Pediatric parenteral nutrition M safarian MSc MD Ph
























- Slides: 39


Pediatric parenteral nutrition M. safarian, MSc, MD, Ph. D Mashhad University of Medical Sciences, Nutrition Department

Indication: �Unsafe or non functional GI �Malnurished children �Increased risk of malnutrition: �They include infants who have gone: 2 -3 days without adequate intake �older children who have gone 4 -5 days

Peripheral parenteral nutrition: � • the patient is not fluid-restricted � • nutrient needs can be met, and � • central PN is not feasible.

Central parenteral nutrition: �the patient is fluid-restricted �peripheral access is limited, and �nutritional needs cannot be met by peripheral PN.

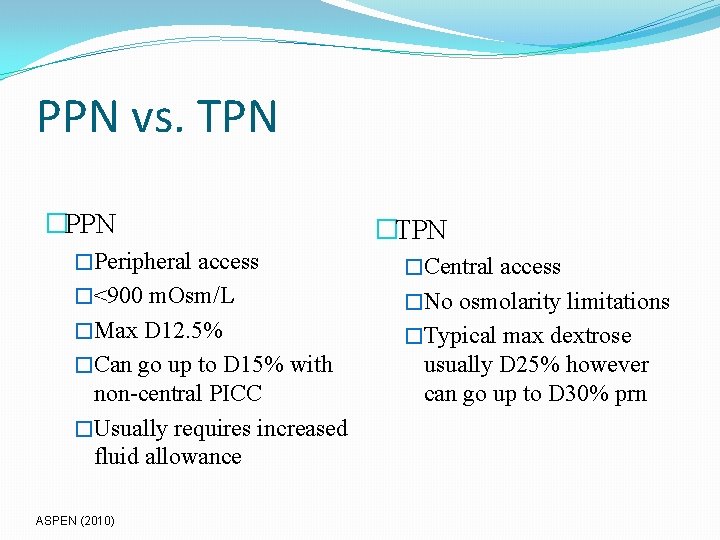
PPN vs. TPN �PPN �TPN �Peripheral access �Central access �<900 m. Osm/L �No osmolarity limitations �Max D 12. 5% �Typical max dextrose �Can go up to D 15% with non-central PICC �Usually requires increased fluid allowance ASPEN (2010) usually D 25% however can go up to D 30% prn

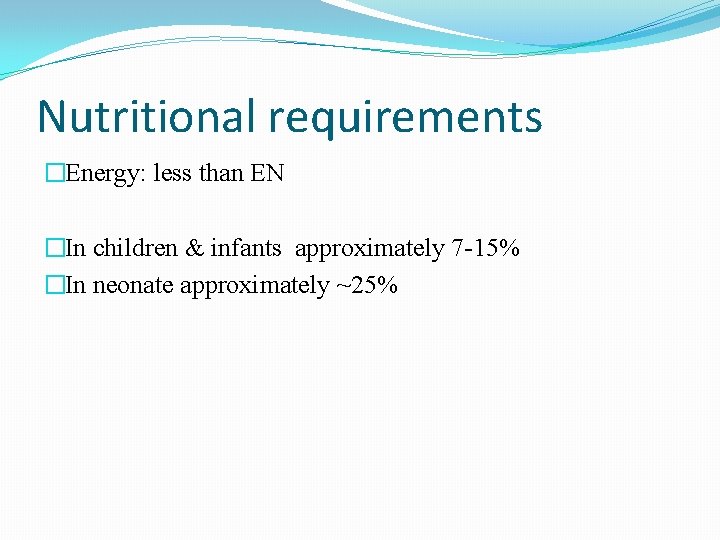
Nutritional requirements �Energy: less than EN �In children & infants approximately 7 -15% �In neonate approximately ~25%

Nutritional requirements �Energy: increased when : �compromised respiratory status, �sepsis, �thermal burns, �cardiac failure, �chronic growth failure, �who are recovering from surgery

Nutritional requirements �Energy: Assessment: �Weight change for short periods �Growth pattern for long term �Also : other anthropometrics

Parenteral Nutrition Kcal �Goal kcal dictate macronutrient goals �Extubated: provide ~10% < DRIs due to lack of thermogenesis �Intubated: REE or ~80% DRI (dependent on pt’s age) usually appropriate Fung (2000)

Resting Energy Expenditure Age (years) REE (kcal/kg/day) 0– 1 55 1– 3 57 4 – 6 48 7 – 10 40 11 -14 (Male/Female) 32 15 -18 (Male/Female) 27

