photons Physics 100 Chapt 21 Photoelectric effect cathode












- Slides: 34

photons Physics 100 Chapt 21

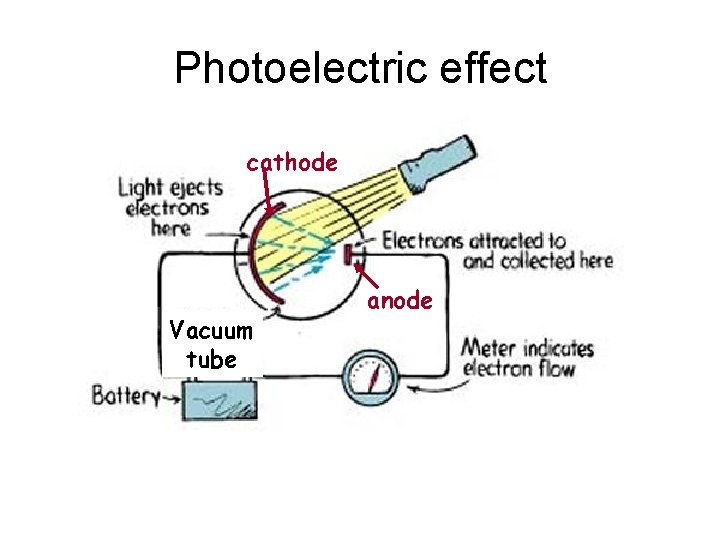
Photoelectric effect cathode Vacuum tube anode

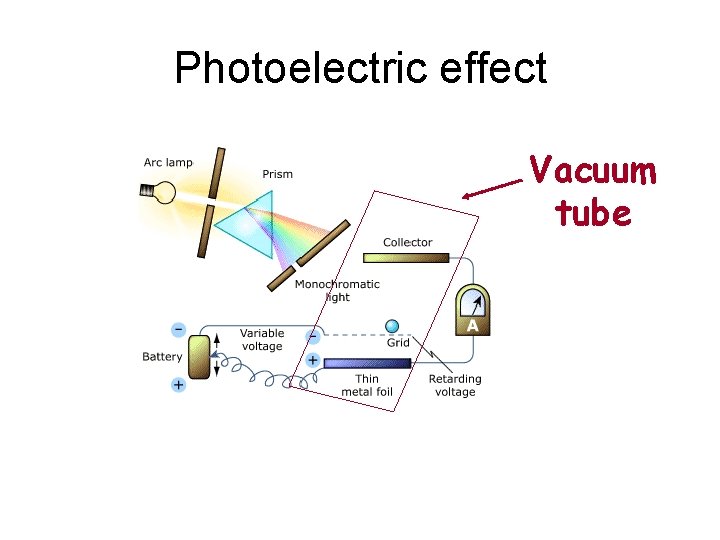
Photoelectric effect Vacuum tube

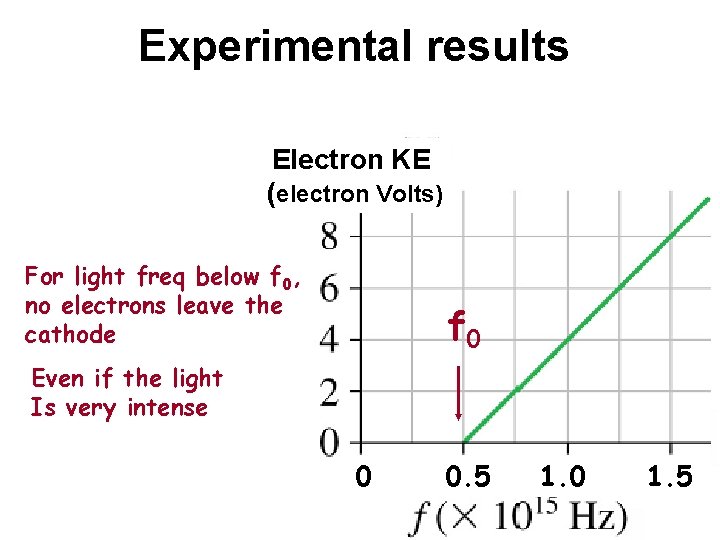
Experimental results Electron KE (electron Volts) For light freq below f 0, no electrons leave the cathode f 0 Even if the light Is very intense 0 0. 5 1. 0 1. 5

