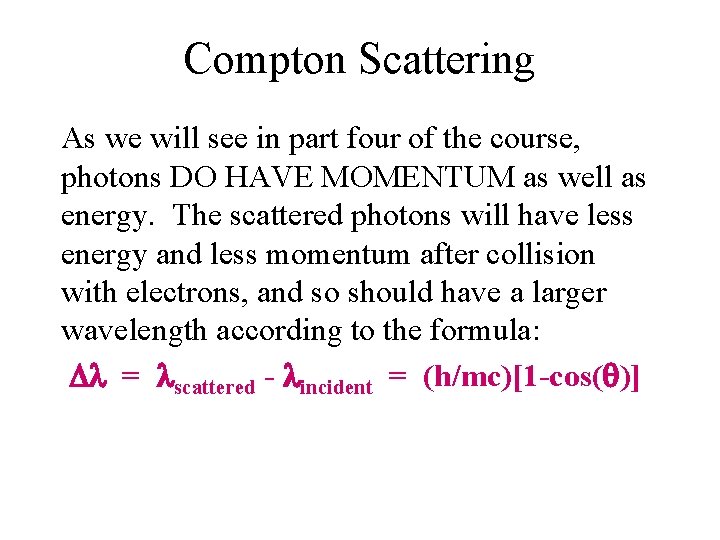
Compton Scattering As we will see in part four of the course, photons DO HAVE MOMENTUM as well as energy. The scattered photons will have less energy and less momentum after collision with electrons, and so should have a larger wavelength according to the formula: = scattered - incident = (h/mc)[1 -cos( )]
![Compton Scattering scattered incident hmc1 cos Note that Plancks Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] • Note that Planck’s](https://slidetodoc.com/presentation_image/453639b76009729452ff40ef16e7d3ff/image-20.jpg)
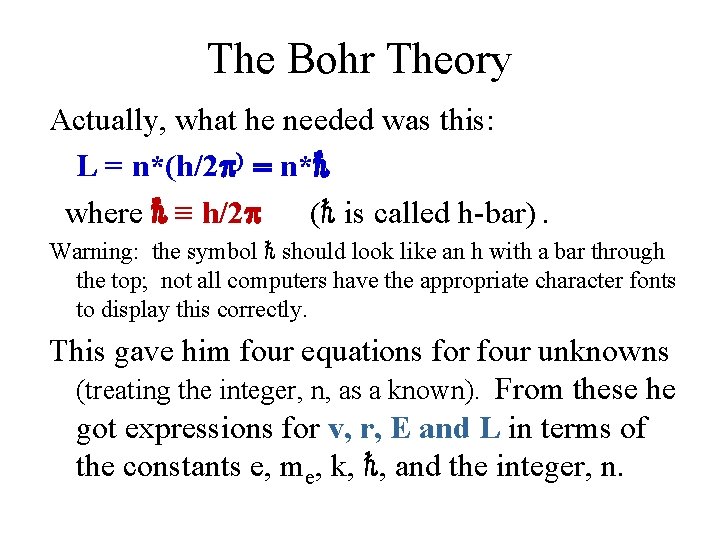
Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] • Note that Planck’s constant is in this relation as well, and gives a further experimental way of getting this value. • Again, the photon theory provides a nice explanation of a phenomenon involving light.
![Compton Scattering scattered incident hmc1 cos Note that the maximum Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] Note that the maximum](https://slidetodoc.com/presentation_image/453639b76009729452ff40ef16e7d3ff/image-21.jpg)
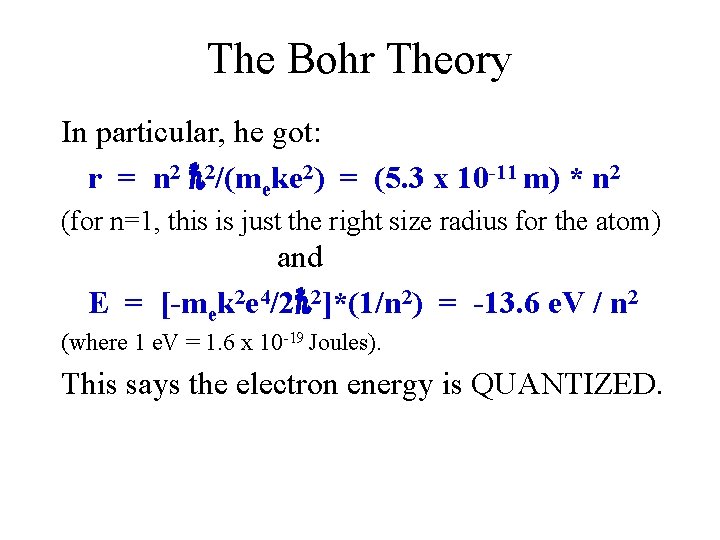
Compton Scattering = scattered - incident = (h/mc)[1 -cos( )] Note that the maximum change in wavelength is when = 180 o (photon hits an electron and bounces straight back) so (for maximum scattering from an electron) 2 h/mec = 2(6. 63 x 10 -34 J-s) / (9. 1 x 10 -31 kg * 3 x 108 m/s) = 4. 86 x 10 -12 m = which would be insignificant for visible light (with of 10 -7 m) but NOT for x-rays (with of 10 -10 m or smaller).