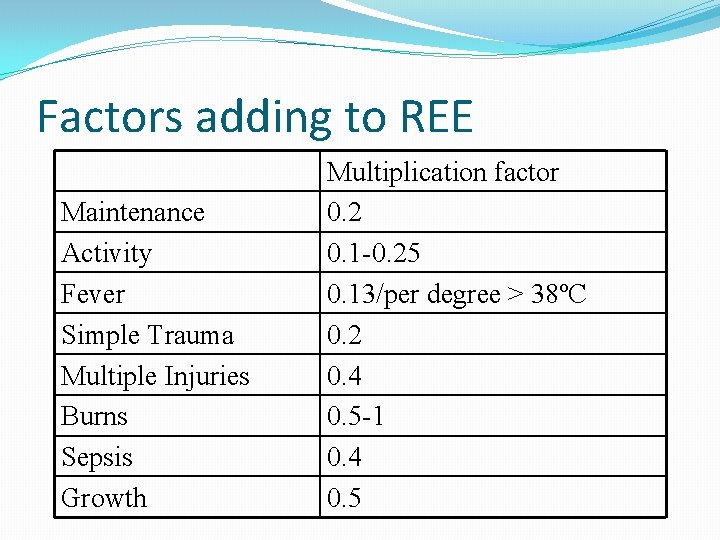
Factors adding to REE Maintenance Activity Fever Simple Trauma Multiple Injuries Burns Sepsis Growth Multiplication factor 0. 2 0. 1 -0. 25 0. 13/per degree > 38ºC 0. 2 0. 4 0. 5 -1 0. 4 0. 5

Nutritional requirements �Protein: �AA in parenteral nutrition


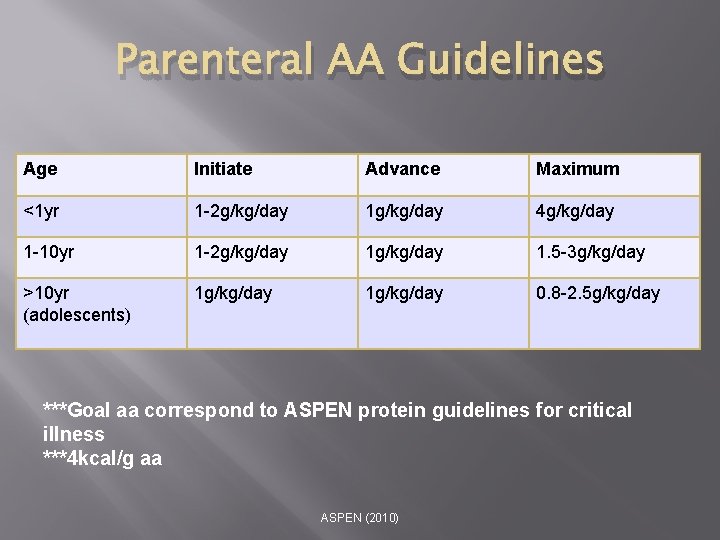
Parenteral AA Guidelines Age Initiate Advance Maximum <1 yr 1 -2 g/kg/day 1 g/kg/day 4 g/kg/day 1 -10 yr 1 -2 g/kg/day 1. 5 -3 g/kg/day >10 yr (adolescents) 1 g/kg/day 0. 8 -2. 5 g/kg/day ***Goal aa correspond to ASPEN protein guidelines for critical illness ***4 kcal/g aa ASPEN (2010)

Nutritional requirements �Protein: �Assessment: �There is no good marker

Nutritional requirements �Carbohydrate: �Solutions greater than 12. 5% dextrose should not be infused �should be initiated in a stepwise fashion �Assessment: �evaluation of serum glucose levels


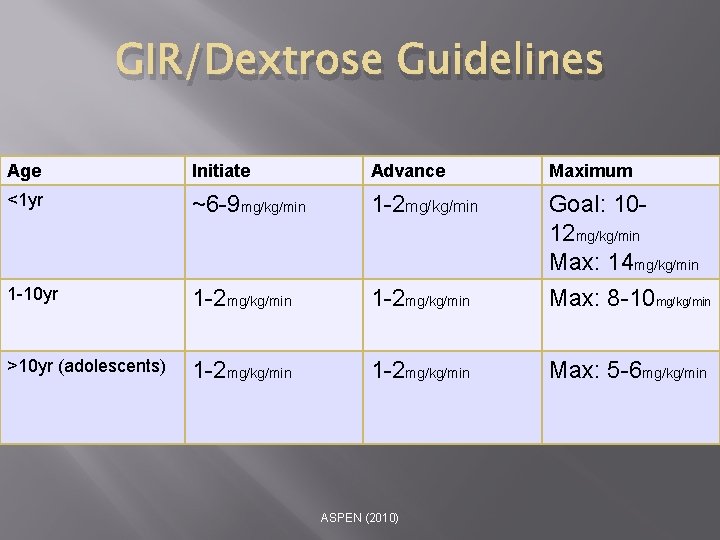
GIR/Dextrose Guidelines Age Initiate Advance Maximum <1 yr ~6 -9 mg/kg/min 1 -2 mg/kg/min Goal: 1012 mg/kg/min Max: 14 mg/kg/min 1 -10 yr 1 -2 mg/kg/min Max: 8 -10 mg/kg/min >10 yr (adolescents) 1 -2 mg/kg/min Max: 5 -6 mg/kg/min ASPEN (2010)