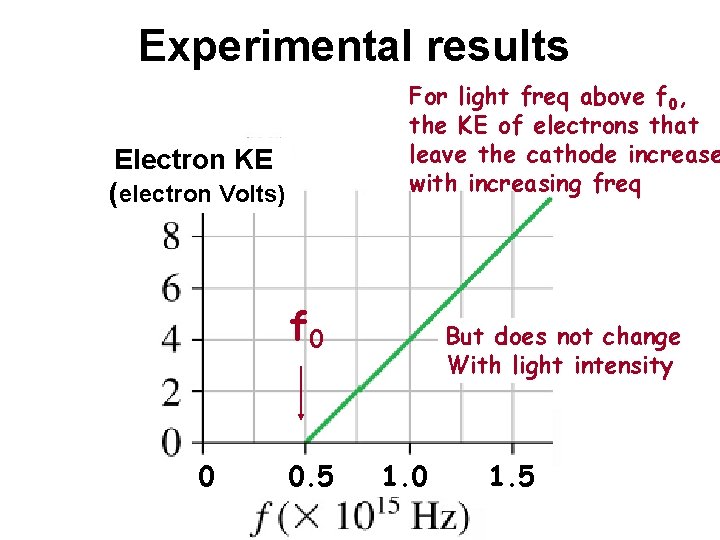
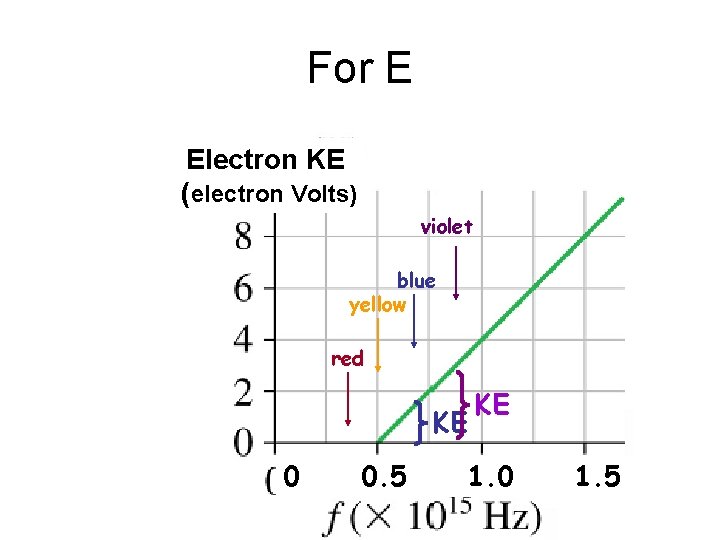
Experimental results For light freq above f 0, the KE of electrons that leave the cathode increase with increasing freq Electron KE (electron Volts) f 0 0 0. 5 But does not change With light intensity 1. 0 1. 5