Compton Scattering Compton scattering also has an “h” in the formula and so provides another way of determining h. All the ways so far: blackbody radiation, photoelectric effect, and Compton scattering, use the same idea, ΔE = hf, and give the same value for h: h = 6. 63 x 10 -34 J-s. We’ll next consider a 2 nd way of making light – from atoms – and see if this supports the wave or the particle or this new combination/apparent contradiction of ΔE = hf.

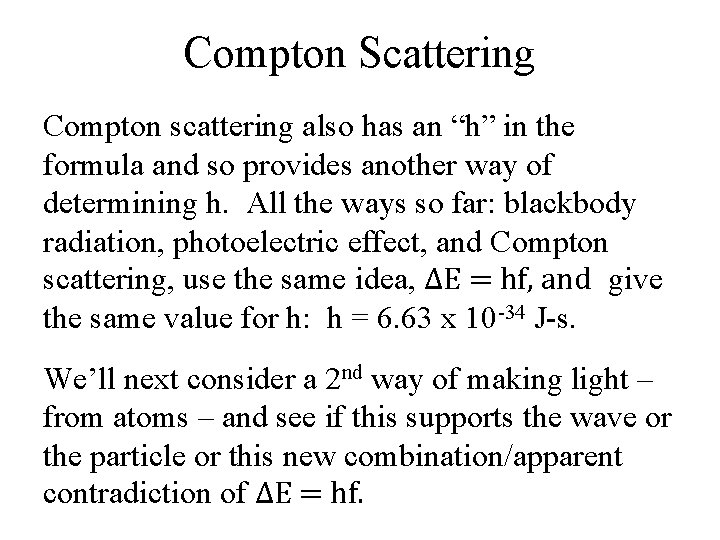
Making Light: Exciting atoms Besides using heat (incandescent light) we can also make light by exciting atoms (fluorescent light). From experiment, we see that different atoms emit different light. But each type of atom emits a very specific set of wavelengths called a discrete spectrum. The hydrogen atoms emit three visible wavelengths: one in the red, one in the bluegreen, and one in the violet.

Atoms and Light Hydrogen’s spectrum (in the visible) consists of just three lines: purple, blue-green, and red. Helium has quite a bit different set of lines in its spectrum. Image is from placing a diffraction grating over the lens of a digital camera.

