CH 221 Computer Simulation Activity Photoelectric Effect Physics






- Slides: 48

CH 221 Computer Simulation Activity Photoelectric Effect – Physics • Electromagnetic Spectrum • n = c/l

Practical applications of the photoelectric effect • solar cells • smoke detectors • security systems

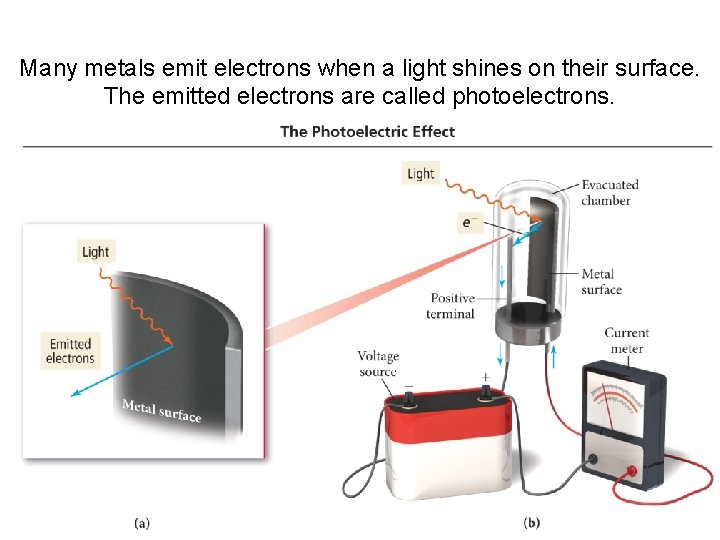
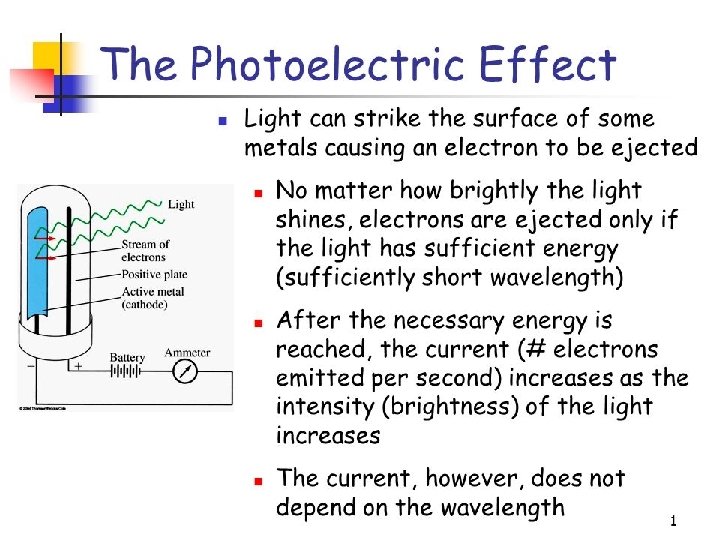
Many metals emit electrons when a light shines on their surface. The emitted electrons are called photoelectrons.

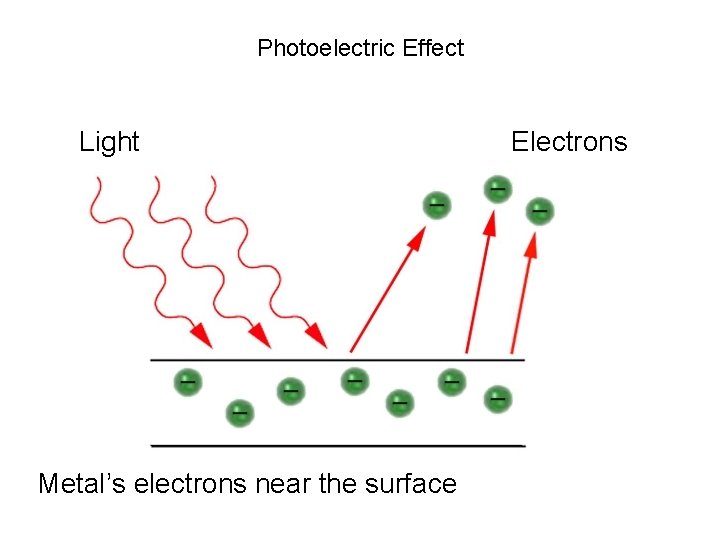
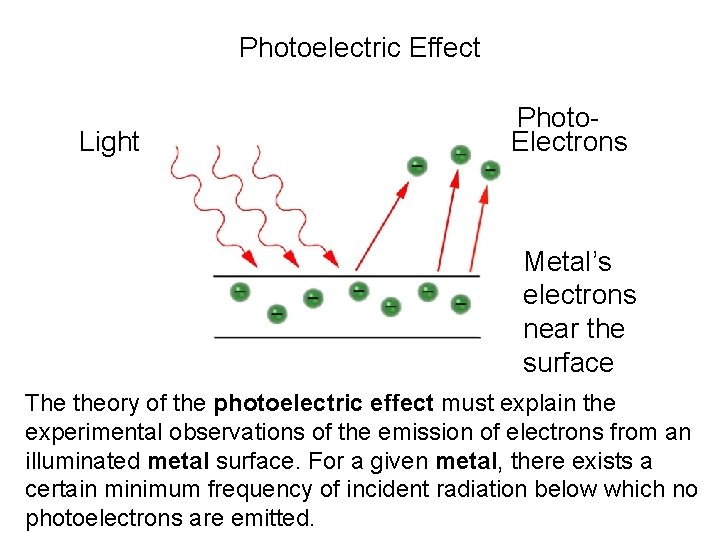
Photoelectric Effect Light Metal’s electrons near the surface Electrons

Photoelectric effect: Many metals emit electrons when a light shines on their surface. http: //www. youtube. com/watch? v=j. At 4 Liq 3 bgc What to look for during the experiment if light has wave behavior: 1. when the wavelength of light is made shorter, more electrons should be ejected. Why? 2. when the light wave’s intensity made brighter, more electrons should be ejected. Why? 3. when the light is dim, there should be a lag time in the ejection of electrons. Why?

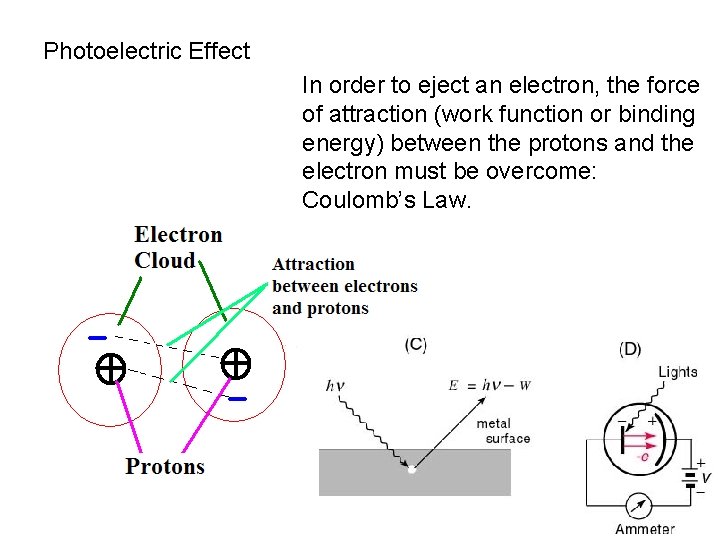
Photoelectric Effect In order to eject an electron, the force of attraction (work function or binding energy) between the protons and the electron must be overcome: Coulomb’s Law.

