Chapter 26 Photoelectric Effect 26 1 Photoelectric Effect











- Slides: 59

Chapter 26: Photoelectric Effect 26 -1 Photoelectric Effect T. Norah Ali Al moneef 1

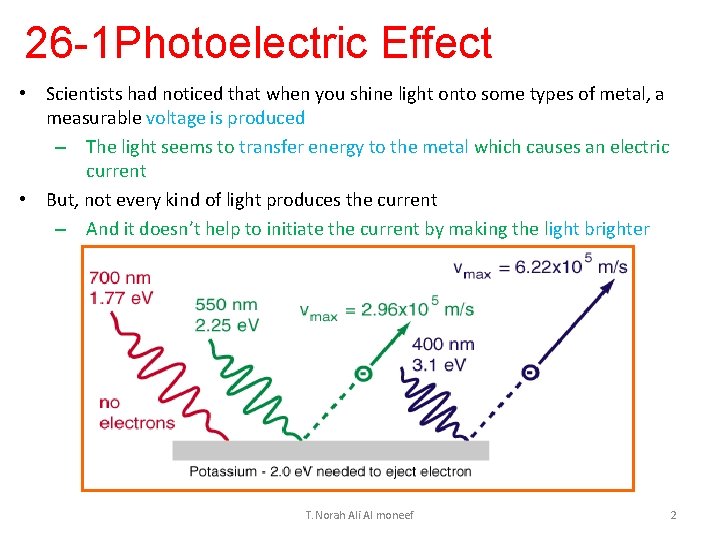
26 -1 Photoelectric Effect • Scientists had noticed that when you shine light onto some types of metal, a measurable voltage is produced – The light seems to transfer energy to the metal which causes an electric current • But, not every kind of light produces the current – And it doesn’t help to initiate the current by making the light brighter T. Norah Ali Al moneef 2

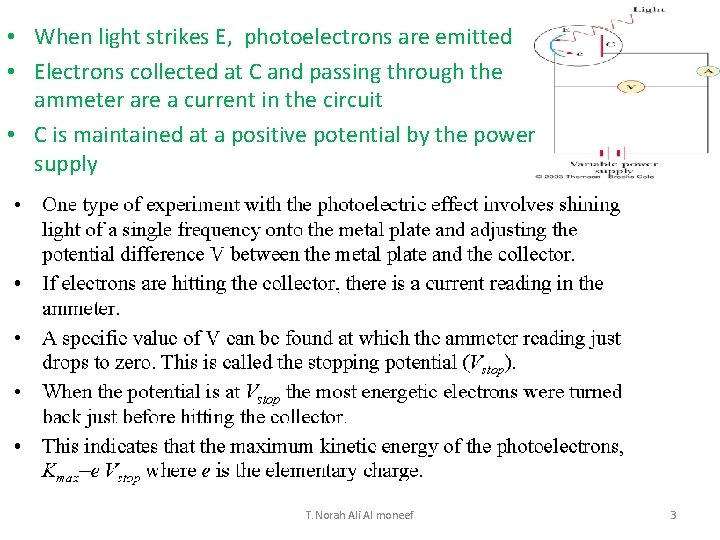
• When light strikes E, photoelectrons are emitted • Electrons collected at C and passing through the ammeter are a current in the circuit • C is maintained at a positive potential by the power supply T. Norah Ali Al moneef 3

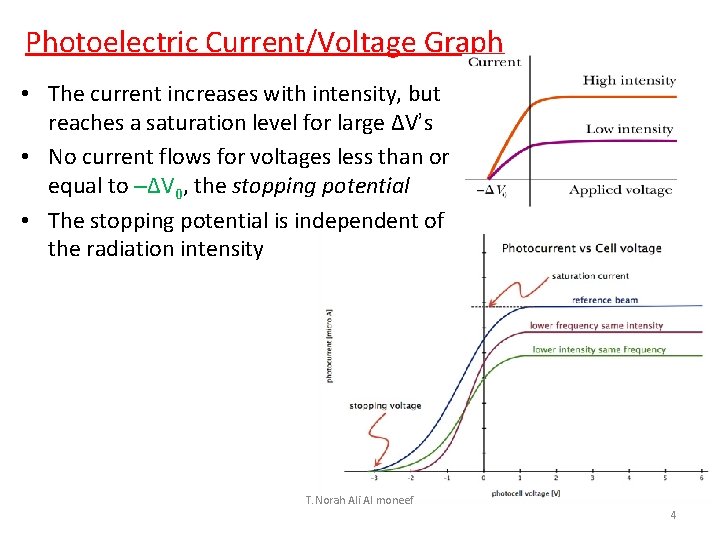
Photoelectric Current/Voltage Graph • The current increases with intensity, but reaches a saturation level for large ΔV’s • No current flows for voltages less than or equal to –ΔV 0, the stopping potential • The stopping potential is independent of the radiation intensity T. Norah Ali Al moneef 4

A specific value of V can be found at which the ammeter reading just drops to zero. This is called the stopping potential (Vstop). • When the potential is at Vstop the most energetic electrons were turned back just before hitting the collector. • This indicates that the maximum kinetic energy of the photoelectrons, Kmax= e Vstop where e is the elementary charge. Interestingly, it was found that Kmax does not depend upon the intensity of the incident light. • It is difficult to explain this observation with classical physics where light is viewed as a continuous wave. • In classical physics, the electrons would be viewed as oscillating under the influence of an alternating electric field. If the intensity of the light is increased, so is the amplitude of the electric field, which should make it easier for the electron to acquire enough energy to break free of the surface of the metal plate. This, however, does not agree with experiment T. Norah Ali Al moneef 5

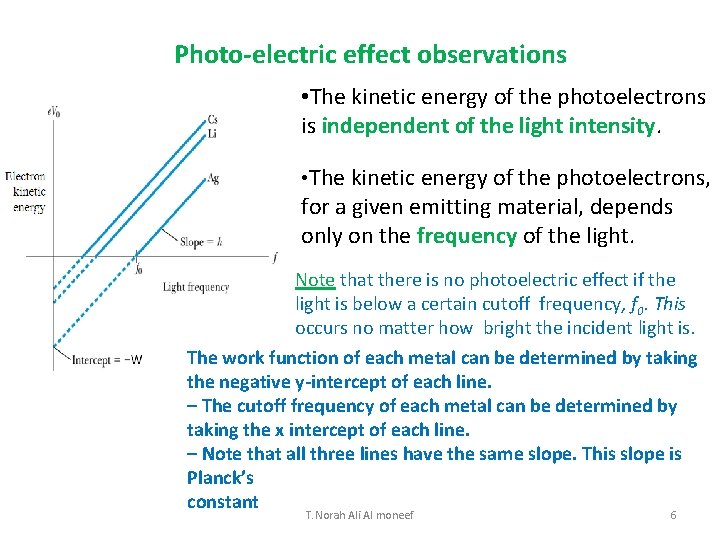
Photo-electric effect observations • The kinetic energy of the photoelectrons is independent of the light intensity. • The kinetic energy of the photoelectrons, for a given emitting material, depends only on the frequency of the light. Note that there is no photoelectric effect if the light is below a certain cutoff frequency, f 0. This occurs no matter how bright the incident light is. The work function of each metal can be determined by taking the negative y-intercept of each line. – The cutoff frequency of each metal can be determined by taking the x intercept of each line. – Note that all three lines have the same slope. This slope is Planck’s constant T. Norah Ali Al moneef 6

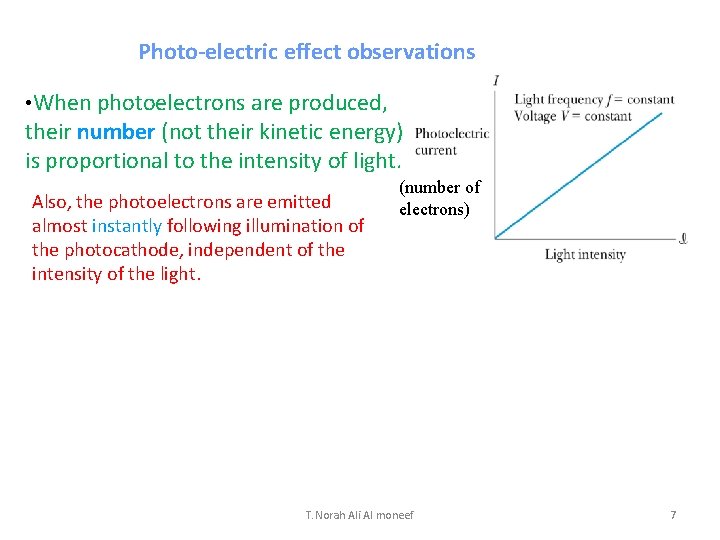
Photo-electric effect observations • When photoelectrons are produced, their number (not their kinetic energy) is proportional to the intensity of light. Also, the photoelectrons are emitted almost instantly following illumination of the photocathode, independent of the intensity of the light. (number of electrons) T. Norah Ali Al moneef 7