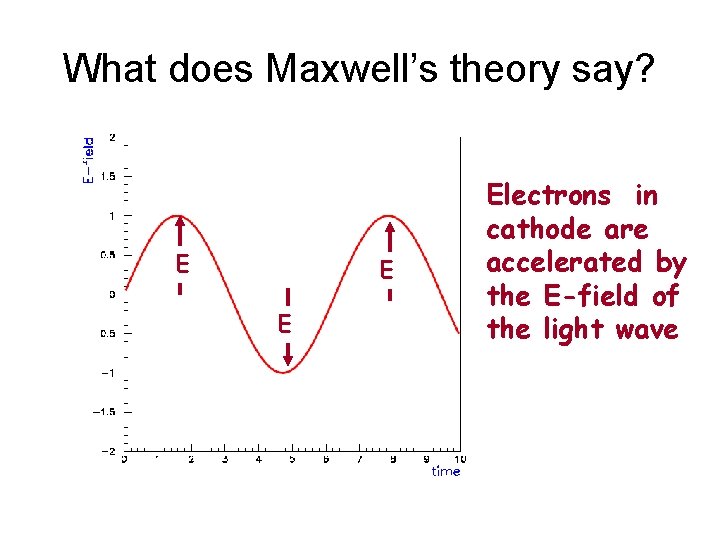
What does Maxwell’s theory say? E Electrons in cathode are accelerated by the E-field of the light wave

More intense light has bigger E-fields E E E And, therefore Larger acceleration

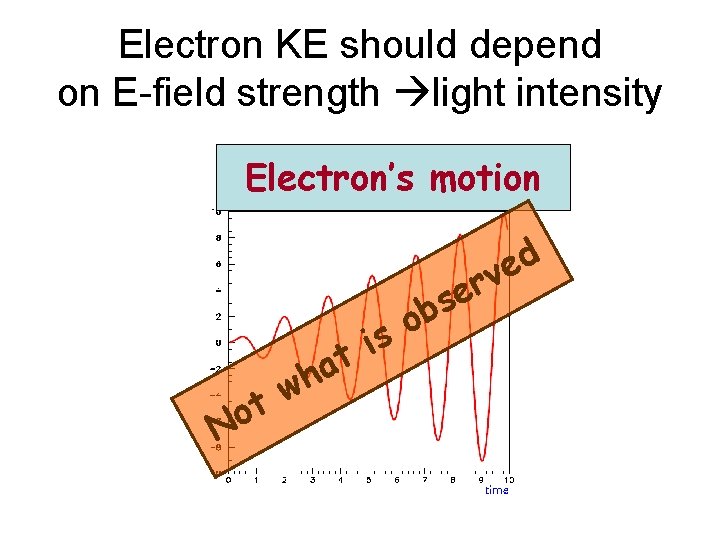
Electron KE should depend on E-field strength light intensity Electron’s motion e s b d e rv t o N o s i t a wh

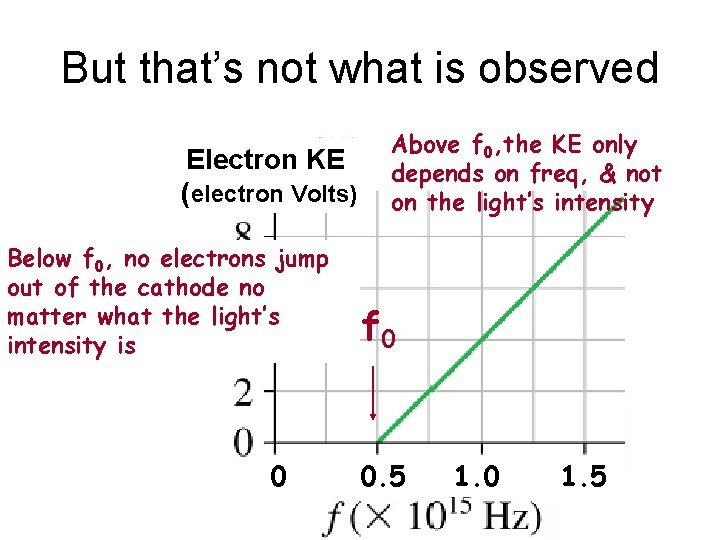
But that’s not what is observed Electron KE (electron Volts) Below f 0, no electrons jump out of the cathode no matter what the light’s intensity is 0 Above f 0, the KE only depends on freq, & not on the light’s intensity f 0 0. 5 1. 0 1. 5