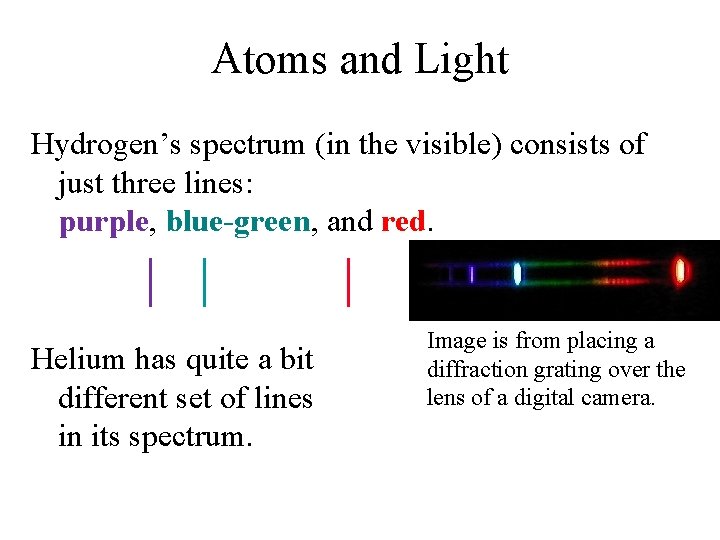
Atoms and Light To contrast with the spectrum of hydrogen, here are the spectra of mercury (on top) and helium. The bright lines on the left are the full light, and the colored lines on the right are the individual spectral lines.

Making Light We need a model of the atom that will explain why atoms emit only certain wavelengths. Let’s look at what we know. First of all, what is the size of a typical atom? Are the heavier atoms bigger than the lighter atoms?

Size of atoms Gold and lead are very heavy (very high density) metals and have very heavy atoms (we know this from the mass spec). If the heavy atoms were bigger, then the density of these metals wouldn’t be that much different than the densities of metals with lighter atoms. So, at least roughly, atoms are about the same size. Let’s take water, although water is a molecule, since we know a lot about water: its mass density: 1 gm / 1 cc; it is H 2 O so it has 18 grams/mole; and we know Avogadro's number = 6. 02 x 1023 molecules/mole. [cc = cm 3 = 10 -6 m 3]

Making Light from Atoms (1 cc / 1 gm) * (18 gms / mole) * (1 mole / 6. 02 x 1023 molecules) = 18 x 10 -6 m 3 / 6 x 1023 molecules = 3 x 10 -29 m 3 = 30 x 10 -30 m 3. Therefore, the size is about (30 x 10 -30 m 3)1/3 = 3 x 10 -10 m. Thus the size of an atom should roughly be about 0. 1 nm. [0. 1 nm is sometimes called an Angstrom]

Making Light from Atoms Now that we know the size of an atom, how much mass does the atom have? From the mass spectrograph, we know that the mass of an atom comes in integer values of 1 amu = 1. 66 x 10 -27 kg. (In fact, this is important in getting Avogadro’s number!)

Making Light from Atoms Now that we know the size and mass, does an atom have parts? We know that the atom has electrons of very small mass (me = 9. 1 x 10 -31 kg), about 2, 000 times smaller than one amu (recall we did this in the charge to mass ratio of the electron experiment in PHYS 251 lab); and a negative charge, qe = -1. 6 x 10 -19 Coul.

Making Light from Atoms We also know that the atom is neutral, so the part of the atom that is not the electrons must have essentially all the mass and a positive charge to cancel the charges of the electrons. But what is the structure of these electrons and this other part of the atom?

Making Light from Atoms But what is the structure of these electrons and this other part of the atom? Two possibilities come to mind: • The planetary model, where the very light electrons orbit the heavy central nucleus. • The plum pudding model, where the very light and small electrons are embedded (like plums) in the much more massive pudding of the rest of the atom.

Making Light from Atoms The Planetary Model: If the light electrons do go around the central, heavy nucleus, then the electrons are accelerating (changing the directions of their velocities). But accelerating electrons should emit electromagnetic radiation (since their electric fields will be wobbling).

Making Light from Atoms • If an electron is emitting E&M radiation, it is emitting energy. • If the electron is emitting energy, it should then lose kinetic energy and fall closer to the nucleus. • The process should continue until all the electrons fall into the nucleus and we have the plum pudding model.