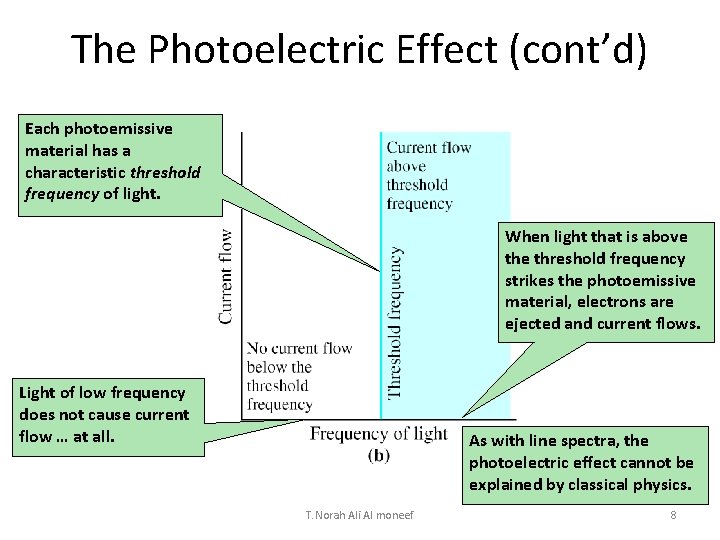
The Photoelectric Effect (cont’d) Each photoemissive material has a characteristic threshold frequency of light. When light that is above threshold frequency strikes the photoemissive material, electrons are ejected and current flows. Light of low frequency does not cause current flow … at all. As with line spectra, the photoelectric effect cannot be explained by classical physics. T. Norah Ali Al moneef 8

The Photoelectric Effect • Albert Einstein won the 1921 Nobel Prize in Physics for explaining the photoelectric effect. • He applied Planck’s quantum theory: electromagnetic energy occurs in little “packets” he called photons. Energy of a photon (E) = hv • The photoelectric effect arises when photons of light striking a surface transfer their energy to surface electrons. • The energized electrons can overcome their attraction for the nucleus and escape from the surface … • … but an electron can escape only if the photon provides enough energy. T. Norah Ali Al moneef 9

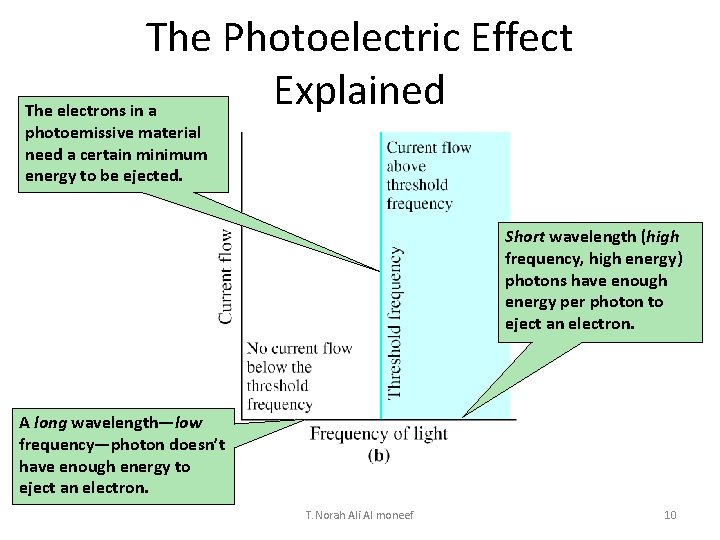
The Photoelectric Effect Explained The electrons in a photoemissive material need a certain minimum energy to be ejected. Short wavelength (high frequency, high energy) photons have enough energy per photon to eject an electron. A long wavelength—low frequency—photon doesn’t have enough energy to eject an electron. T. Norah Ali Al moneef 10

Features Not Explained by Classical Physics/Wave Theory • No electrons are emitted if the incident light frequency is below some cutoff frequency f 0 that is characteristic of the material being illuminatedsince the photon energy must be greater than or equal to the work function • Without this, electrons are not emitted, regardless of the intensity of the light • The maximum kinetic energy kmax of the photoelectrons is independent of the light intensity • The maximum kinetic energy of the photoelectrons increases with increasing light frequency, The maximum KE depends only on the frequency and the work function, • Electrons are emitted from the surface almost instantaneously, even at low intensities T. Norah Ali Al moneef 11

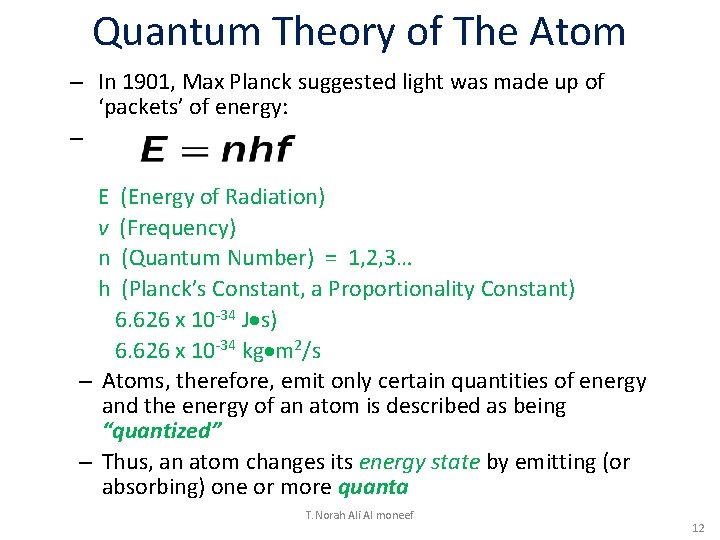
Quantum Theory of The Atom – In 1901, Max Planck suggested light was made up of ‘packets’ of energy: – E (Energy of Radiation) v (Frequency) n (Quantum Number) = 1, 2, 3… h (Planck’s Constant, a Proportionality Constant) 6. 626 x 10 -34 J s) 6. 626 x 10 -34 kg m 2/s – Atoms, therefore, emit only certain quantities of energy and the energy of an atom is described as being “quantized” – Thus, an atom changes its energy state by emitting (or absorbing) one or more quanta T. Norah Ali Al moneef 12

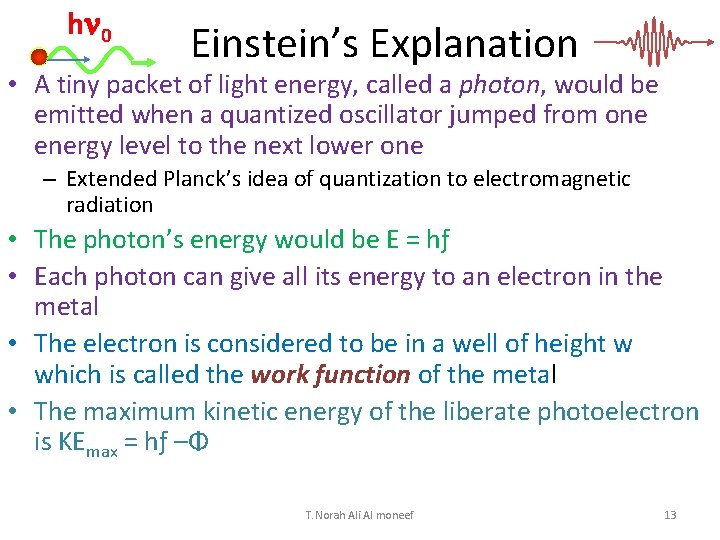
h 0 Einstein’s Explanation • A tiny packet of light energy, called a photon, would be emitted when a quantized oscillator jumped from one energy level to the next lower one – Extended Planck’s idea of quantization to electromagnetic radiation • The photon’s energy would be E = hƒ • Each photon can give all its energy to an electron in the metal • The electron is considered to be in a well of height w which is called the work function of the metal • The maximum kinetic energy of the liberate photoelectron is KEmax = hƒ –Φ T. Norah Ali Al moneef 13

Photons easily explain the results of both photoelectric effect experiments. • Light energy transferred as photons is independent of intensity as shown in both experiments • Furthermore, the existence of a cutoff frequency indicates that below that frequency electrons are not receiving enough energy to overcome the electric forces that bind them to the metal, supporting the idea that the energy is proportional to the frequency • The minimum energy that an electron needs to escape the metal plate depends on the material plate is made out of. This minimum energy is called the work function (w) of the material. • Einstein was able to sum up the results of the two experiments with the photoelectric equation – h f= Kmax + Φ. Note that if this equation is rearranged to Kmax=h f - Φ it is obviously linear in nature, agreeing with the photoelectric effect graph seen earlier T. Norah Ali Al moneef 14

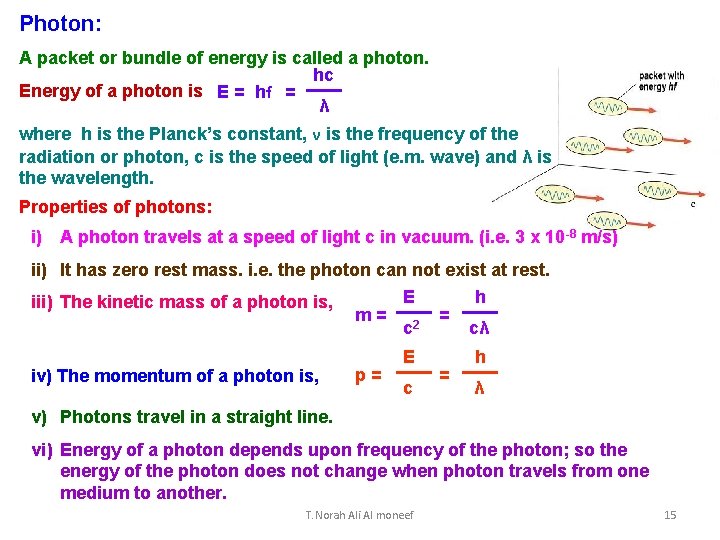
Photon: A packet or bundle of energy is called a photon. hc Energy of a photon is E = hf = λ where h is the Planck’s constant, ν is the frequency of the radiation or photon, c is the speed of light (e. m. wave) and λ is the wavelength. Properties of photons: i) A photon travels at a speed of light c in vacuum. (i. e. 3 x 10 -8 m/s) ii) It has zero rest mass. i. e. the photon can not exist at rest. E h iii) The kinetic mass of a photon is, m= 2 = c cλ iv) The momentum of a photon is, p= E c = h λ v) Photons travel in a straight line. vi) Energy of a photon depends upon frequency of the photon; so the energy of the photon does not change when photon travels from one medium to another. T. Norah Ali Al moneef 15