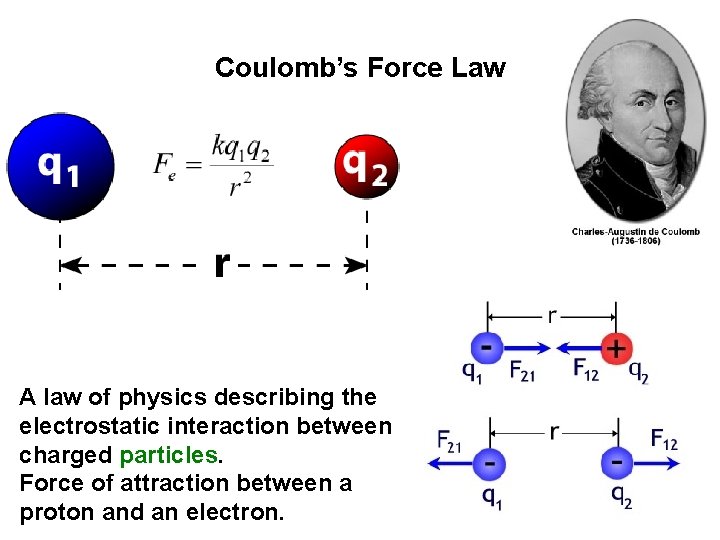
Coulomb’s Force Law A law of physics describing the electrostatic interaction between charged particles. Force of attraction between a proton and an electron.

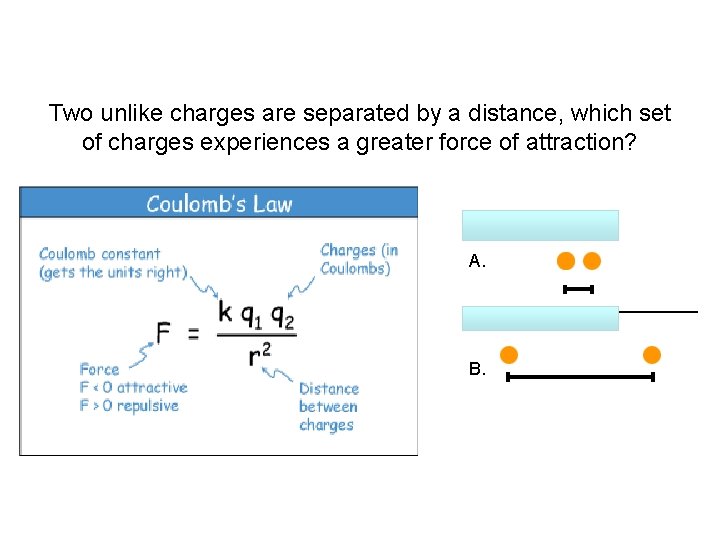
Two unlike charges are separated by a distance, which set of charges experiences a greater force of attraction? A. B.

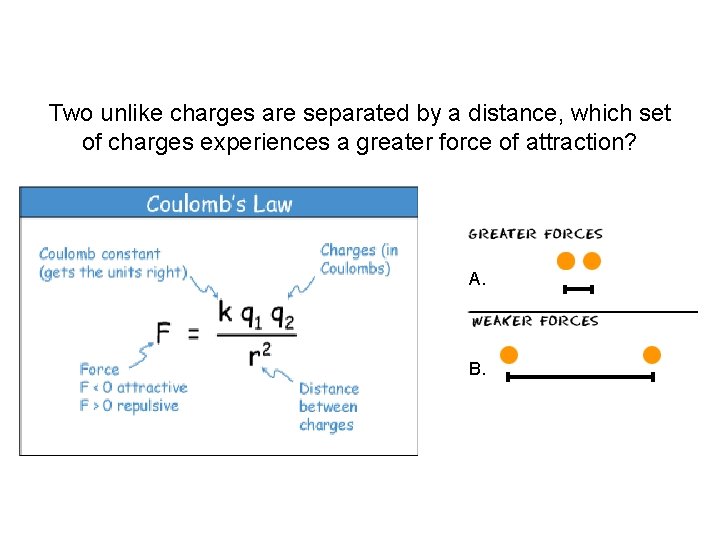
Two unlike charges are separated by a distance, which set of charges experiences a greater force of attraction? A. B.

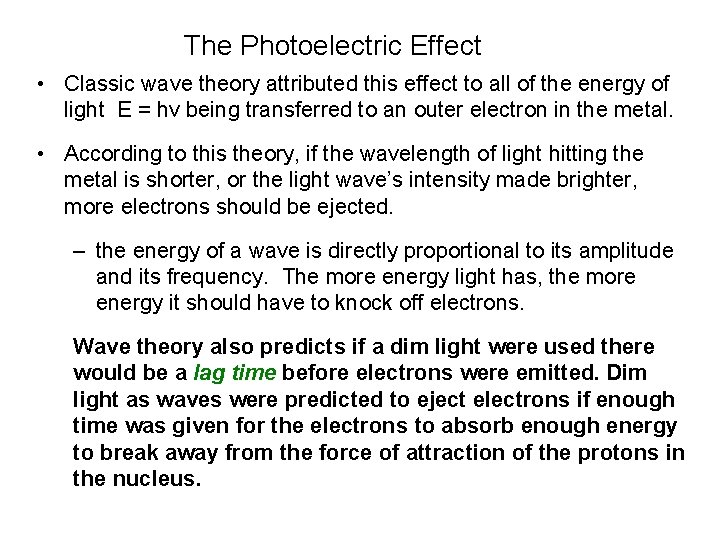
The Photoelectric Effect • Classic wave theory attributed this effect to all of the energy of light E = hv being transferred to an outer electron in the metal. • According to this theory, if the wavelength of light hitting the metal is shorter, or the light wave’s intensity made brighter, more electrons should be ejected. – the energy of a wave is directly proportional to its amplitude and its frequency. The more energy light has, the more energy it should have to knock off electrons. Wave theory also predicts if a dim light were used there would be a lag time before electrons were emitted. Dim light as waves were predicted to eject electrons if enough time was given for the electrons to absorb enough energy to break away from the force of attraction of the protons in the nucleus.