Making Light from Atoms In addition, the frequency of the E&M radiation (light) emitted by the accelerating (orbiting) electrons should continuously vary in frequency as the frequencies of the electrons continuously vary as they spiral into the nucleus. This does not agree with the experimental results: the spectra of the atoms.

Making Light from Atoms The plum pudding model has no such problem with accelerating electrons, since the electrons are just sitting like plums in the pudding. However, we’re still left with the problem of explaining the spectra of atoms.

Rutherford Scattering To test the plum pudding model, Rutherford decided to shoot alpha particles (mass = 4 amu’s; charge = +2 e; moving very fast) at a thin gold foil and see what happens to the alpha particles. (Gold does not oxidize so it remains pure, and it can be made very thin - only several atoms thick; thus there should be very few multiple scatterings. )

Rutherford Scattering Prediction: the positive charge of the atom according to the Plum Pudding Model is spread out, so by symmetry it should have little effect on the alphas. The electrons are so light (8, 000 times lighter than an alpha) that they should deflect the massive alpha very little. If the plum pudding model is correct, then the alphas should pretty much go straight through - like shooting a cannon ball at a piece of tissue paper.

Rutherford Scattering Results: • Most of the alphas did indeed go almost straight through the foil. • However, a few were deflected at significant angles. • A very few even bounced back! Once in a while a cannot ball bounced back off the tissue paper!

Rutherford Scattering The results of the scattering were consistent with the alphas scattering off a tiny positively charged and massive nucleus rather than the diffuse neutral (containing the negative electrons and the positive pudding) atoms. If the electric repulsion of the gold nucleus is the only force acting on the alpha (remember both alpha and the nucleus are positively charged), then the deflection of the alpha can be predicted. We look at this scattering in detail in PHYS 380 Advanced Mechanics.

Rutherford Scattering The faster we fire the alpha, the closer the alpha should come to the gold nucleus. [Kinetic energy goes into electrical potential energy. ] 1/2 mαvα 2 = q (kqgold/rα-gold) where rα-gold is the distance of closest approach of the alpha to the gold nucleus. We will know that we have “hit” the nucleus (and hence know its size) when the scattering differs from that due to the purely electric repulsion. This also means that there must be a “nuclear force” if the scattering differs from the purely electric!

Rutherford Scattering The results indicated that the positive charge and heavy mass were located in a nucleus on the order of 10 -14 m (recall the atom size is on the order of 10 -10 m). 0 Later results with other atoms indicated that the nucleus for heavier atoms is bigger than the nucleus of lighter atoms. The electron “cloud” for heavier atoms, however, is not necessarily bigger since the electrons are held tighter due to the increased positive charge of the heavier nucleus. We’ll look at nuclei in much more detail in Part 5 of the course.

Rutherford Scattering Note how small the nucleus is in relation to the atom: the nuclear radius is 10 -14 m versus the atomic radius of 10 -10 m - a difference in size of 10, 000 and a difference in volume of (104)3 = 1012 (a trillion!). The electron is even smaller. It is so small that we can’t yet say how small, but it is less than 10 -17 meters in radius. (More on this later in this section. )

Rutherford Scattering If the mass takes up only 1 trillionth of the space, why can’t I walk right through the wall?

Rutherford Scattering Why can’t I walk right through the wall? The electric repulsion between the orbiting electrons of the wall and the orbiting electrons of me - and the electric repulsion between the nuclei of the atoms in the wall and the nuclei of my atoms, these repulsions keep me and the wall separate. The nuclear force does not come into play. We’ll say more about the nuclear force in part 5 of this course.

Making Light from Atoms We now know that the atom seems to have a very tiny nucleus with the electrons somehow filling out the size of the atom just what the planetary model of the atom would suggest. However, we still have the problem of how the electrons stay in those orbits, and how the atom emits characteristic spectrum of light.