Cutoff Wavelength • The cutoff wavelength is related to the work function • Wavelengths greater than l. C incident on a material with a work function w don’t result in the emission of photoelectrons Photoemission • The number of electrons emitted by a surface is proportional to the number of incident photons • An electron is emitted as soon as a photon reaches the surface hf = Φ + ½ m. V 2 max Φ = work function ½ m. V 2 max = max kinetic energy T. Norah Ali Al moneef 16

Threshold Frequency ( f 0) hfo = Φ hc = Φ λo c = speed of light λo = wavelength Work Function (Φ ) The minimum amount of energy that has to be given to an electron to release it from the surface of the material and varies depending on the material T. Norah Ali Al moneef 17

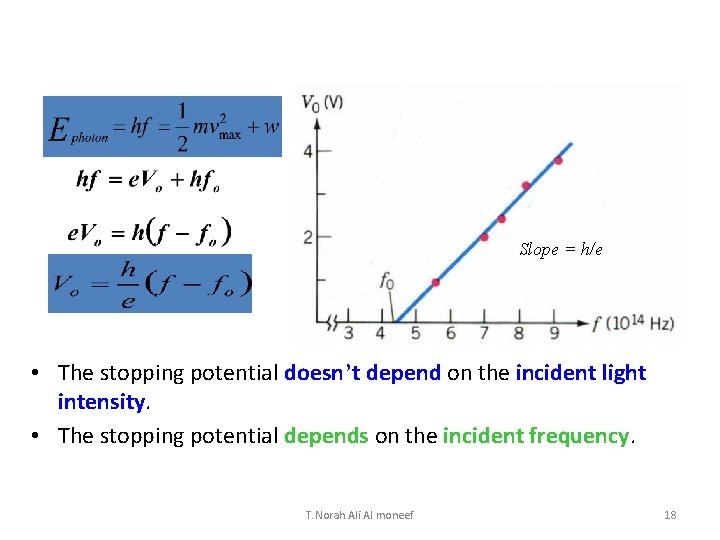
Slope = h/e • The stopping potential doesn’t depend on the incident light intensity. • The stopping potential depends on the incident frequency. T. Norah Ali Al moneef 18

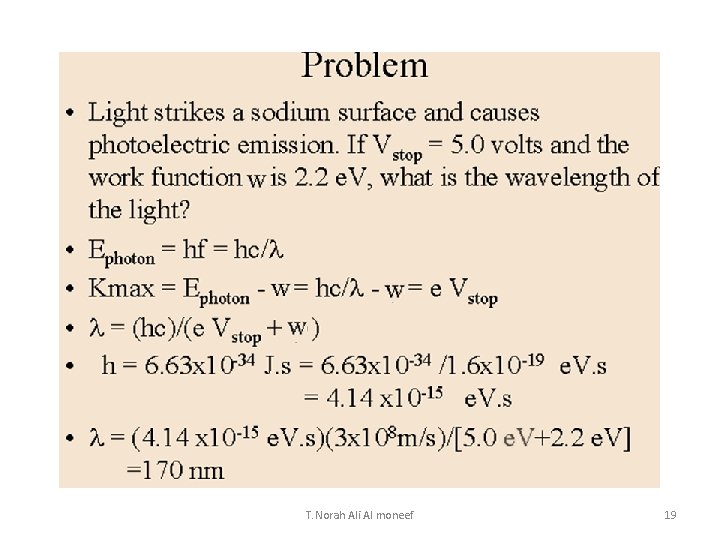
T. Norah Ali Al moneef 19

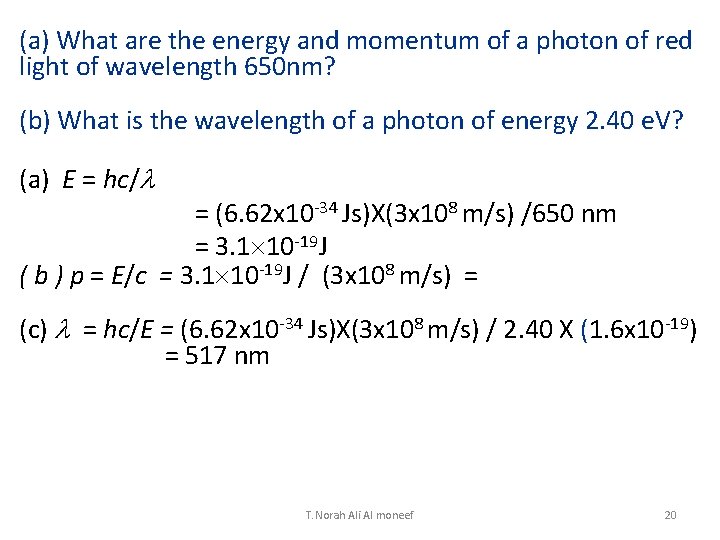
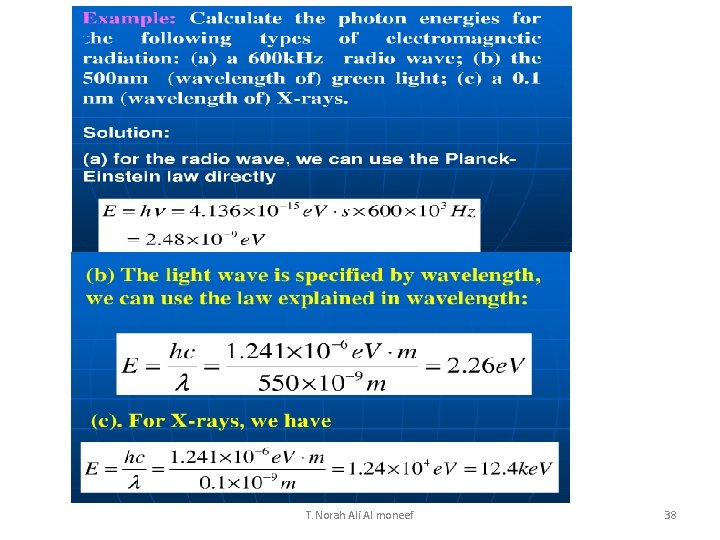
(a) What are the energy and momentum of a photon of red light of wavelength 650 nm? (b) What is the wavelength of a photon of energy 2. 40 e. V? (a) E = hc/l = (6. 62 x 10 -34 Js)X(3 x 108 m/s) /650 nm = 3. 1 10 -19 J ( b ) p = E/c = 3. 1 10 -19 J / (3 x 108 m/s) = (c) l = hc/E = (6. 62 x 10 -34 Js)X(3 x 108 m/s) / 2. 40 X (1. 6 x 10 -19) = 517 nm T. Norah Ali Al moneef 20

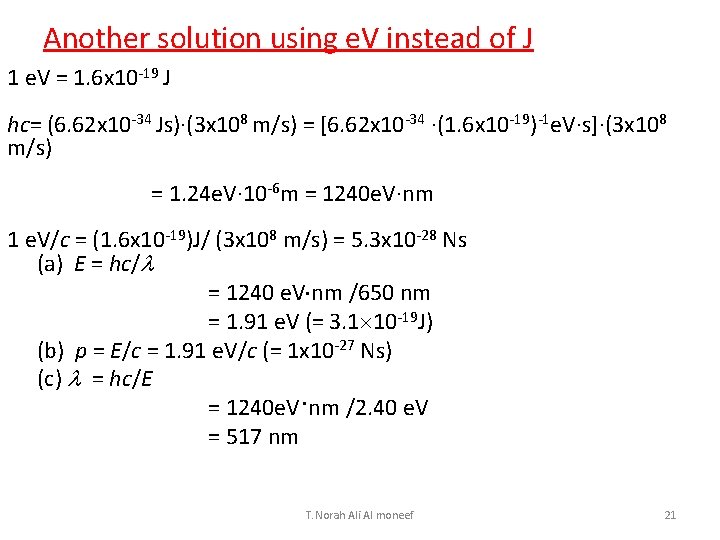
Another solution using e. V instead of J 1 e. V = 1. 6 x 10 -19 J hc= (6. 62 x 10 -34 Js)·(3 x 108 m/s) = [6. 62 x 10 -34 ·(1. 6 x 10 -19)-1 e. V·s]·(3 x 108 m/s) = 1. 24 e. V· 10 -6 m = 1240 e. V·nm 1 e. V/c = (1. 6 x 10 -19)J/ (3 x 108 m/s) = 5. 3 x 10 -28 Ns (a) E = hc/l = 1240 e. V nm /650 nm = 1. 91 e. V (= 3. 1 10 -19 J) (b) p = E/c = 1. 91 e. V/c (= 1 x 10 -27 Ns) (c) l = hc/E = 1240 e. V·nm /2. 40 e. V = 517 nm T. Norah Ali Al moneef 21