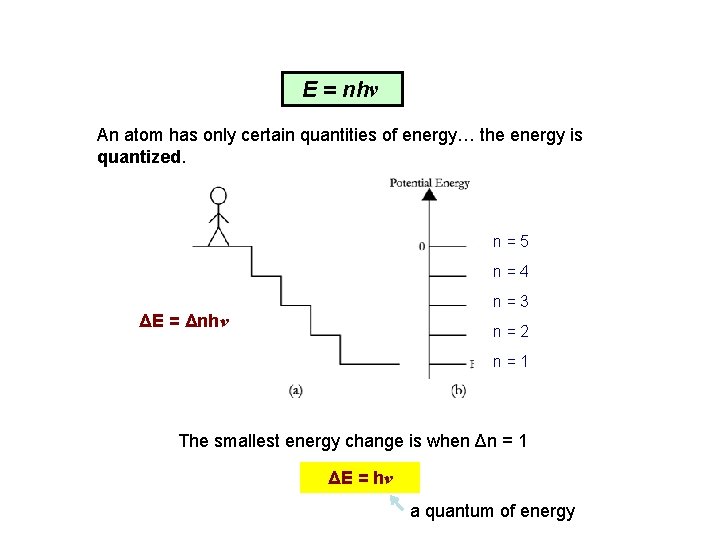
E = nhν An atom has only certain quantities of energy… the energy is quantized. n = 5 n = 4 n = 3 ΔE = Δnhν n = 2 n = 1 The smallest energy change is when Δn = 1 ΔE = hν a quantum of energy

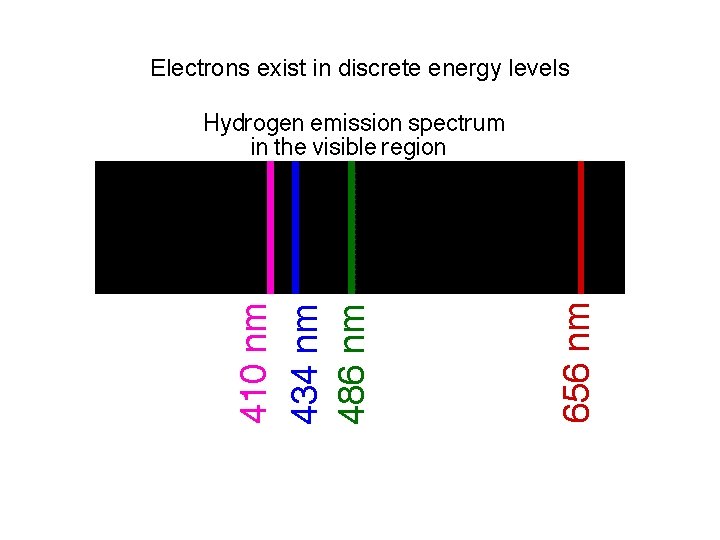
Electrons exist in discrete energy levels

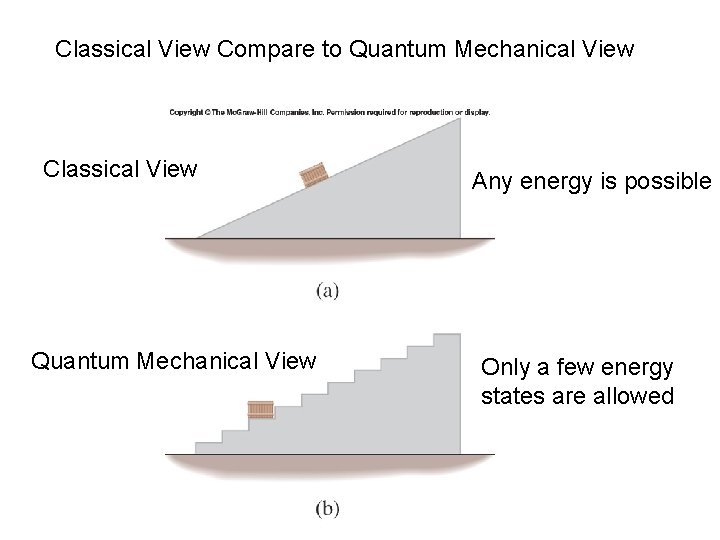
Classical View Compare to Quantum Mechanical View Classical View Quantum Mechanical View Any energy is possible Only a few energy states are allowed

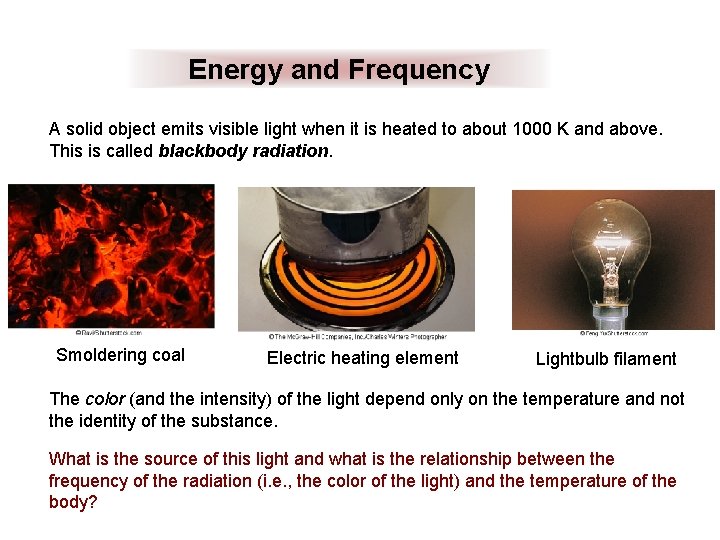
Energy and Frequency A solid object emits visible light when it is heated to about 1000 K and above. This is called blackbody radiation. Smoldering coal Electric heating element Lightbulb filament The color (and the intensity) of the light depend only on the temperature and not the identity of the substance. What is the source of this light and what is the relationship between the frequency of the radiation (i. e. , the color of the light) and the temperature of the body?

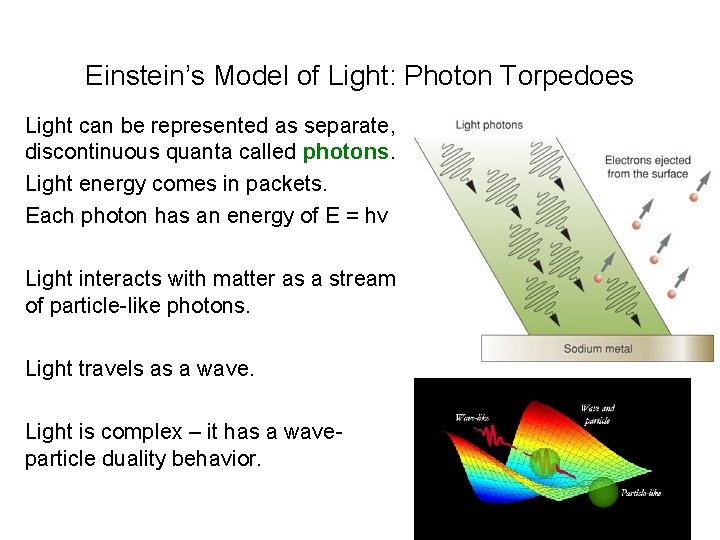
• Historically, light was thought of as a stream of particles until Young’s experiments proved light has wave-like properties. • Planck was working with the wave notion of light when he related the energy of blackbody radiation to the frequency of emitted light. E = hv • Einstein began to consider the particle viewpoint again when trying to explain the photoelectric effect. Max Planck and Albert Einstein Berlin, June 1929


