When light shines on a metal electrons can




- Slides: 12

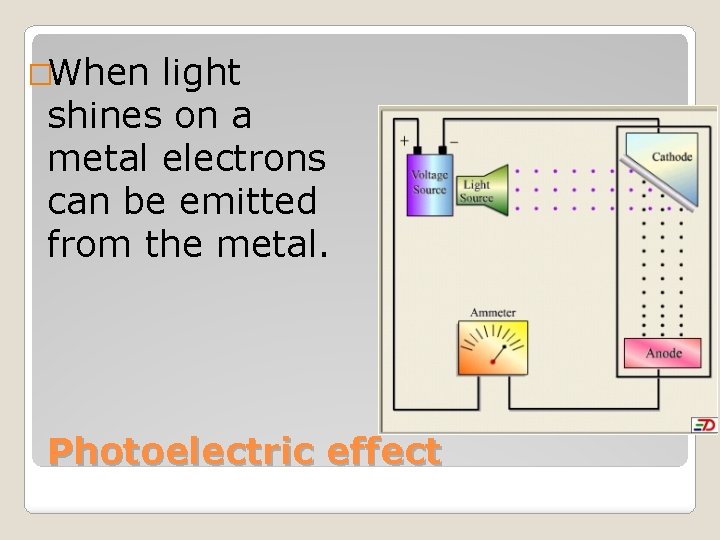
�When light shines on a metal electrons can be emitted from the metal. Photoelectric effect

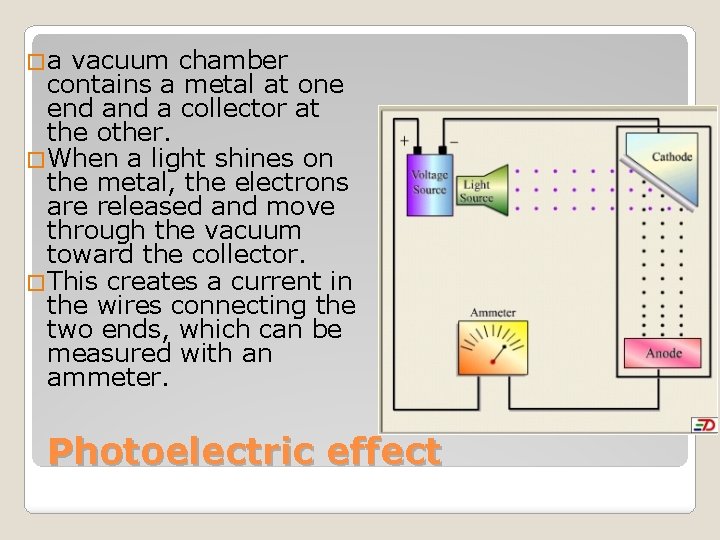
�a vacuum chamber contains a metal at one end a collector at the other. �When a light shines on the metal, the electrons are released and move through the vacuum toward the collector. �This creates a current in the wires connecting the two ends, which can be measured with an ammeter. Photoelectric effect

�In classical wave theory, the energy of electromagnetic radiation is carried within the wave itself. �So when the EM wave hits the metal, the electron should absorb energy from the wave until it exceeds the binding energy, at which point an electron would be released from the metal. Predictions based on wave theory

�The amount of energy needed to remove the electron is dependent on the type of metal used. �The prediction said that the photoelectric effect should occur for any type of light, regardless of frequency or wavelength, because the electron could keep absorbing energy from the wave until it had enough to break free of the metal. Predictions based on wave theory

�For a given metal, no electrons were emitted if the light’s frequency was below a certain amount, no matter how long the light shone on the metal. �Scientists could not explain why the light had to be a minimum frequency… The Conundrum