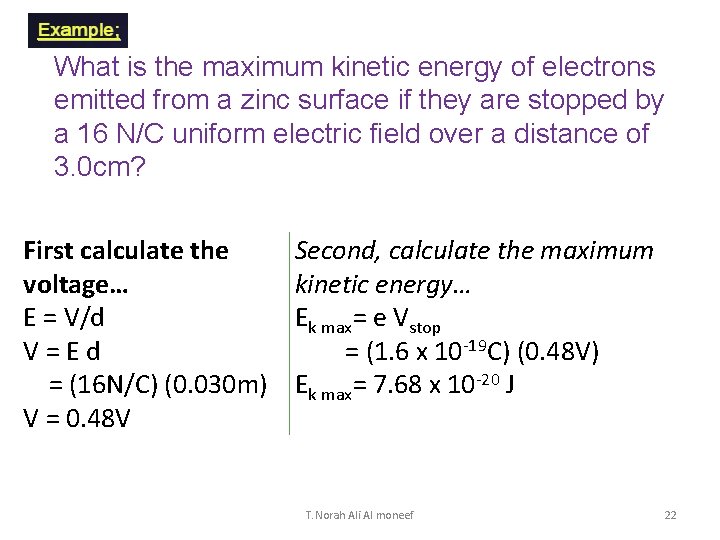
What is the maximum kinetic energy of electrons emitted from a zinc surface if they are stopped by a 16 N/C uniform electric field over a distance of 3. 0 cm? First calculate the voltage… E = V/d V = E d = (16 N/C) (0. 030 m) V = 0. 48 V Second, calculate the maximum kinetic energy… Ek max= e Vstop = (1. 6 x 10 -19 C) (0. 48 V) Ek max= 7. 68 x 10 -20 J T. Norah Ali Al moneef 22

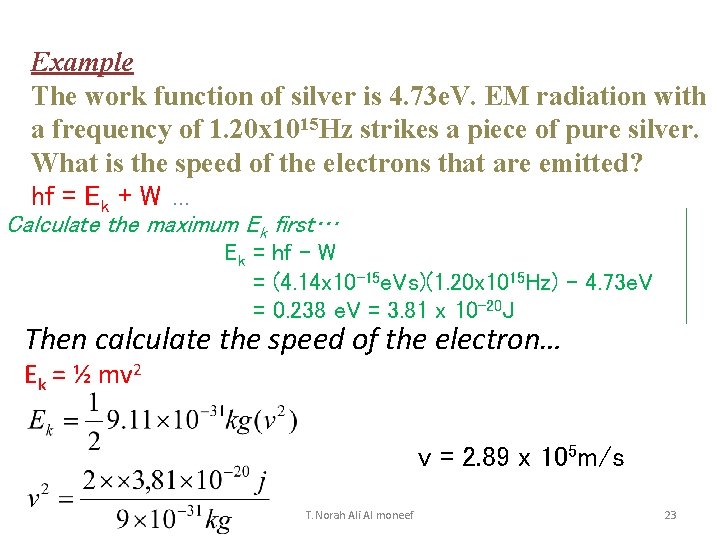
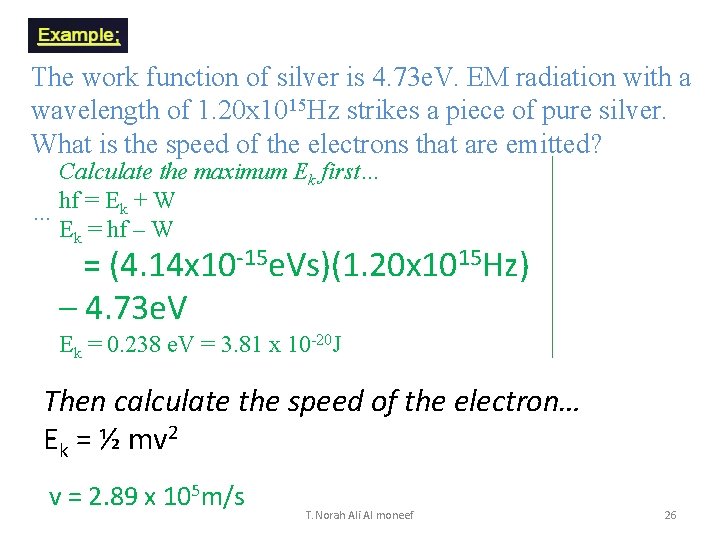
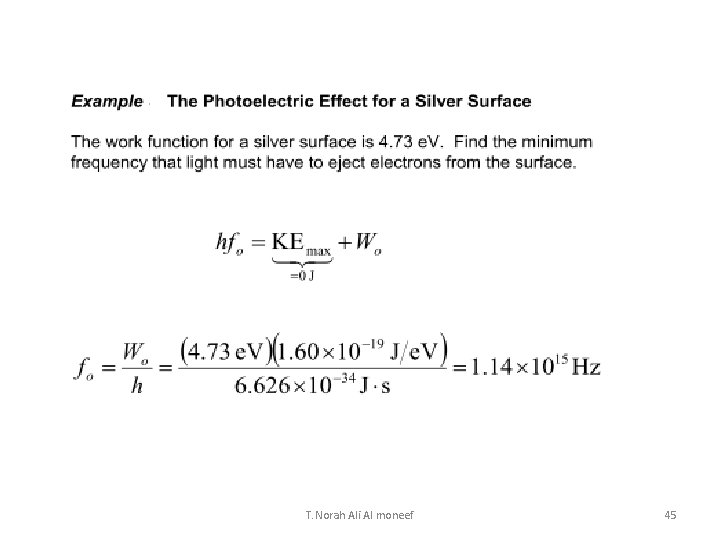
Example The work function of silver is 4. 73 e. V. EM radiation with a frequency of 1. 20 x 1015 Hz strikes a piece of pure silver. What is the speed of the electrons that are emitted? hf = Ek + W … Calculate the maximum Ek first… Ek = hf – W = (4. 14 x 10 -15 e. Vs)(1. 20 x 1015 Hz) – 4. 73 e. V = 0. 238 e. V = 3. 81 x 10 -20 J Then calculate the speed of the electron… Ek = ½ mv 2 v = 2. 89 x 105 m/s T. Norah Ali Al moneef 23

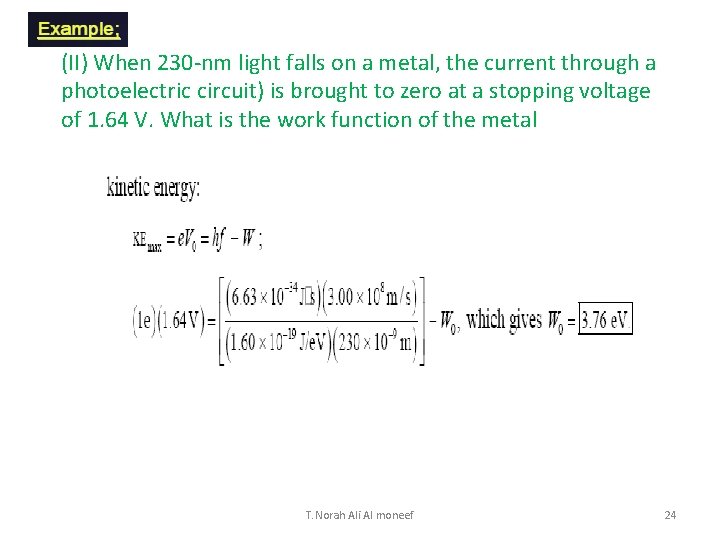
(II) When 230 -nm light falls on a metal, the current through a photoelectric circuit) is brought to zero at a stopping voltage of 1. 64 V. What is the work function of the metal T. Norah Ali Al moneef 24

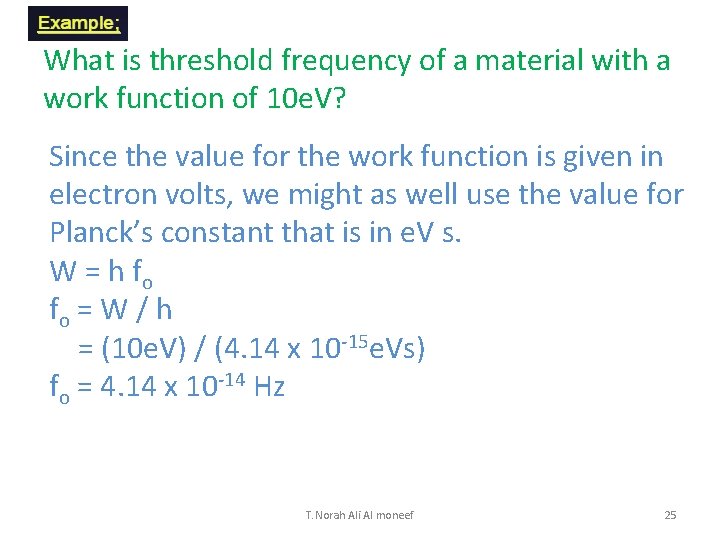
What is threshold frequency of a material with a work function of 10 e. V? Since the value for the work function is given in electron volts, we might as well use the value for Planck’s constant that is in e. V s. W = h fo fo = W / h = (10 e. V) / (4. 14 x 10 -15 e. Vs) fo = 4. 14 x 10 -14 Hz T. Norah Ali Al moneef 25