Photoelectric Effect Experiment Work the Questions of the Activity Worksheet posted on the Canvas web site Download and work the computer simulation http: //phet. colorado. edu/en/simulations/category/physics

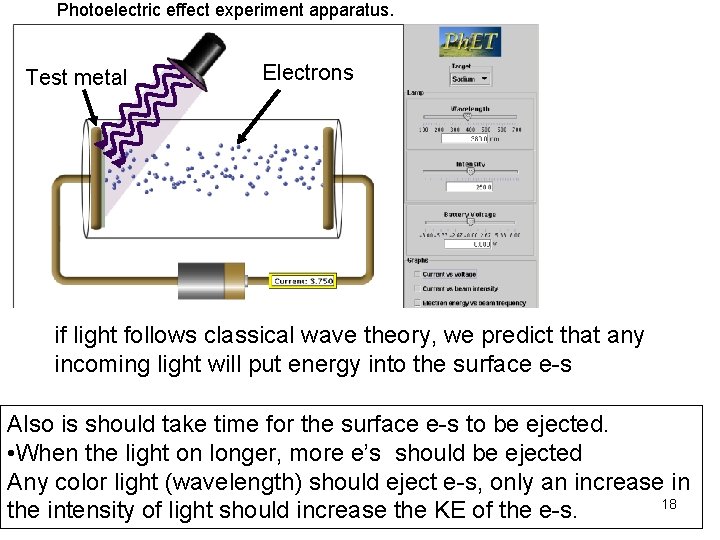
Photoelectric effect experiment apparatus. Test metal Electrons if light follows classical wave theory, we predict that any incoming light will put energy into the surface e-s Also is should take time for the surface e-s to be ejected. • When the light on longer, more e’s should be ejected Any color light (wavelength) should eject e-s, only an increase in 18 the intensity of light should increase the KE of the e-s.

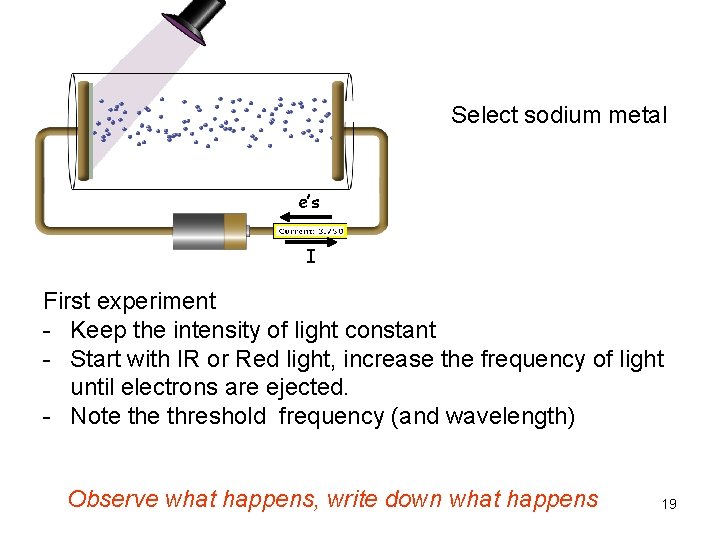
Select sodium metal e’s I First experiment - Keep the intensity of light constant - Start with IR or Red light, increase the frequency of light until electrons are ejected. - Note threshold frequency (and wavelength) Observe what happens, write down what happens 19

e’s I First experiment Part 2 - Select two different metals - Design an experiment to determine threshold frequency of each metal. - What variables will you keep constant? Observe what happens, write down what happens 20

Select sodium metal e’s I Second experiment - Select a frequency of light below the threshold frequency. - Increase the intensity of light - Select a frequency of light above threshold frequency. - Increase the intensity of light - Make note of the speed and number of ejected electrons. Observe what happens, write down what happens 21

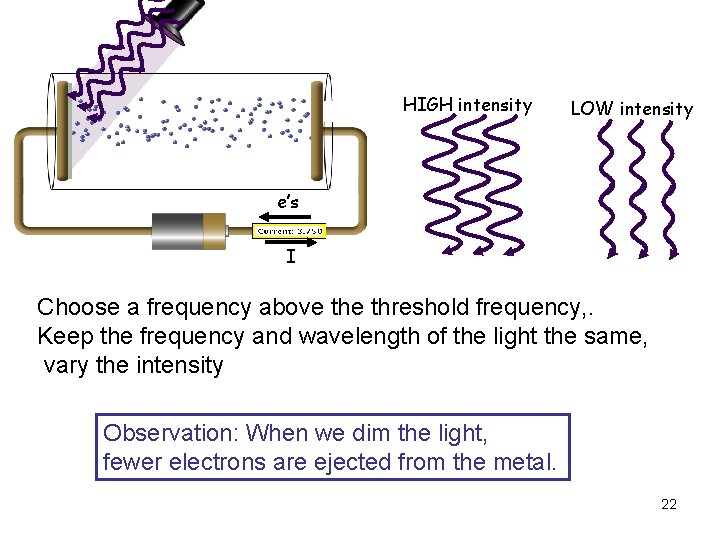
HIGH intensity LOW intensity e’s I Choose a frequency above threshold frequency, . Keep the frequency and wavelength of the light the same, vary the intensity Observation: When we dim the light, fewer electrons are ejected from the metal. 22

e’s I Third experiment - Work with the two other different metals from Experiment 2 - Determine threshold frequency of each metal Observe what happens, write down what happens 23

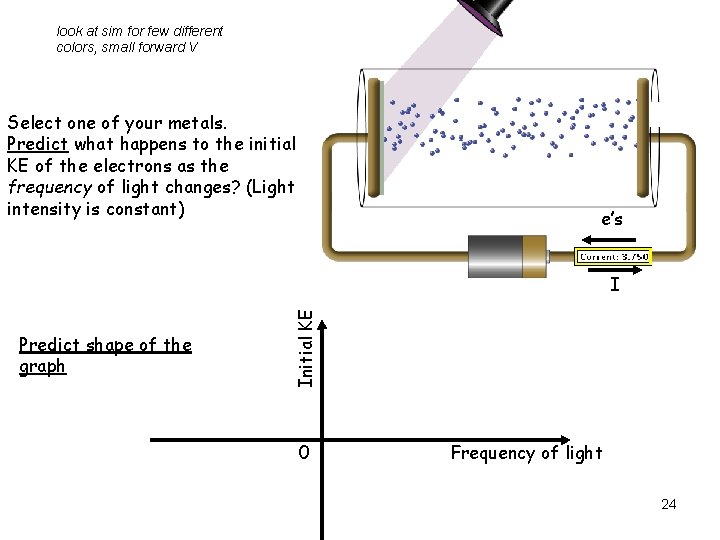
look at sim for few different colors, small forward V Select one of your metals. Predict what happens to the initial KE of the electrons as the frequency of light changes? (Light intensity is constant) e’s Predict shape of the graph Initial KE I 0 Frequency of light 24