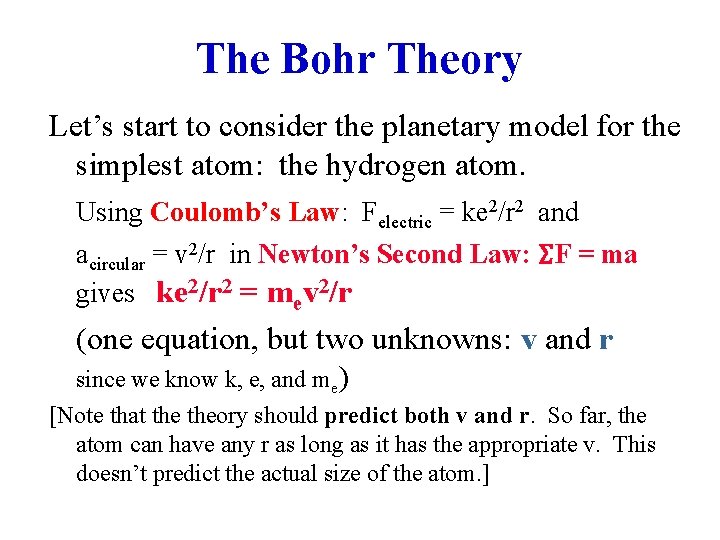
The Bohr Theory Let’s start to consider the planetary model for the simplest atom: the hydrogen atom. Using Coulomb’s Law: Felectric = ke 2/r 2 and acircular = v 2/r in Newton’s Second Law: ΣF = ma gives ke 2/r 2 = mev 2/r (one equation, but two unknowns: v and r since we know k, e, and me) [Note that theory should predict both v and r. So far, the atom can have any r as long as it has the appropriate v. This doesn’t predict the actual size of the atom. ]

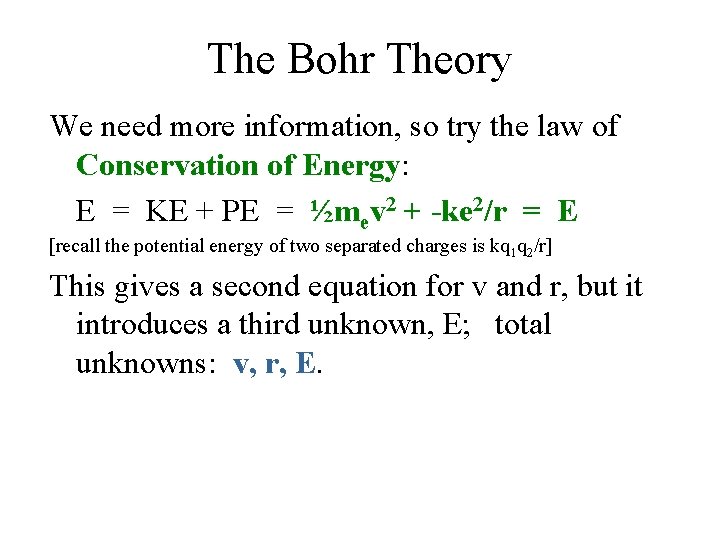
The Bohr Theory We need more information, so try the law of Conservation of Energy: E = KE + PE = ½mev 2 + -ke 2/r = E [recall the potential energy of two separated charges is kq 1 q 2/r] This gives a second equation for v and r, but it introduces a third unknown, E; total unknowns: v, r, E.