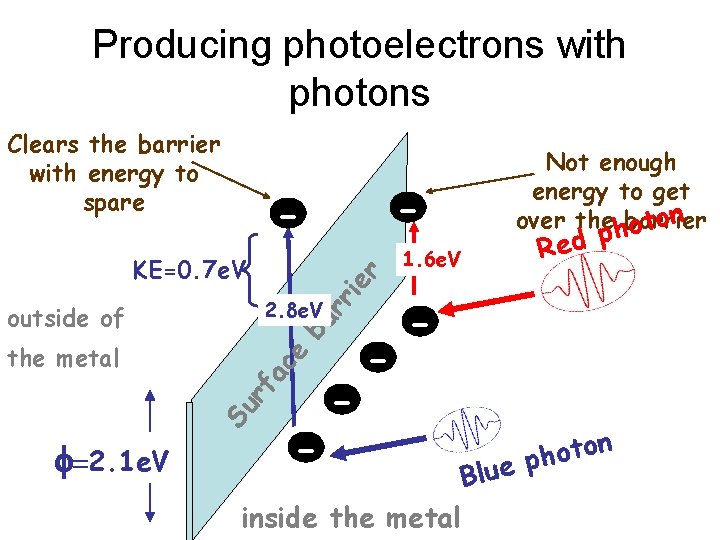
Einstein’s explanation Light is comprised of particle-like quanta each with energy Equant = hf The quanta collide with electrons & Transfer all their energy to them Each electron needs a minimum energy to escape the cathode. This is called f If Equant is less than f, the electron can’t escape If Equant is greater than f, the electron escapes & the quantum energy in excess of f becomes electron KE KEelectron = hf - f f

Light quanta “photons” Einstein’s light quanta were given the name “photons” by Arthur Compton

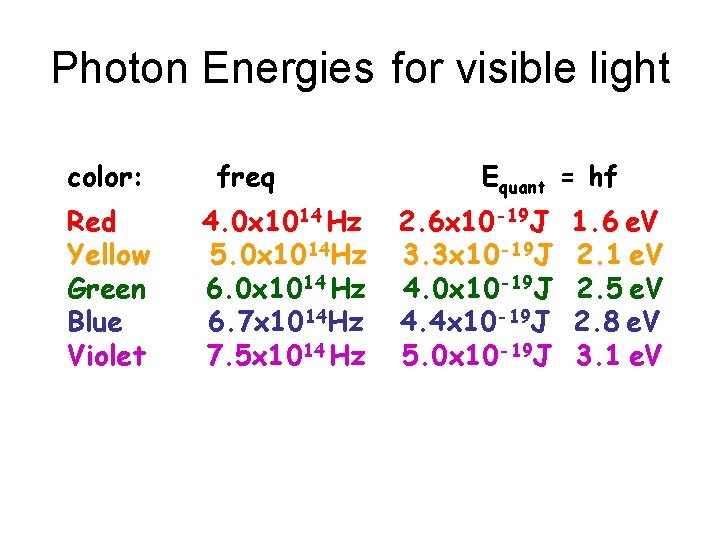
Photon Energy for red light Red light: f = 4. 0 x 1014 Hz Ephoton = hf = (6. 6 x 10 -34 Js) x (4. 0 x 1014 Hz) = (6. 6 x 4. 0)x 10 -34+14 J = 2. 6 x 10 -19 J = 2. 6 e. V 1. 6 x = 26 x 10 -20 J 1 e. V 1. 6 x 10 -19 J =1. 6 e. V

Photon Energies for visible light color: Red Yellow Green Blue Violet freq 4. 0 x 1014 Hz 5. 0 x 1014 Hz 6. 0 x 1014 Hz 6. 7 x 1014 Hz 7. 5 x 1014 Hz Equant = hf 2. 6 x 10 -19 J 3. 3 x 10 -19 J 4. 0 x 10 -19 J 4. 4 x 10 -19 J 5. 0 x 10 -19 J 1. 6 e. V 2. 1 e. V 2. 5 e. V 2. 8 e. V 3. 1 e. V