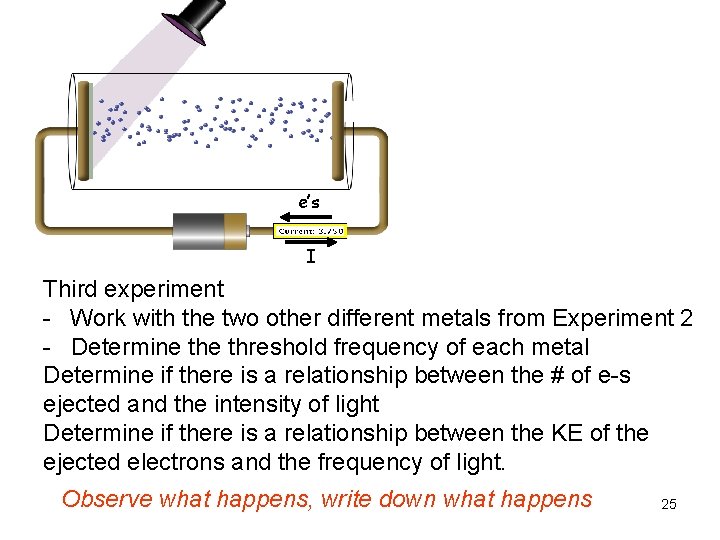
e’s I Third experiment - Work with the two other different metals from Experiment 2 - Determine threshold frequency of each metal Determine if there is a relationship between the # of e-s ejected and the intensity of light Determine if there is a relationship between the KE of the ejected electrons and the frequency of light. Observe what happens, write down what happens 25

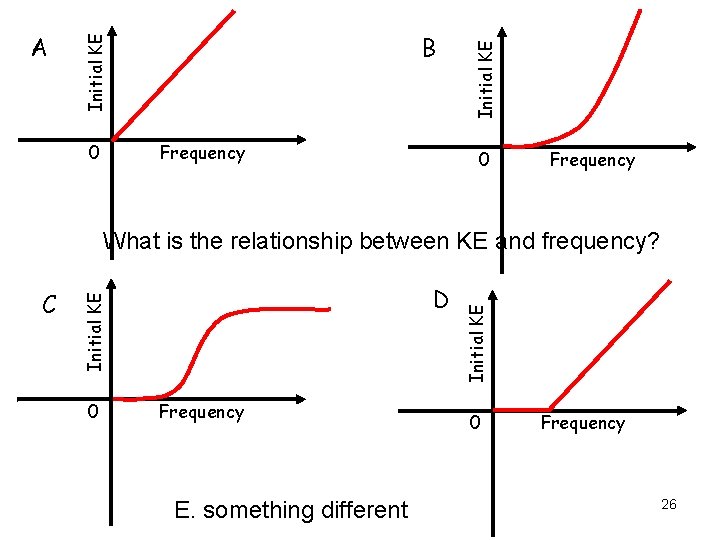
0 Frequency Initial KE B Initial KE A 0 Frequency D Initial KE C 0 Frequency E. something different Initial KE What is the relationship between KE and frequency? 0 Frequency 26

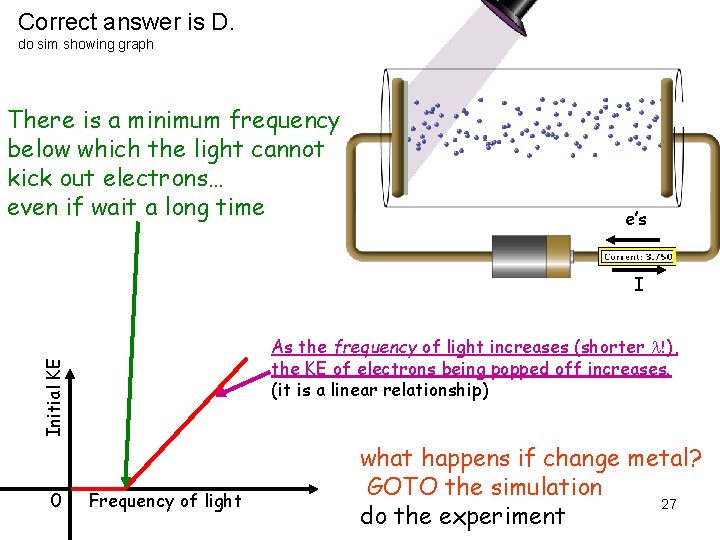
Correct answer is D. do sim showing graph There is a minimum frequency below which the light cannot kick out electrons… even if wait a long time e’s I Initial KE As the frequency of light increases (shorter l!), the KE of electrons being popped off increases. (it is a linear relationship) 0 Frequency of light what happens if change metal? GOTO the simulation 27 do the experiment

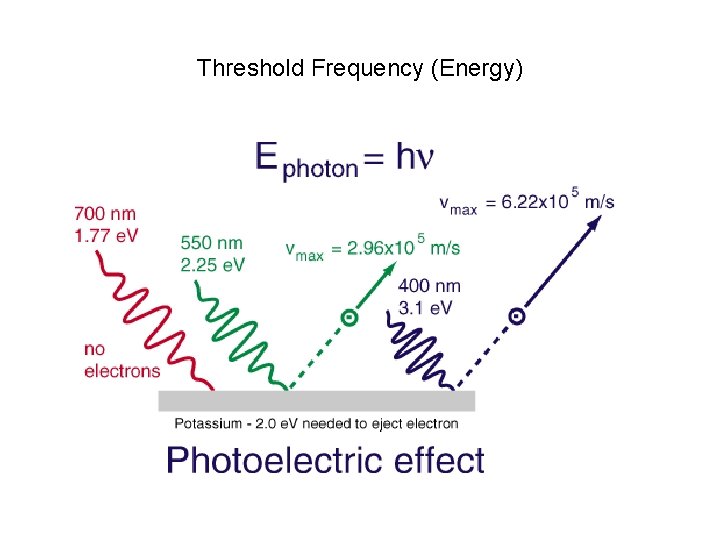
Threshold Frequency (Energy)

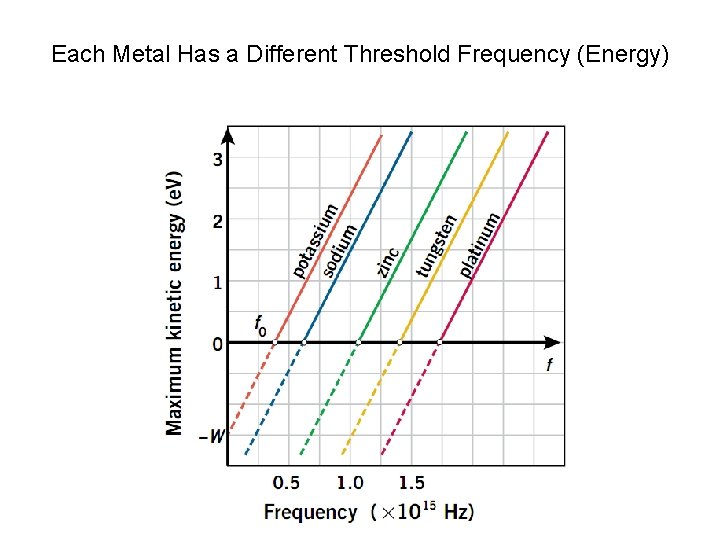
Each Metal Has a Different Threshold Frequency (Energy)