The work function of silver is 4. 73 e. V. EM radiation with a wavelength of 1. 20 x 1015 Hz strikes a piece of pure silver. What is the speed of the electrons that are emitted? Calculate the maximum Ek first… hf = Ek + W … Ek = hf – W -15 15 = (4. 14 x 10 – 4. 73 e. Vs)(1. 20 x 10 Hz) Ek = 0. 238 e. V = 3. 81 x 10 -20 J Then calculate the speed of the electron… Ek = ½ mv 2 v = 2. 89 x 105 m/s T. Norah Ali Al moneef 26

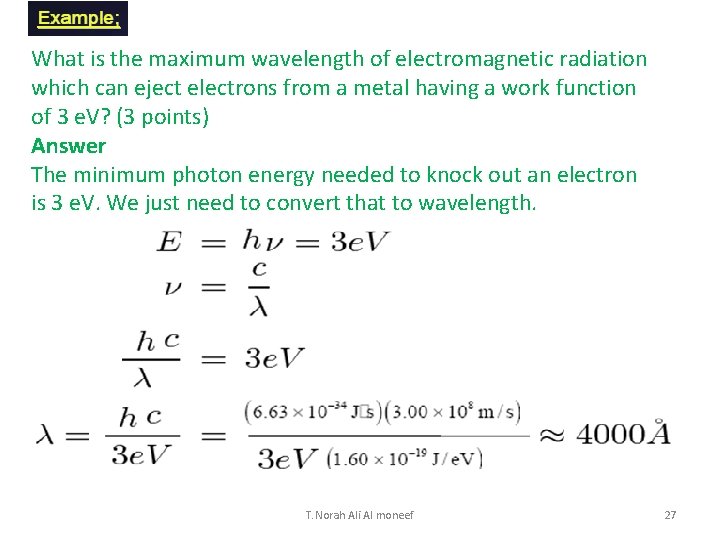
What is the maximum wavelength of electromagnetic radiation which can eject electrons from a metal having a work function of 3 e. V? (3 points) Answer The minimum photon energy needed to knock out an electron is 3 e. V. We just need to convert that to wavelength. T. Norah Ali Al moneef 27

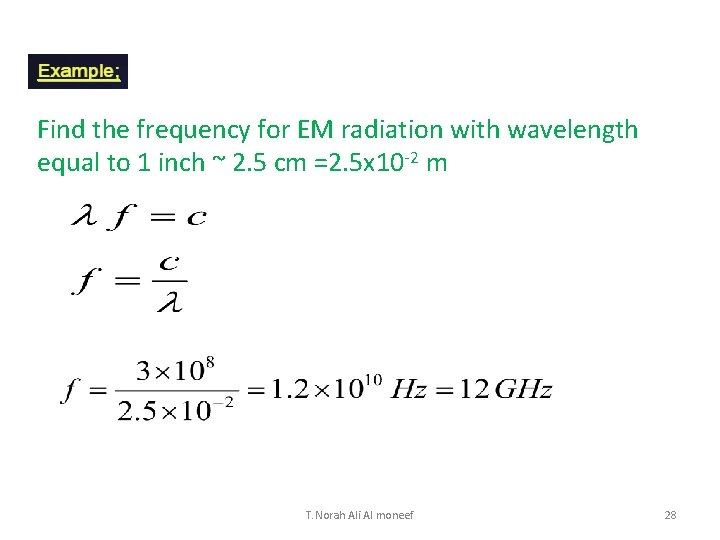
Find the frequency for EM radiation with wavelength equal to 1 inch ~ 2. 5 cm =2. 5 x 10 -2 m T. Norah Ali Al moneef 28

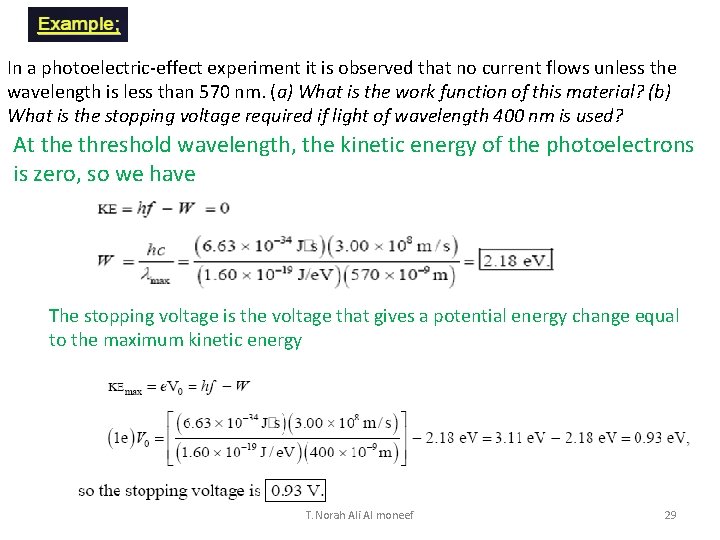
In a photoelectric-effect experiment it is observed that no current flows unless the wavelength is less than 570 nm. (a) What is the work function of this material? (b) What is the stopping voltage required if light of wavelength 400 nm is used? At the threshold wavelength, the kinetic energy of the photoelectrons is zero, so we have The stopping voltage is the voltage that gives a potential energy change equal to the maximum kinetic energy T. Norah Ali Al moneef 29

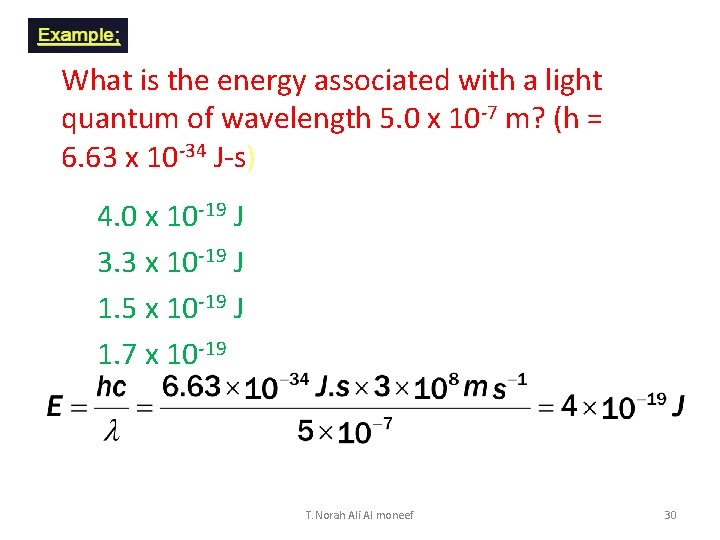
What is the energy associated with a light quantum of wavelength 5. 0 x 10 -7 m? (h = 6. 63 x 10 -34 J-s) 1. 2. 3. 4. 0 x 10 -19 J 3. 3 x 10 -19 J 1. 5 x 10 -19 J 1. 7 x 10 -19 J T. Norah Ali Al moneef 30

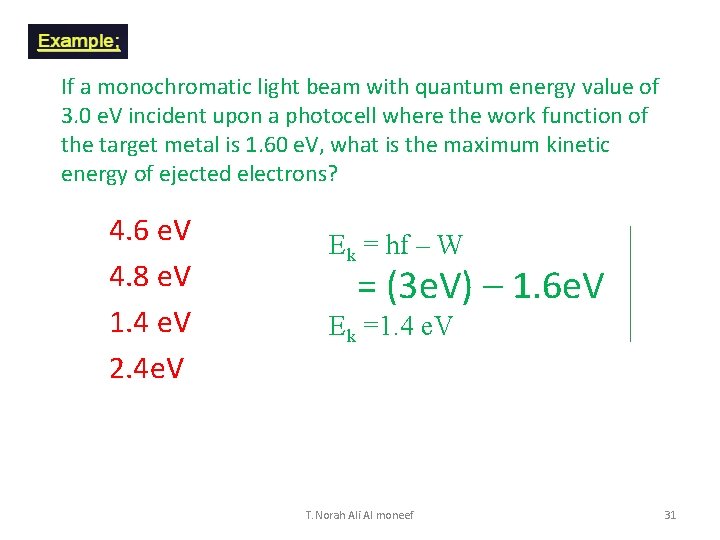
If a monochromatic light beam with quantum energy value of 3. 0 e. V incident upon a photocell where the work function of the target metal is 1. 60 e. V, what is the maximum kinetic energy of ejected electrons? 1. 2. 3. 4. 6 e. V 4. 8 e. V 1. 4 e. V 2. 4 e. V Ek = hf – W = (3 e. V) – 1. 6 e. V Ek =1. 4 e. V T. Norah Ali Al moneef 31