Producing photoelectrons with photons Clears the barrier with energy to spare rr ie r KE=0. 7 e. V 2. 8 e. V Su rf a ce ba outside of the metal f=2. 1 e. V - - - 1. 6 e. V Not enough energy to get over thehbarrier oton p d e R - inside the metal oto h p Blue n

For E Electron KE (electron Volts) violet blue yellow red KE 0 0. 5 KE 1. 0 1. 5

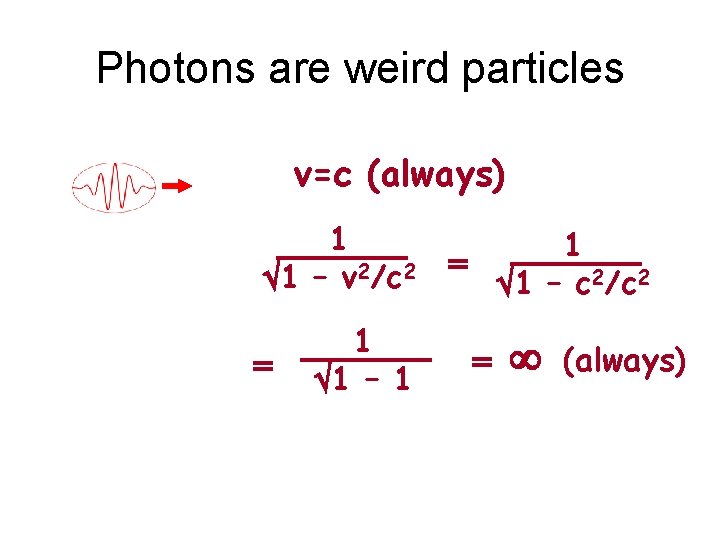
Photons are weird particles v=c (always) 1 1 – v 2/c 2 = 1 1 – 1 1 = 1 – c 2/c 2 = (always)

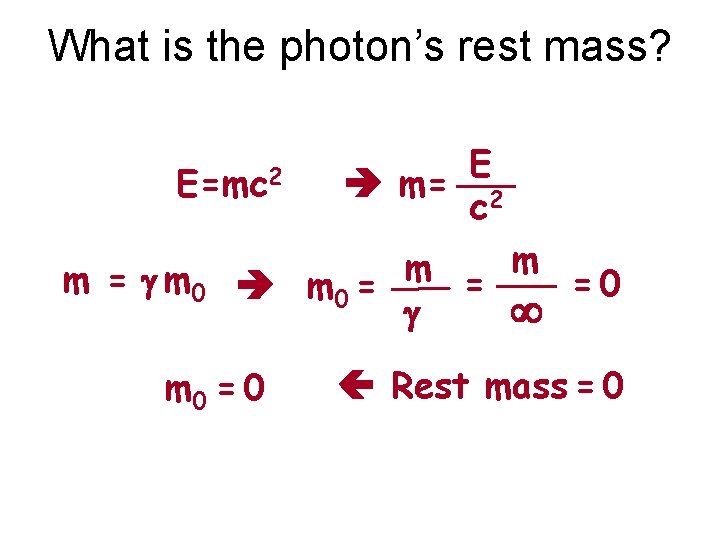
What is the photon’s rest mass? E=mc 2 m = g m 0 E m= 2 c m m = =0 m 0 = g m 0 = 0 Rest mass = 0