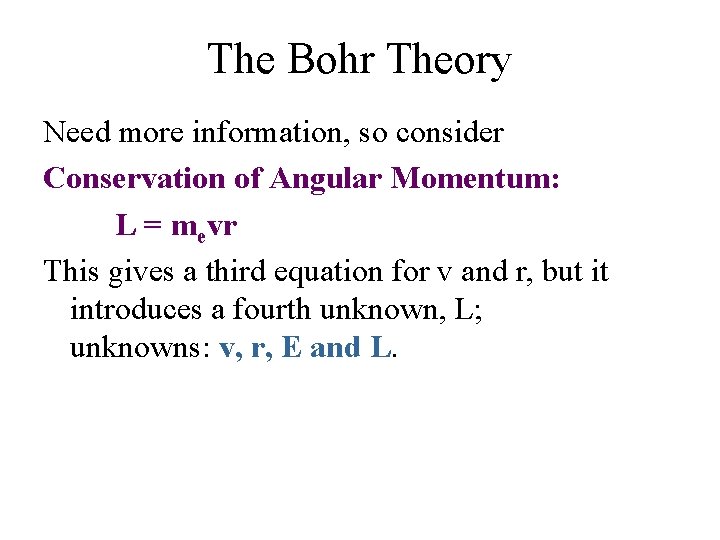
The Bohr Theory Need more information, so consider Conservation of Angular Momentum: L = mevr This gives a third equation for v and r, but it introduces a fourth unknown, L; unknowns: v, r, E and L.

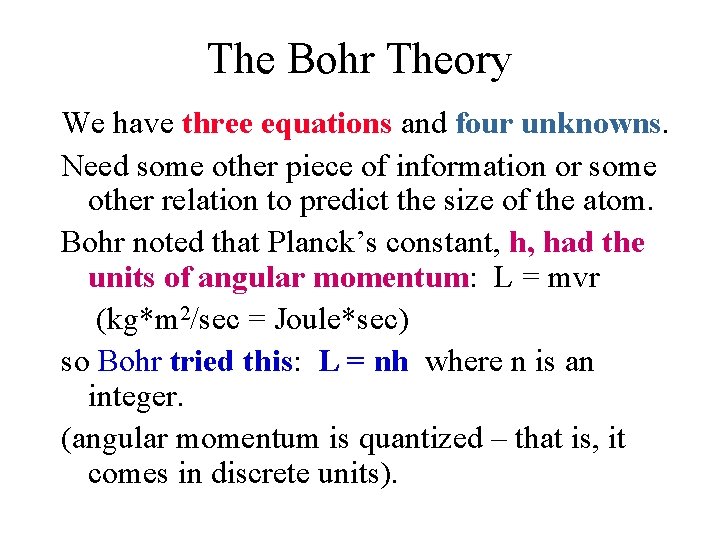
The Bohr Theory We have three equations and four unknowns. Need some other piece of information or some other relation to predict the size of the atom. Bohr noted that Planck’s constant, h, had the units of angular momentum: L = mvr (kg*m 2/sec = Joule*sec) so Bohr tried this: L = nh where n is an integer. (angular momentum is quantized – that is, it comes in discrete units).

The Bohr Theory Actually, what he needed was this: L = n*(h/2 n*ℏ where ℏ ≡ h/2 (ℏ is called h-bar) Warning: the symbol ℏ should look like an h with a bar through the top; not all computers have the appropriate character fonts to display this correctly. This gave him four equations for four unknowns (treating the integer, n, as a known). From these he got expressions for v, r, E and L in terms of the constants e, me, k, ℏ, and the integer, n.

The Bohr Theory In particular, he got: r = n 2 ℏ 2/(meke 2) = (5. 3 x 10 -11 m) * n 2 (for n=1, this is just the right size radius for the atom) and E = [-mek 2 e 4/2ℏ 2]*(1/n 2) = -13. 6 e. V / n 2 (where 1 e. V = 1. 6 x 10 -19 Joules). This says the electron energy is QUANTIZED.