T. Norah Ali Al moneef 32

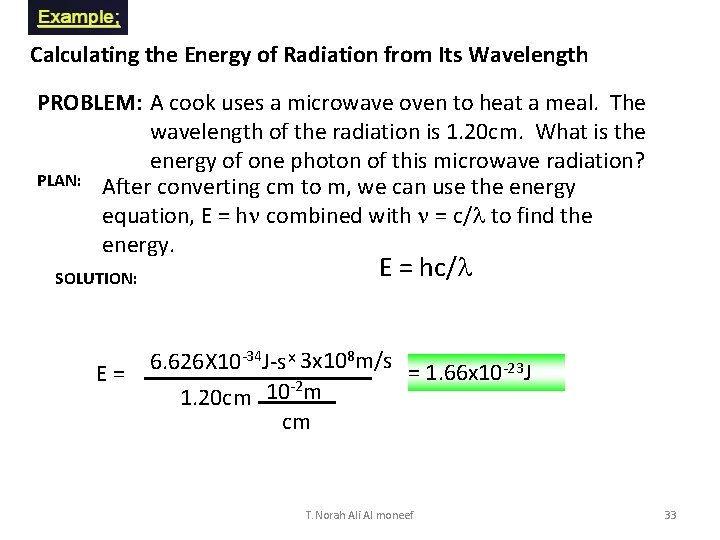
Calculating the Energy of Radiation from Its Wavelength PROBLEM: A cook uses a microwave oven to heat a meal. The wavelength of the radiation is 1. 20 cm. What is the energy of one photon of this microwave radiation? PLAN: After converting cm to m, we can use the energy equation, E = hn combined with n = c/l to find the energy. SOLUTION: E = hc/l 6. 626 X 10 -34 J-s x 3 x 108 m/s = 1. 66 x 10 -23 J -2 m 10 1. 20 cm cm T. Norah Ali Al moneef 33

CQ: A photon at 300 nm will kick out an electron with an amount of kinetic energy, KE 300. If the wavelength is halved to 150 nm and the photon hits an electron in the metal with same energy as the previous electron, the energy of the electron coming out is a. less than ½ KE 300. b. ½ KE 300 c. = KE 300 d. 2 x KE 300 e. more than 2 x KE 300 KE = photon energy-energy to get out = hf – energy to get out if l is ½ then, f twice as big, Ephot =2 hf 300 New KEnew= 2 hf 300 - energy to get out Old KE 300 =hf 300 - energy to get out so KE new is more than twice as big. T. Norah Ali Al moneef 34

CQ: Shine in light of 300 nm, most energetic electrons come out with kinetic energy, KE 300. A voltage diff of 1. 8 V is required to stop these electrons. What is the work function for this plate? (e. g. the minimum amount of energy needed to kick e out of metal? ) a. 1. 2 e. V b. 2. 9 e. V c. 6. 4 e. V d. 11. 3 e. V e. none Energy is conserved so: Ephot= energy need to exit (w) + electron’s left over energy so w= Ephot – electron’s energy When electron stops, all of initial KE has been converted to electrostatic potential energy: electron energy = q. DV = e x 1. 8 V = 1. 8 e. V, and Ephot = 1240 e. V nm/300 nm = 4. 1 e. V. So w = 4. 1 e. V - 1. 8 e. V = 2. 3 e. V T. Norah Ali Al moneef 35

T. Norah Ali Al moneef 36

T. Norah Ali Al moneef 37

T. Norah Ali Al moneef 38

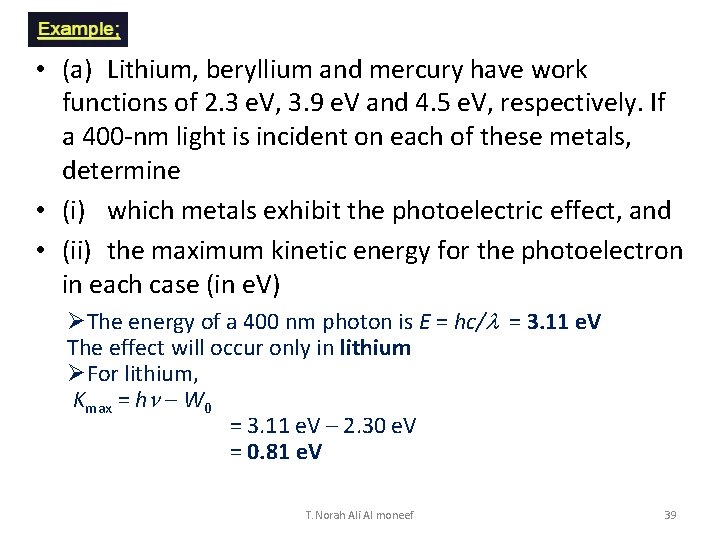
• (a) Lithium, beryllium and mercury have work functions of 2. 3 e. V, 3. 9 e. V and 4. 5 e. V, respectively. If a 400 -nm light is incident on each of these metals, determine • (i) which metals exhibit the photoelectric effect, and • (ii) the maximum kinetic energy for the photoelectron in each case (in e. V) ØThe energy of a 400 nm photon is E = hc/l = 3. 11 e. V The effect will occur only in lithium ØFor lithium, Kmax = hn – W 0 = 3. 11 e. V – 2. 30 e. V = 0. 81 e. V T. Norah Ali Al moneef 39

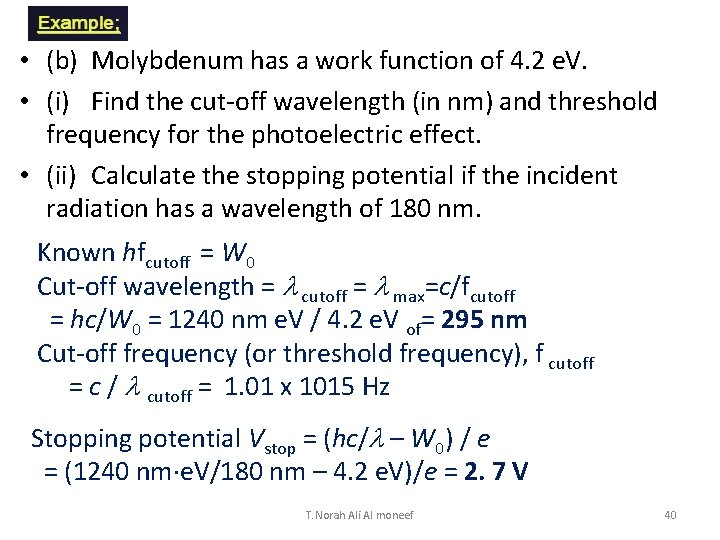
• (b) Molybdenum has a work function of 4. 2 e. V. • (i) Find the cut-off wavelength (in nm) and threshold frequency for the photoelectric effect. • (ii) Calculate the stopping potential if the incident radiation has a wavelength of 180 nm. Known hfcutoff = W 0 Cut-off wavelength = l cutoff = l max=c/fcutoff = hc/W 0 = 1240 nm e. V / 4. 2 e. V of= 295 nm Cut-off frequency (or threshold frequency), f cutoff = c / l cutoff = 1. 01 x 1015 Hz Stopping potential Vstop = (hc/l – W 0) / e = (1240 nm e. V/180 nm – 4. 2 e. V)/e = 2. 7 V T. Norah Ali Al moneef 40

• Light of wavelength 400 nm is incident upon lithium (W 0 = 2. 9 e. V). Calculate • (a) the photon energy and • (b) the stopping potential, Vs • (c) What frequency of light is needed to produce electrons of kinetic energy 3 e. V from illumination of lithium? • (a) E= hn = hc/l = 1240 e. V·nm/400 nm = 3. 1 e. V • (b) The stopping potential x e = Max Kinetic energy of the photon • => e. Vs = Kmax = hf - W 0 = (3. 1 - 2. 9) e. V • Hence, Vs = 0. 2 V • a retarding potential of 0. 2 V will stop all photoelectrons • (c) hf = Kmax + W 0 = 3 e. V + 2. 9 e. V = 5. 9 e. V. Hence the frequency of the photon is f= 5. 9 x (1. 6 x 10 -19 J) / 6. 63 x 10 -34 Js = 1. 42 x 1015 Hz T. Norah Ali Al moneef 41

Which of the following statement(s) is (are) true? I-The energy of the quantum of light is proportional to the frequency of the wave model of light II-In photoelectricity, the photoelectrons has as much energy as the quantum of light which causes it to be ejected III-In photoelectricity, no time delay in the emission of photoelectrons would be expected in the quantum theory A. II, III B. I, III C. I, III D. I ONLY E. Non of the above Ans: B T. Norah Ali Al moneef 42

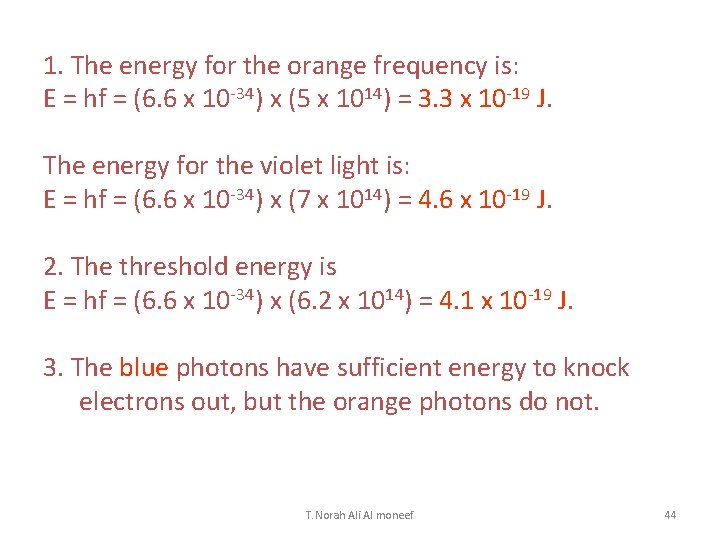
Light made of two colors (two frequencies) shines on a metal surface whose photoelectric threshold frequency is 6. 2 x 1014 Hz. The two frequencies are 5 x 1014 Hz (orange) 7 x 1014 Hz (violet). (1) Find the energies of the orange and violet photons. (2) Find the amount of energy a photon needs to knock electrons out of this surface. (3) Do either the orange photons or the violet photons have this much energy? T. Norah Ali Al moneef 43