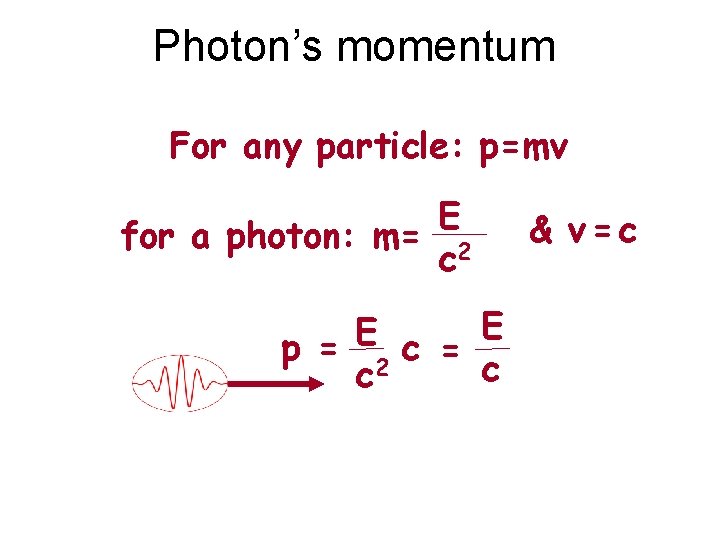
Photon’s momentum For any particle: p=mv E for a photon: m= 2 c E E p = 2 c = c c & v=c

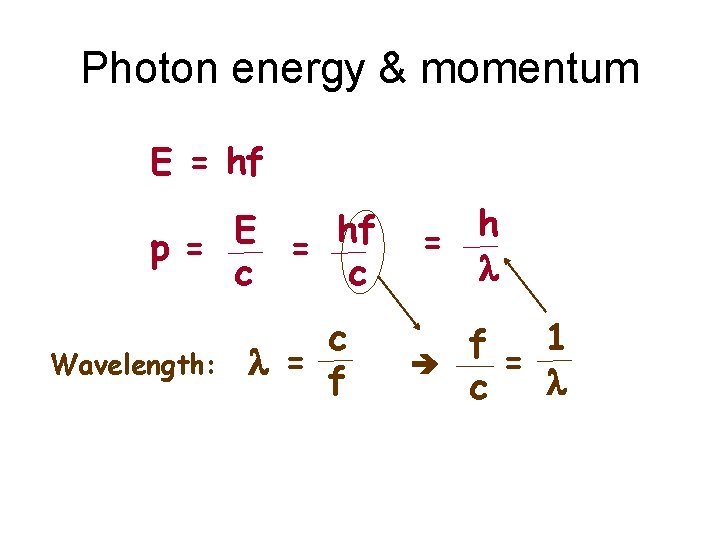
Photon energy & momentum E = hf E hf p= = c c Wavelength: c l = f h = l f= 1 l c