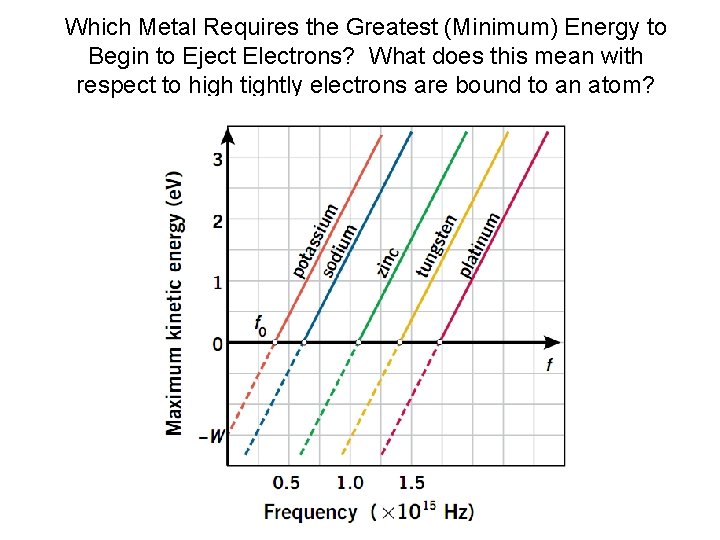
Which Metal Requires the Greatest (Minimum) Energy to Begin to Eject Electrons? What does this mean with respect to high tightly electrons are bound to an atom?

Photoelectric Effect Light Photo. Electrons Metal’s electrons near the surface The theory of the photoelectric effect must explain the experimental observations of the emission of electrons from an illuminated metal surface. For a given metal, there exists a certain minimum frequency of incident radiation below which no photoelectrons are emitted.

Summary of Photoelectric experiment results. (play with sim to check and thoroughly understand) http: //phet. colorado. edu/new/simulations/sims. php? sim=Photoelectric_Effect 1. # of e-s ejected linearly proportional to intensity of light. 2. # of e-s ejected appears with no delay. 3. Electrons only emitted if frequency of light exceeds a threshold. (same as “if wavelength short enough”). 4. Maximum energy that electrons come off with increases linearly with frequency (=1/wavelength). 5. Threshold frequency depends on the type of metal - - which is an indication of how tightly the surface e-s are held. how do these compare with classical wave predictions? 32


Classical wave predictions vs. experimental observations • Increase intensity, increase # of electrons. experiment matches • Wavelength of light does not matter, only intensity. experiment shows strong dependence on wavelength • Takes time to heat up ⇒ # of e-s coming off start low and should increase with time (energy added). experiment: with the threshold frequency electrons come off the surface immediately, no time delay to heat up 34

Summary If light can kick out electrons, then even smallest intensities of that light will continue to kick out electrons. KE of electrons does not depend on the intensity of the light of a specific wave length. (Light energy must be getting concentrated/focused somehow) 2. At lower frequencies, initial KE of the ejected e-s is low. KE of the ejected e-s changes linearly with the frequency of the incoming light. (This concentrated energy is linearly related to frequency) 3. There is a minimum frequency below which light won’t kick out electrons. (Need a certain amount of energy to free electron from metal) (Einstein) Need “photon” picture of light to explain observations: - Light comes in chunks (“particle-like”) of energy (“photon”) - a photon interacts only with single electron - Photon energy depends on frequency of light, … for lower frequencies, photon energy not enough to free an electron 35

Einstein’s Model of Light: Photon Torpedoes Light can be represented as separate, discontinuous quanta called photons. Light energy comes in packets. Each photon has an energy of E = hv Light interacts with matter as a stream of particle-like photons. Light travels as a wave. Light is complex – it has a waveparticle duality behavior.

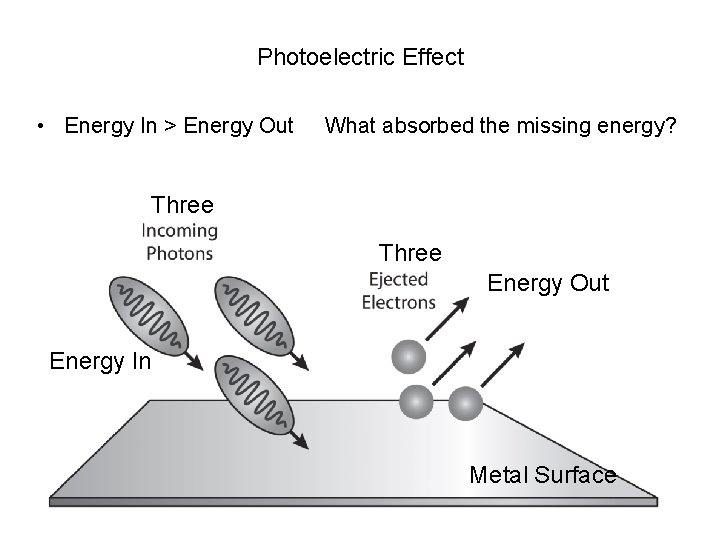
Photoelectric Effect • Energy In > Energy Out What absorbed the missing energy? Three Energy Out Energy In Metal Surface

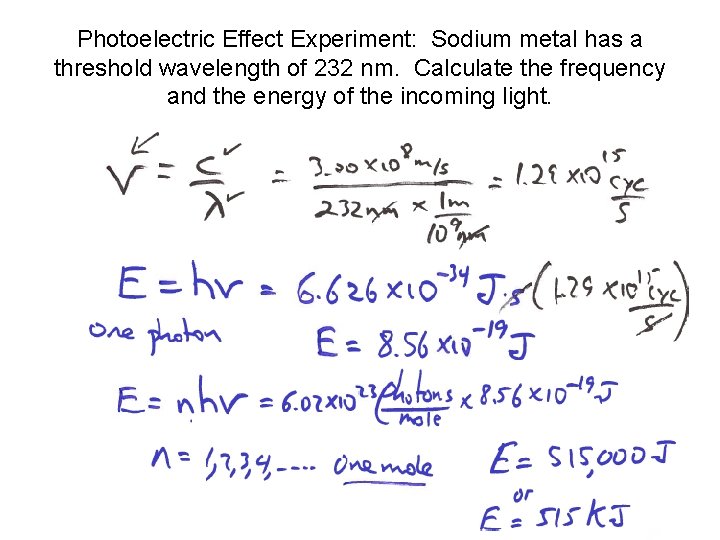
Photoelectric Effect Experiment: Sodium metal has a threshold wavelength of 232 nm. Calculate the frequency and the energy of the incoming light.