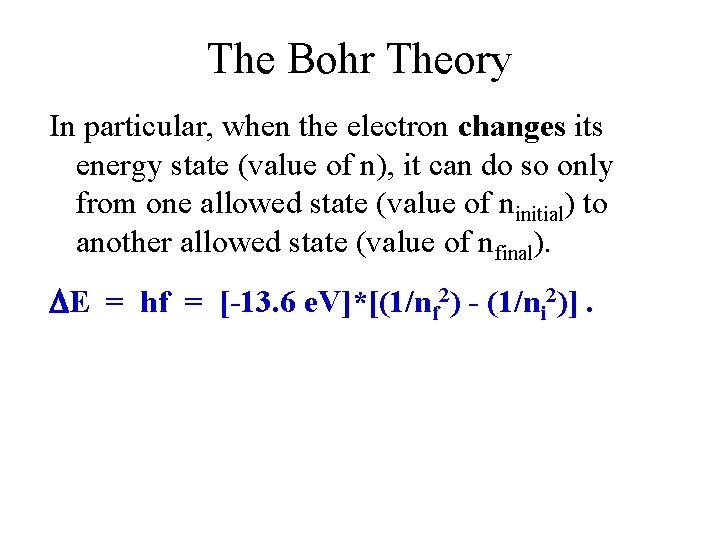
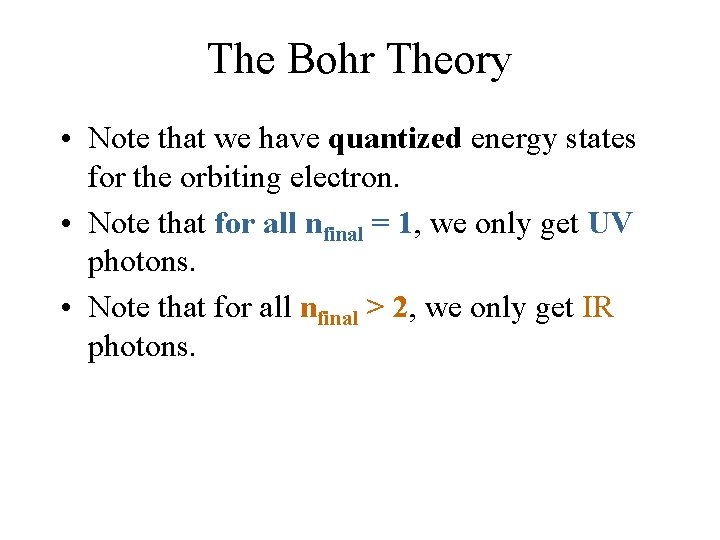
The Bohr Theory In particular, when the electron changes its energy state (value of n), it can do so only from one allowed state (value of ninitial) to another allowed state (value of nfinal). E = hf = [-13. 6 e. V]*[(1/nf 2) - (1/ni 2)].
![The Bohr Theory E hf 13 6 e V1nf 2 1ni The Bohr Theory E = hf = [-13. 6 e. V]*[(1/nf 2) - (1/ni](https://slidetodoc.com/presentation_image/453639b76009729452ff40ef16e7d3ff/image-54.jpg)
The Bohr Theory E = hf = [-13. 6 e. V]*[(1/nf 2) - (1/ni 2)] In the case of ni = 3, and nf = 2, E = (-13. 6 e. V)*(1/22 - 1/32) = -1. 89 e. V The negative means that the atom is losing this much energy by emitting light of this positive amount of energy. E = hf = hc/ , so in this case, emitted = hc/ E = (6. 63 x 10 -34 J-sec)*(3 x 108 m/s)/(1. 89 x 1. 6 x 10 -19 J) = 658 nm (red light).

The Bohr Theory Similarly, when ni = 4 and nf = 2, we get E = 2. 55 e. V, and emitted = 488 nm (blue-green); and when ni = 5 and nf = 2, we get E = 3. 01 e. V, and emitted = 413 nm (violet). ALL THREE MATCH THE ACTUAL SPECTRUM OF HYDROGEN!

Spectrum of Hydrogen’s spectrum (in the visible) consists of just three lines: purple, blue-green, and red. Helium has quite a bit different set of lines in its spectrum.

The Bohr Theory This matching of theory with experiment is the reason Bohr made his assumption that L = nℏ (instead of L = nh).

The Bohr Theory • Note that we have quantized energy states for the orbiting electron. • Note that for all nfinal = 1, we only get UV photons. • Note that for all nfinal > 2, we only get IR photons.