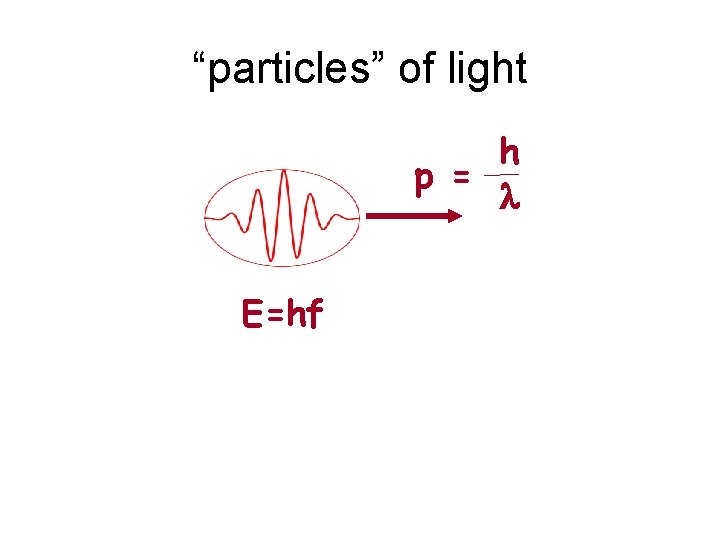
“particles” of light h p = l E=hf

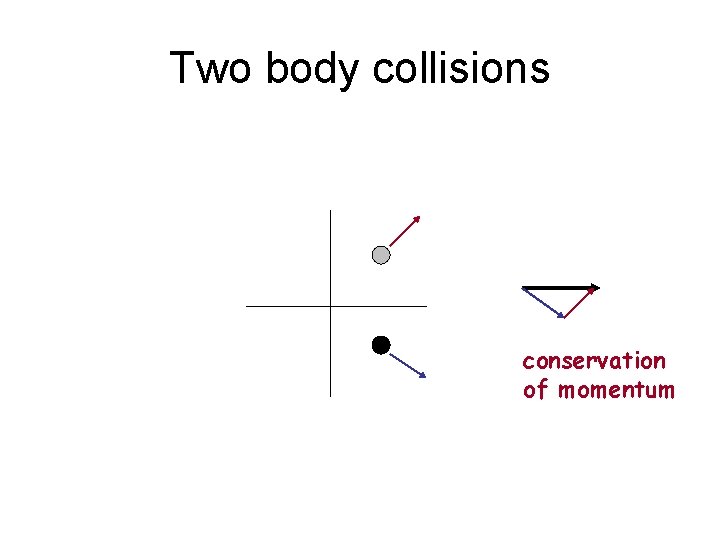
Two body collisions conservation of momentum

Compton scattering Scatter X-rays from electrons p=h/li - p= h/ Recoil electron & scattered photon conserve momentum l f

Compton’s expt proved the existence of photons & won him the 1927 Nobel Prize (Physics)

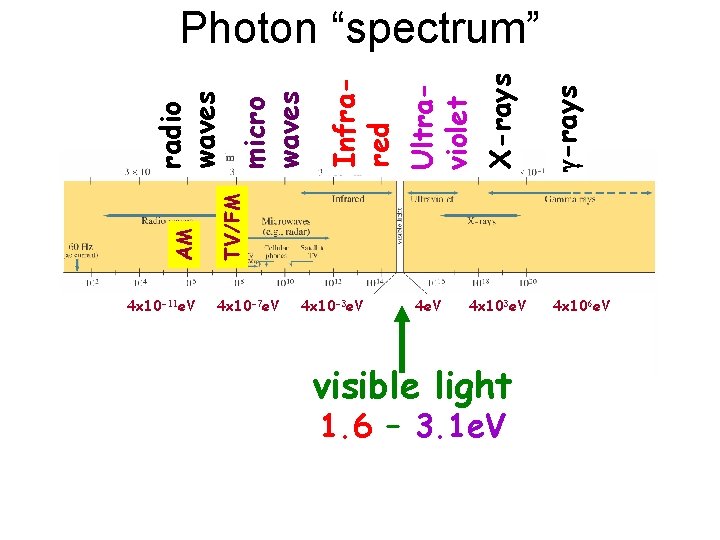
4 x 10 -11 e. V g-rays X-rays Ultraviolet Infrared micro waves TV/FM AM radio waves Photon “spectrum” 4 x 10 -7 e. V 4 x 10 -3 e. V 4 x 103 e. V visible light 1. 6 – 3. 1 e. V 4 x 106 e. V