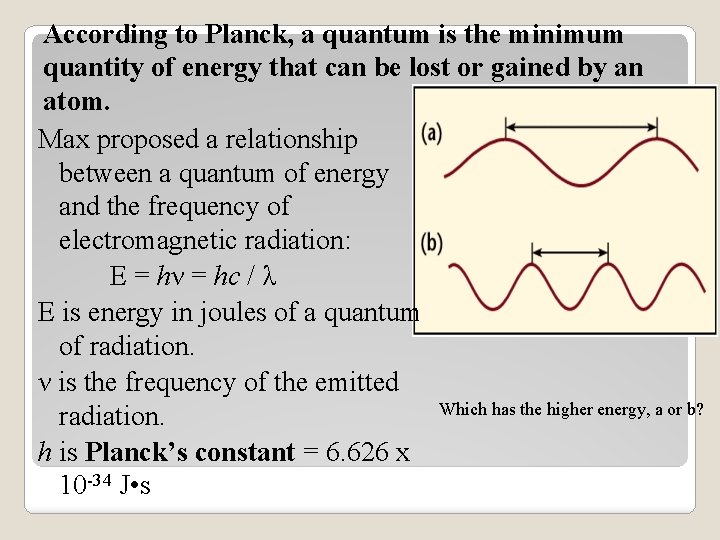
According to Planck, a quantum is the minimum quantity of energy that can be lost or gained by an atom. Max proposed a relationship between a quantum of energy and the frequency of electromagnetic radiation: E = hν = hc / λ E is energy in joules of a quantum of radiation. ν is the frequency of the emitted Which has the higher energy, a or b? radiation. h is Planck’s constant = 6. 626 x 10 -34 J • s

1905 – Einstein expands theory by introducing idea of wave-particle duality of light Einstein called the light particles photons. A photon is a particle of electromagnetic radiation having zero rest mass and carrying a quantum of energy such that Ephoton = hν = hc / λ

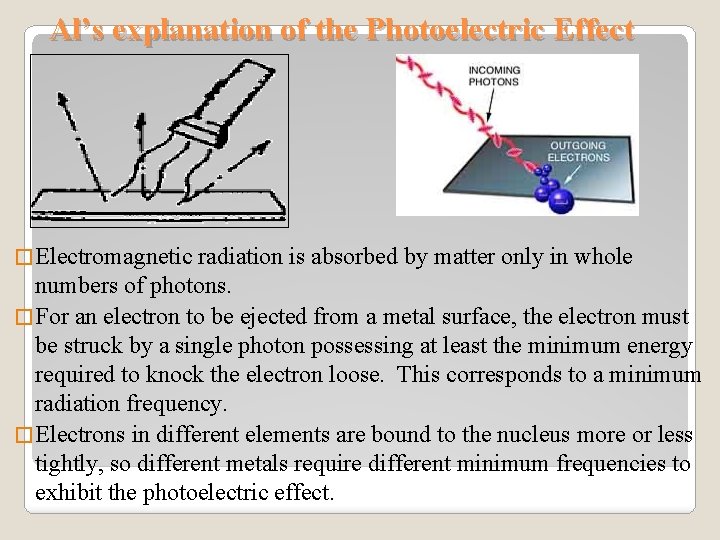
Al’s explanation of the Photoelectric Effect � Electromagnetic radiation is absorbed by matter only in whole numbers of photons. � For an electron to be ejected from a metal surface, the electron must be struck by a single photon possessing at least the minimum energy required to knock the electron loose. This corresponds to a minimum radiation frequency. � Electrons in different elements are bound to the nucleus more or less tightly, so different metals require different minimum frequencies to exhibit the photoelectric effect.

Sample Problem #3 �Sodium emits yellow light with a wavelength of approximately 570 nm. What is the frequency of this radiation in Hz?

Sample Problem #4 �Helium emits light at 420 nm. What is the energy of a single photon of this radiation in joules?

Sample Problem #5 �From problem #4, what is the energy given off by 1. 56 mol of helium atoms, in joules?

Assignment �Light Energy Problem Set �Due BOP Fri.