Photoelectric Effect Experiment: Sodium metal has a threshold wavelength of 232 nm. Calculate the frequency and the energy of the incoming light.

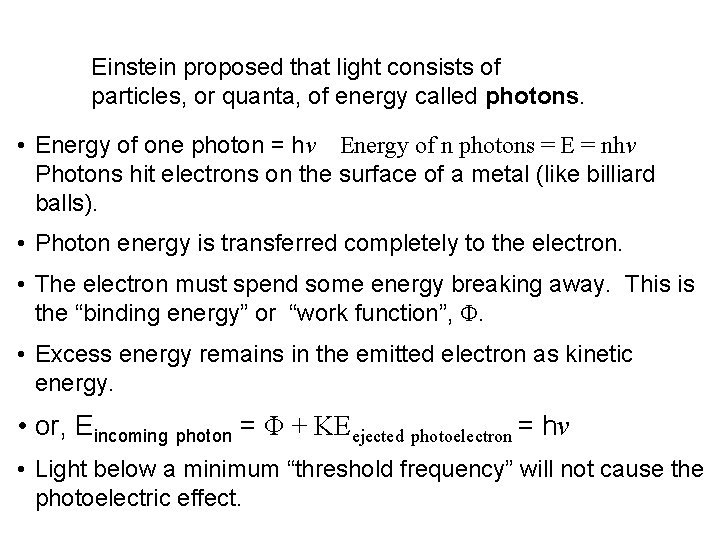
Einstein proposed that light consists of particles, or quanta, of energy called photons. • Energy of one photon = hν Energy of n photons = E = nhν Photons hit electrons on the surface of a metal (like billiard balls). • Photon energy is transferred completely to the electron. • The electron must spend some energy breaking away. This is the “binding energy” or “work function”, Φ. • Excess energy remains in the emitted electron as kinetic energy. • or, Eincoming photon = Φ + KEejected photoelectron = hν • Light below a minimum “threshold frequency” will not cause the photoelectric effect.

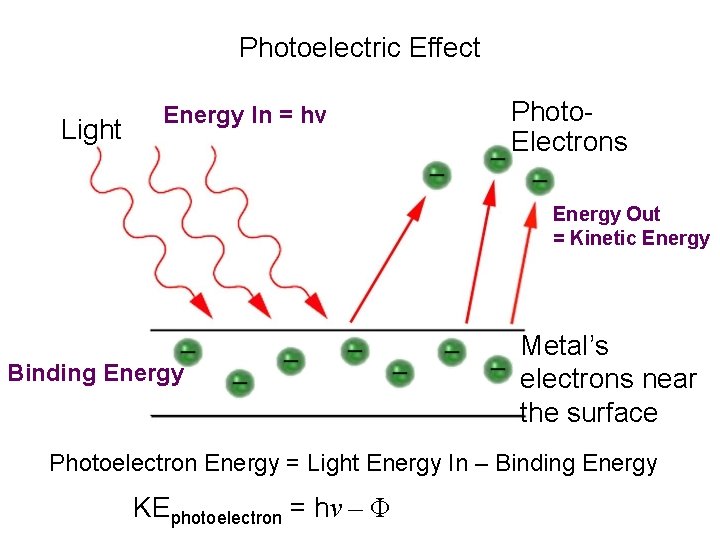
Photoelectric Effect Light Energy In = hv Photo. Electrons Energy Out = Kinetic Energy Binding Energy Metal’s electrons near the surface Photoelectron Energy = Light Energy In – Binding Energy KEphotoelectron = hν – Φ

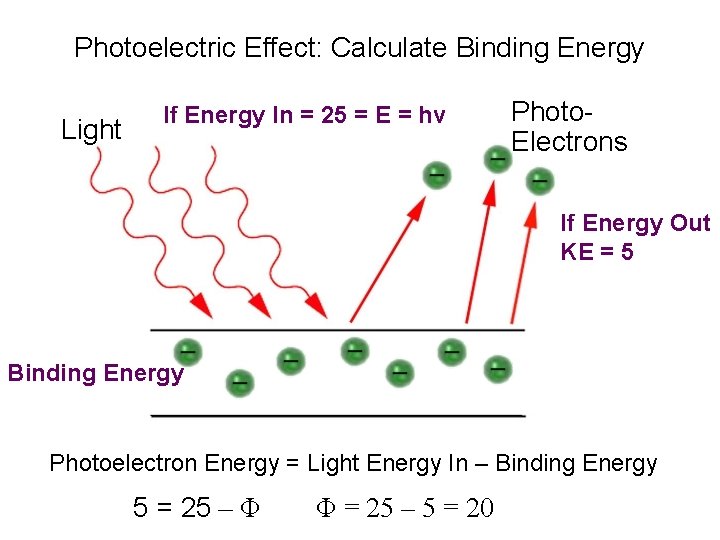
Photoelectric Effect: Calculate Binding Energy Light If Energy In = 25 = E = hv Photo. Electrons If Energy Out KE = 5 Binding Energy Photoelectron Energy = Light Energy In – Binding Energy 5 = 25 – Φ Φ = 25 – 5 = 20

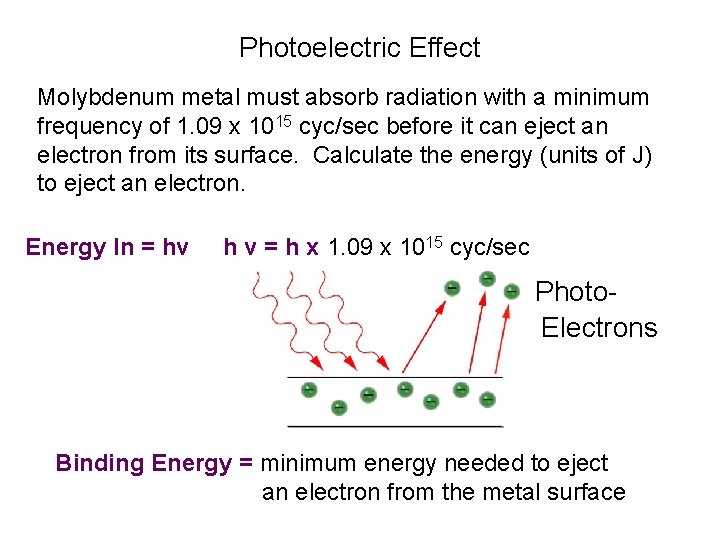
Photoelectric Effect Molybdenum metal must absorb radiation with a minimum frequency of 1. 09 x 1015 cyc/sec before it can eject an electron from its surface. Calculate the energy (units of J) to eject an electron. Energy In = hv h v = h x 1. 09 x 1015 cyc/sec Photo. Electrons Binding Energy = minimum energy needed to eject an electron from the metal surface