1. The energy for the orange frequency is: E = hf = (6. 6 x 10 -34) x (5 x 1014) = 3. 3 x 10 -19 J. The energy for the violet light is: E = hf = (6. 6 x 10 -34) x (7 x 1014) = 4. 6 x 10 -19 J. 2. The threshold energy is E = hf = (6. 6 x 10 -34) x (6. 2 x 1014) = 4. 1 x 10 -19 J. 3. The blue photons have sufficient energy to knock electrons out, but the orange photons do not. T. Norah Ali Al moneef 44

T. Norah Ali Al moneef 45

T. Norah Ali Al moneef 46

T. Norah Ali Al moneef 47

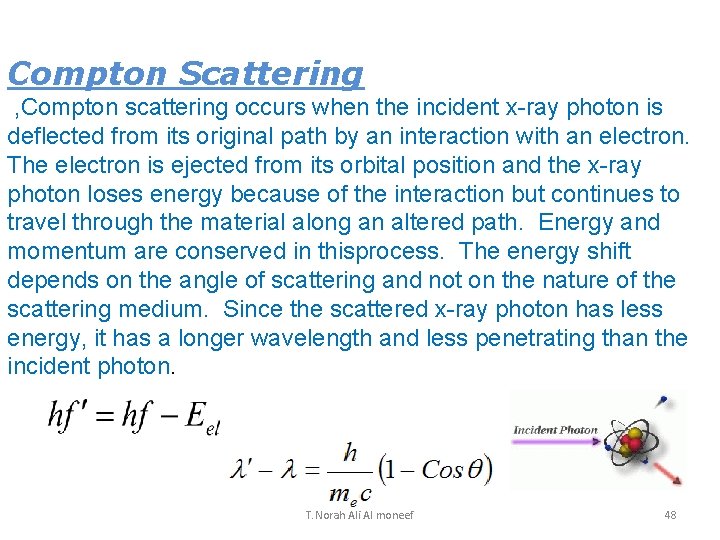
Compton Scattering , Compton scattering occurs when the incident x-ray photon is deflected from its original path by an interaction with an electron. The electron is ejected from its orbital position and the x-ray photon loses energy because of the interaction but continues to travel through the material along an altered path. Energy and momentum are conserved in thisprocess. The energy shift depends on the angle of scattering and not on the nature of the scattering medium. Since the scattered x-ray photon has less energy, it has a longer wavelength and less penetrating than the incident photon. T. Norah Ali Al moneef 48

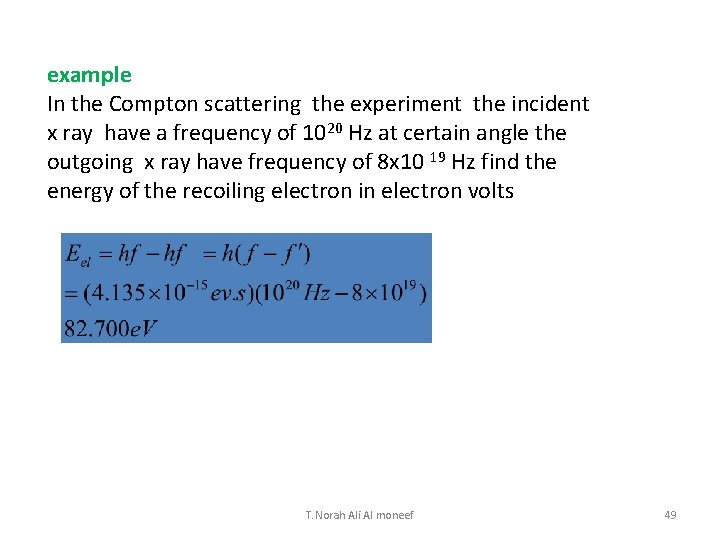
example In the Compton scattering the experiment the incident x ray have a frequency of 1020 Hz at certain angle the outgoing x ray have frequency of 8 x 10 19 Hz find the energy of the recoiling electron in electron volts T. Norah Ali Al moneef 49

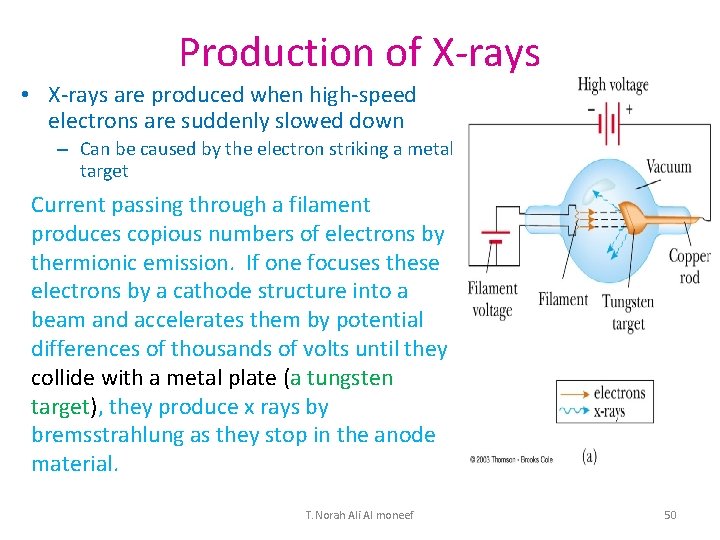
Production of X-rays • X-rays are produced when high-speed electrons are suddenly slowed down – Can be caused by the electron striking a metal target Current passing through a filament produces copious numbers of electrons by thermionic emission. If one focuses these electrons by a cathode structure into a beam and accelerates them by potential differences of thousands of volts until they collide with a metal plate (a tungsten target), they produce x rays by bremsstrahlung as they stop in the anode material. T. Norah Ali Al moneef 50

As a result, we would expect a photon of much larger energy, and hence much higher frequency, to be emitted when the atom returns to its normal state after the displacement of an inner electron. This is, in fact, the case, and it is the displacement of the inner electrons that gives rise to the emission of x-rays. In addition to the x-ray line spectrum there is a background of continuous x-ray radiation from the target of an x-ray tube. This is due to the sudden deceleration of those “cathode rays”. The remarkable feature of the continuous spectrum is that while it extends Indefinitely towards the long wavelength end, it is cut off very sharply at the short wavelength end. The quantum theory furnishes a simple explanation of the short-wave limit of the continuous xray spectrum (it is called Bremsstrahlung T. Norah Ali Al moneef 51

T. Norah Ali Al moneef 52

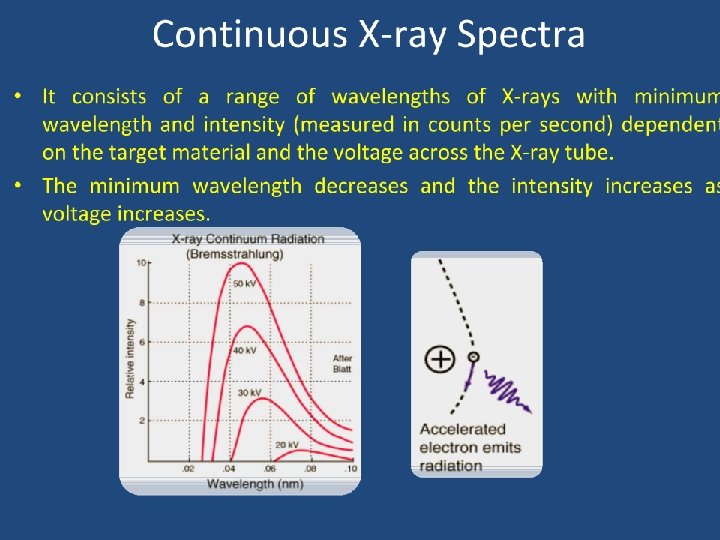
X-rays have a very small wavelength, no larger than 10 -8 to 10 -9. T. Norah Ali Al moneef 53