The Bohr Theory Problems with the Bohr Theory: • WHY is angular momentum quantized? (WHY does L=nℏ need to be true? ) • What do we do with atoms that have more than one electron? (The Bohr theory does work for singly ionized Helium, but what about normal Helium with 2 electrons? )
 Photoelectric and compton effect
Photoelectric and compton effect Photoelectric and compton effect
Photoelectric and compton effect Photon cross section
Photon cross section Laws of photoelectric effect
Laws of photoelectric effect Photoelectric effect สรุป
Photoelectric effect สรุป Kcvs.ca photoelectric effect
Kcvs.ca photoelectric effect Hf = φ + ek
Hf = φ + ek Site:slidetodoc.com
Site:slidetodoc.com Wave superposition worksheet
Wave superposition worksheet Kumar is producing the photoelectric effect by using
Kumar is producing the photoelectric effect by using Multiple choice questions on photoelectric effect
Multiple choice questions on photoelectric effect Photoelectric effect demo
Photoelectric effect demo Photoelectric effect
Photoelectric effect Photoelectric effect
Photoelectric effect Photoelectric effect สรุป
Photoelectric effect สรุป Photoelectric effect
Photoelectric effect Photoelectric effect
Photoelectric effect Photoelectric effect
Photoelectric effect What happen when light hits an object
What happen when light hits an object Why does light not bend when it hits a curved surface
Why does light not bend when it hits a curved surface Light light light chapter 23
Light light light chapter 23 Light light light chapter 22
Light light light chapter 22 Chapter 22
Chapter 22 Pour plate method steps
Pour plate method steps Divergent boundary oreo
Divergent boundary oreo Difference between metal oxides and non metal oxides
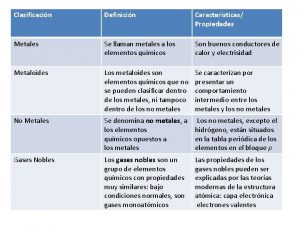
Difference between metal oxides and non metal oxides Compare the properties of metals and nonmetals
Compare the properties of metals and nonmetals Metals are used
Metals are used Liquid elements at room temperature
Liquid elements at room temperature Metal and non metal definition
Metal and non metal definition Metals vs nonmetals
Metals vs nonmetals Metalloids
Metalloids Pure cultures
Pure cultures Streaking method
Streaking method A denser oceanic plate collides with a continental plate
A denser oceanic plate collides with a continental plate What size electrode was used to make the 1f lap joint weld?
What size electrode was used to make the 1f lap joint weld? 3 states of matter venn diagram
3 states of matter venn diagram Pimp nimp
Pimp nimp Metals react with nonmetals to form ionic compounds by
Metals react with nonmetals to form ionic compounds by Metal no metal y metaloide tabla periodica
Metal no metal y metaloide tabla periodica Oxigeno caracteristicas y propiedades
Oxigeno caracteristicas y propiedades El sodio es metal o no metal
El sodio es metal o no metal Einstein equation for photoelectric emission is
Einstein equation for photoelectric emission is Photo electric transducer
Photo electric transducer Tungsten atomic structure
Tungsten atomic structure Photoelectric absorption
Photoelectric absorption Photoelectric ophthalmia
Photoelectric ophthalmia Biochemistry semi auto analyzer
Biochemistry semi auto analyzer Plate effect
Plate effect Who had hits with lego house sing and photograph
Who had hits with lego house sing and photograph Metal semiconductor field effect transistor
Metal semiconductor field effect transistor Specilis
Specilis Mobility ratio
Mobility ratio Bohr's effect in respiration
Bohr's effect in respiration Roy's identity
Roy's identity Does it matter what type of light shines on the metal
Does it matter what type of light shines on the metal Strongest element on earth
Strongest element on earth Put out the light and then put out the light
Put out the light and then put out the light Distinguish between photosystem 1 and photosystem 2
Distinguish between photosystem 1 and photosystem 2 Or the bending of light and the bouncing off of light
Or the bending of light and the bouncing off of light