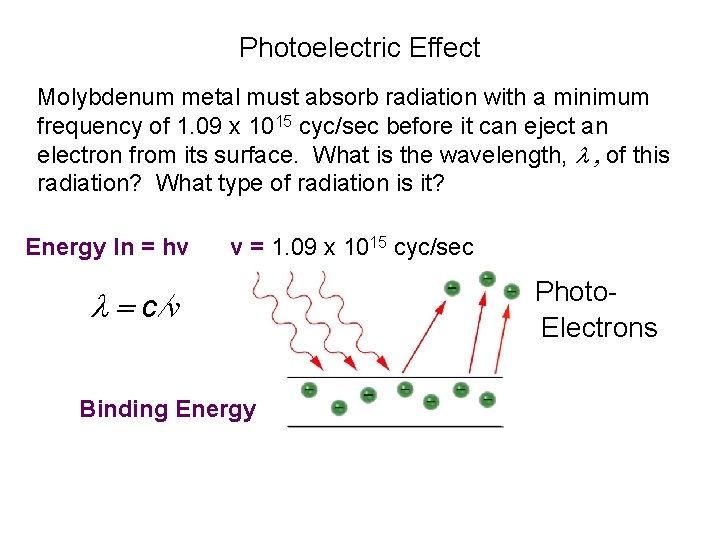
Photoelectric Effect Molybdenum metal must absorb radiation with a minimum frequency of 1. 09 x 1015 cyc/sec before it can eject an electron from its surface. What is the wavelength, l , of this radiation? What type of radiation is it? Energy In = hv v = 1. 09 x 1015 cyc/sec l = c/v Binding Energy Photo. Electrons

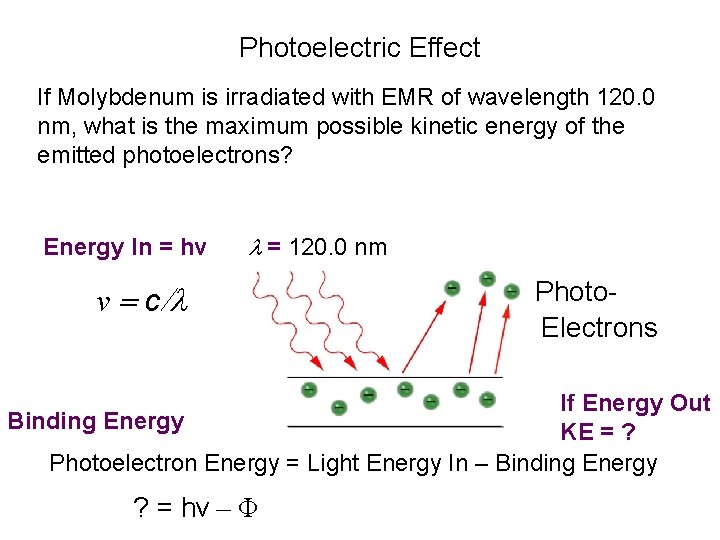
Photoelectric Effect If Molybdenum is irradiated with EMR of wavelength 120. 0 nm, what is the maximum possible kinetic energy of the emitted photoelectrons? Energy In = hv l = 120. 0 nm v = c/l Photo. Electrons If Energy Out Binding Energy KE = ? Photoelectron Energy = Light Energy In – Binding Energy ? = hv – Φ


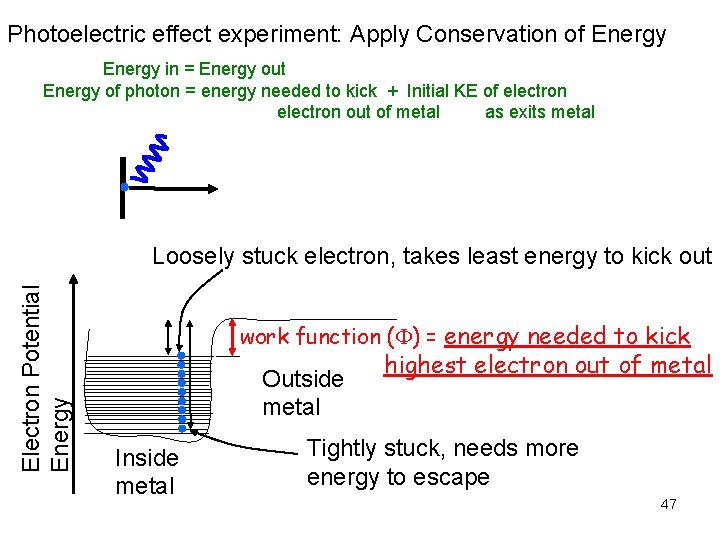
Photoelectric effect experiment: Apply Conservation of Energy in = Energy out Energy of photon = energy needed to kick + Initial KE of electron electron out of metal as exits metal Electron Potential Energy Loosely stuck electron, takes least energy to kick out work function ( ) = energy needed to kick Outside metal Inside metal highest electron out of metal Tightly stuck, needs more energy to escape 47

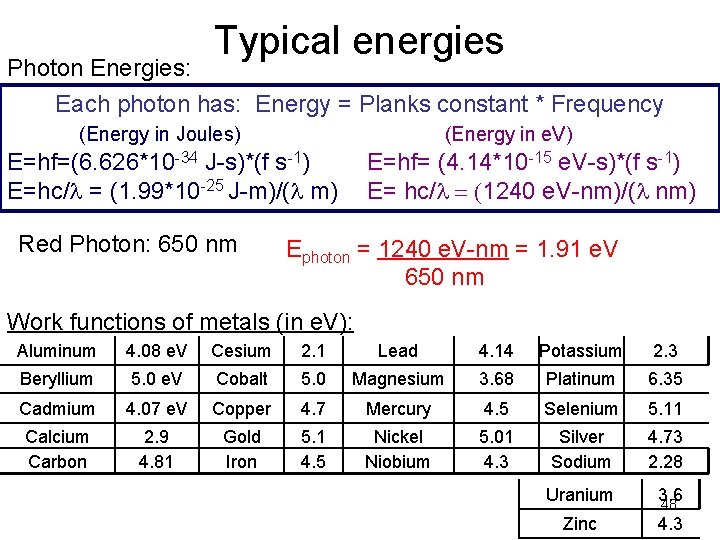
Typical energies Photon Energies: Each photon has: Energy = Planks constant * Frequency (Energy in Joules) (Energy in e. V) E=hf=(6. 626*10 -34 J-s)*(f s-1) E=hf= (4. 14*10 -15 e. V-s)*(f s-1) E=hc/l = (1. 99*10 -25 J-m)/(l m) E= hc/l = (1240 e. V-nm)/(l nm) Red Photon: 650 nm Ephoton = 1240 e. V-nm = 1. 91 e. V 650 nm Work functions of metals (in e. V): Aluminum 4. 08 e. V Cesium 2. 1 Lead 4. 14 Potassium 2. 3 Beryllium 5. 0 e. V Cobalt 5. 0 Magnesium 3. 68 Platinum 6. 35 Cadmium 4. 07 e. V Copper 4. 7 Mercury 4. 5 Selenium 5. 11 Calcium Carbon 2. 9 4. 81 Gold Iron 5. 1 4. 5 Nickel Niobium 5. 01 4. 3 Silver Sodium 4. 73 2. 28 Uranium 3. 6 48 Zinc 4. 3