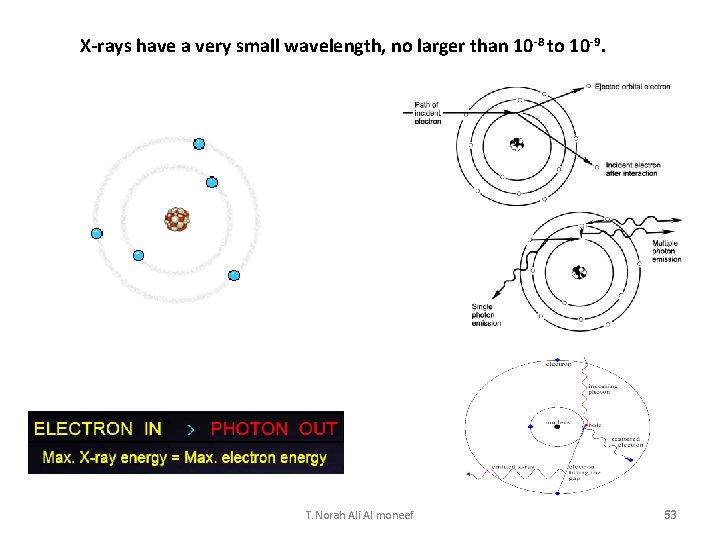
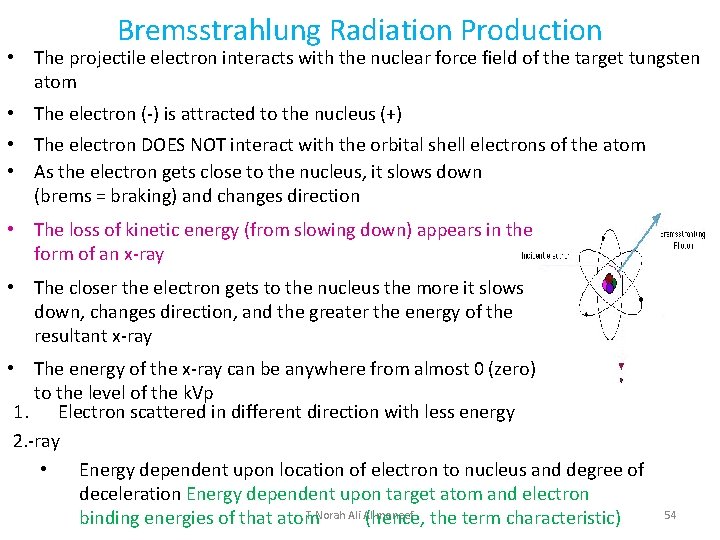
Bremsstrahlung Radiation Production • The projectile electron interacts with the nuclear force field of the target tungsten atom • The electron (-) is attracted to the nucleus (+) • The electron DOES NOT interact with the orbital shell electrons of the atom • As the electron gets close to the nucleus, it slows down (brems = braking) and changes direction • The loss of kinetic energy (from slowing down) appears in the form of an x-ray • The closer the electron gets to the nucleus the more it slows down, changes direction, and the greater the energy of the resultant x-ray • The energy of the x-ray can be anywhere from almost 0 (zero) to the level of the k. Vp 1. Electron scattered in different direction with less energy 2. -ray • Energy dependent upon location of electron to nucleus and degree of deceleration Energy dependent upon target atom and electron T. Norah Ali Al moneef binding energies of that atom (hence, the term characteristic) 54

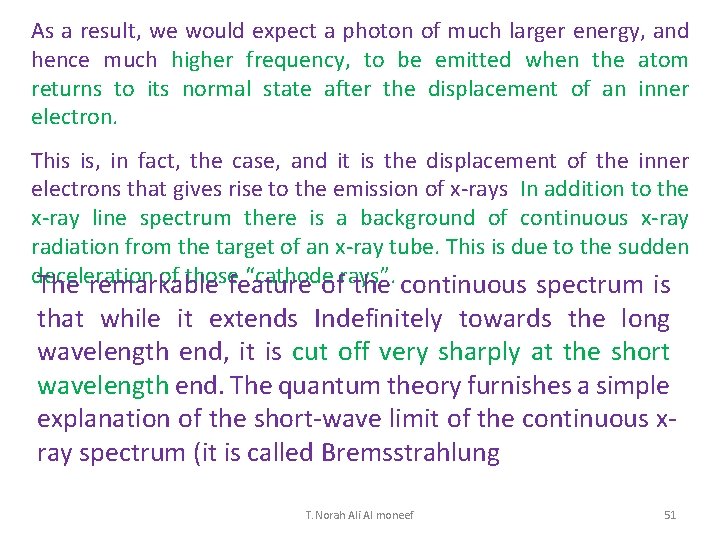
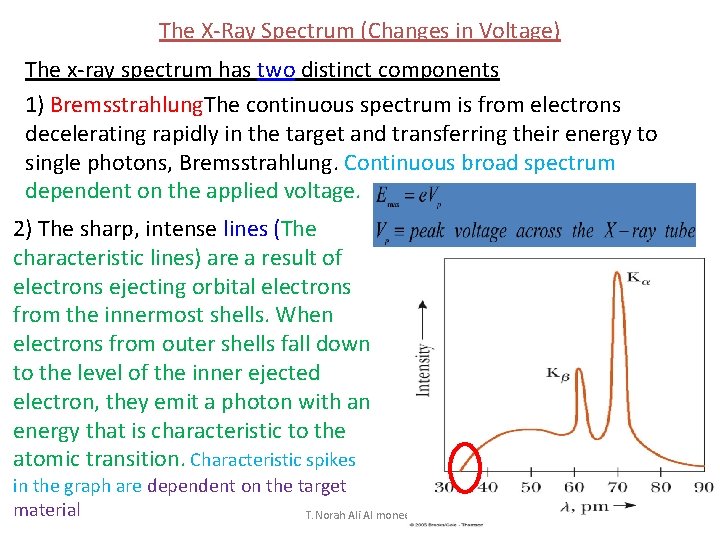
The X-Ray Spectrum (Changes in Voltage) The x-ray spectrum has two distinct components 1) Bremsstrahlung. The continuous spectrum is from electrons decelerating rapidly in the target and transferring their energy to single photons, Bremsstrahlung. Continuous broad spectrum dependent on the applied voltage. 2) The sharp, intense lines (The characteristic lines) are a result of electrons ejecting orbital electrons from the innermost shells. When electrons from outer shells fall down to the level of the inner ejected electron, they emit a photon with an energy that is characteristic to the atomic transition. Characteristic spikes in the graph are dependent on the target material T. Norah Ali Al moneef 55

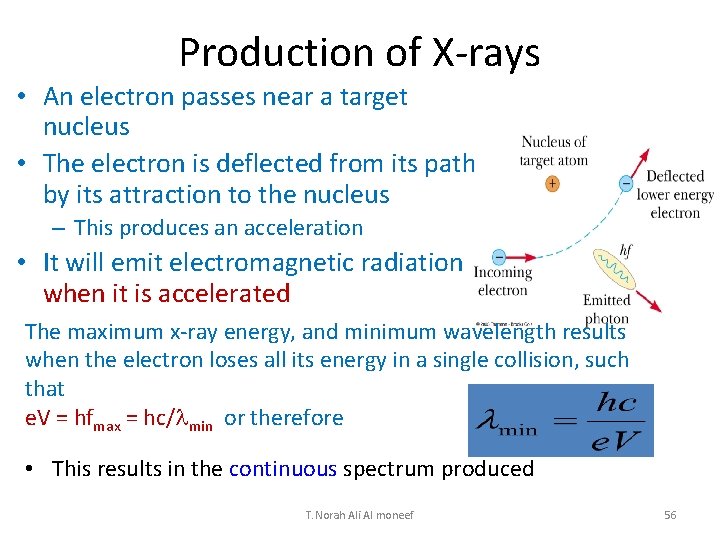
Production of X-rays • An electron passes near a target nucleus • The electron is deflected from its path by its attraction to the nucleus – This produces an acceleration • It will emit electromagnetic radiation when it is accelerated The maximum x-ray energy, and minimum wavelength results when the electron loses all its energy in a single collision, such that e. V = hfmax = hc/lmin or therefore • This results in the continuous spectrum produced T. Norah Ali Al moneef 56

An electron is accelerated through 50, 000 volts What is the minimum wavelength photon it can produce when striking a target? q Compute the energy of the 1. 5 [nm] X-ray photon. E = hc/l = (6. 6 x 10 -34 [J s])(3 x 108 [m/s]) / (1. 5 x 10 -9 [m]) = 1. 3 x 10 -16 [J] T. Norah Ali Al moneef 57

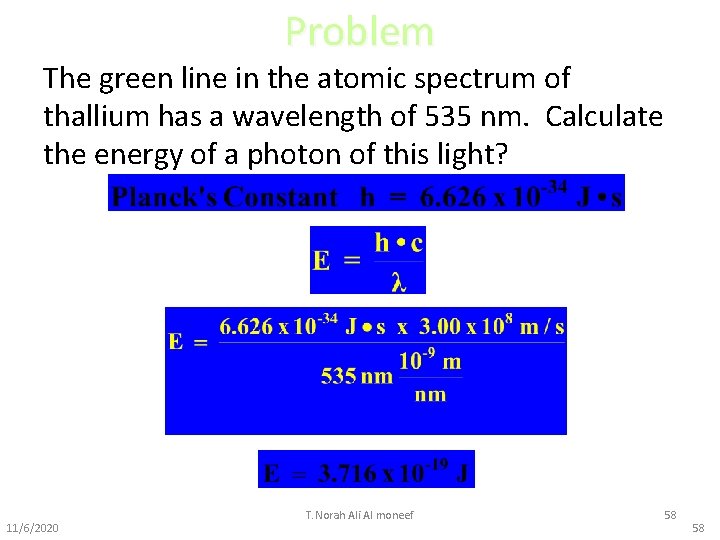
Problem The green line in the atomic spectrum of thallium has a wavelength of 535 nm. Calculate the energy of a photon of this light? 11/6/2020 T. Norah Ali Al moneef 58 58

T. Norah Ali Al moneef 59