Wave? Particles? ? Physics 100 Chapt 22

Maxwell E B James Clerk Maxwell Light is a wave of oscillating E- and B-fields

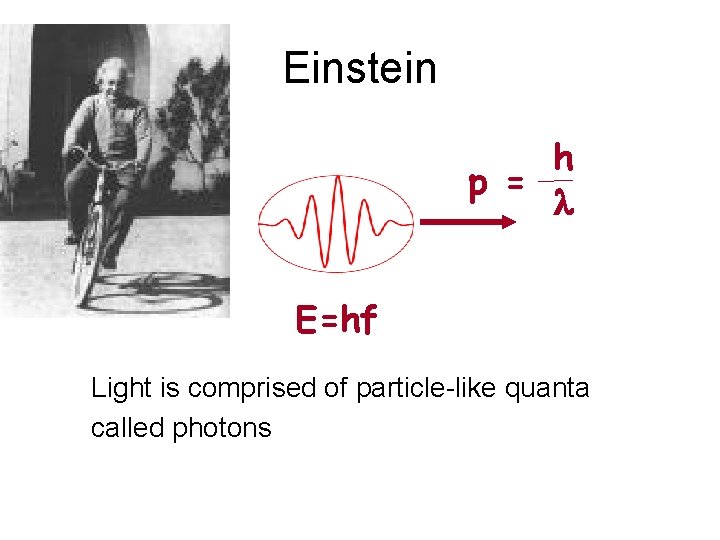
Einstein h p = l E=hf Light is comprised of particle-like quanta called photons

Who’s right? ? Waves explain diffraction & interference Photons explain photoelectric effect & Compton scattering

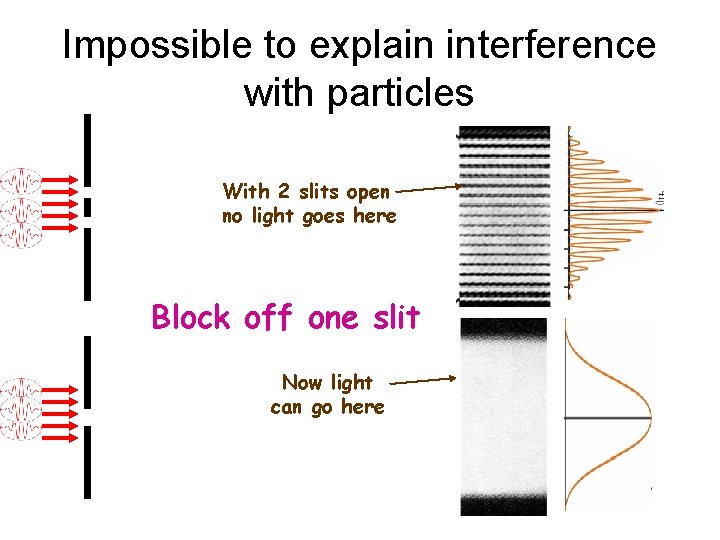
Impossible to explain interference with particles With 2 slits open no light goes here Block off one slit Now light can go here

Impossible to explain PE-effect and Compton scattering with waves Electron KE (electron Volts) yell ow violet blue red 0. 5 1. 0 1. 5

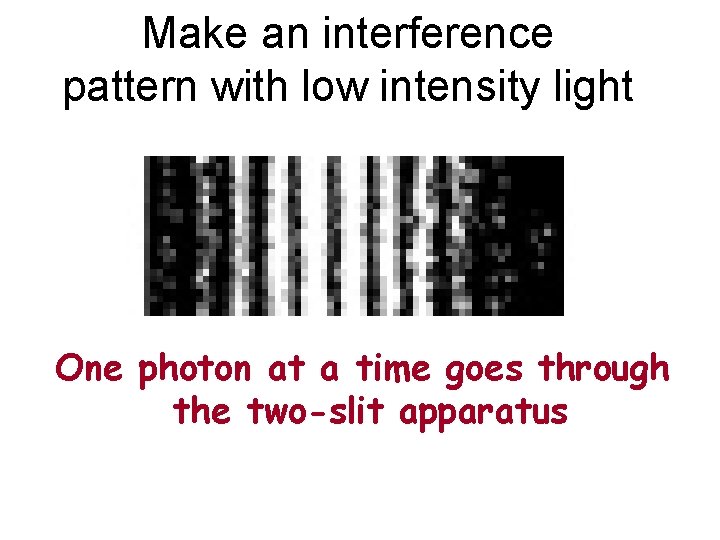
Make an interference pattern with low intensity light One photon at a time goes through the two-slit apparatus

-Light behaves like a wave when it propagates through space -And as a particle when it interacts with matter

Photon photography

Photoelectric effect Vacuum tube