Nutritional requirements �Fat: �Assessment: �Tolerance is measured by an Intralipid level, a measure of unmetabolized intravenous fat or artificial chylomicrons. A level <1. 0 g/L indicates acceptable clearance.

Do not give intravenous lipids to patients with an allergy to egg or soy due to the presence of egg and soy protein in the intravenous preparation.


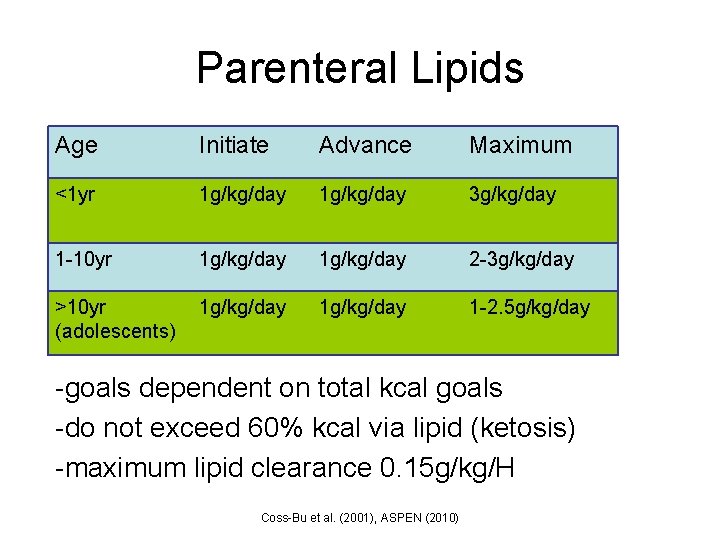
Parenteral Lipids Age Initiate Advance Maximum <1 yr 1 g/kg/day 3 g/kg/day 1 -10 yr 1 g/kg/day 2 -3 g/kg/day >10 yr (adolescents) 1 g/kg/day 1 -2. 5 g/kg/day -goals dependent on total kcal goals -do not exceed 60% kcal via lipid (ketosis) -maximum lipid clearance 0. 15 g/kg/H Coss-Bu et al. (2001), ASPEN (2010)

Fat Emulsion n What TG level is appropriate? n n < 200 if a trial period off < 300 -350 if continuously infusing Lipid calories should not exceed dextrose calories Do not exceed 0. 15 g/kg/hr infusion

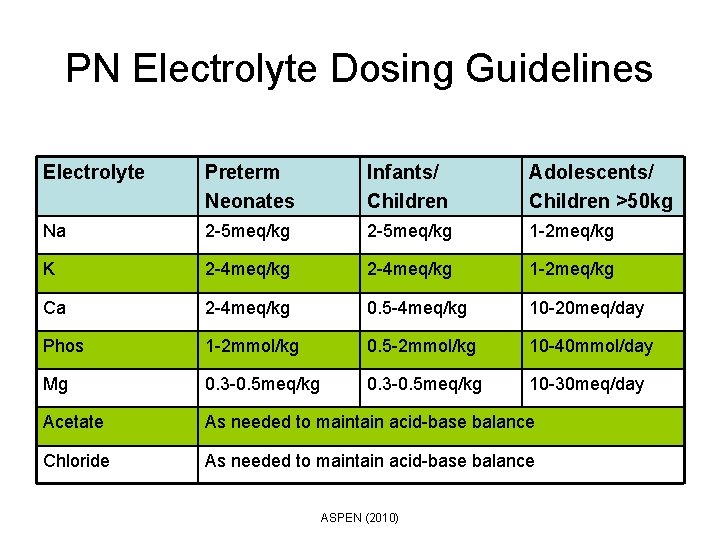
PN Electrolyte Dosing Guidelines Electrolyte Preterm Neonates Infants/ Children Adolescents/ Children >50 kg Na 2 -5 meq/kg 1 -2 meq/kg K 2 -4 meq/kg 1 -2 meq/kg Ca 2 -4 meq/kg 0. 5 -4 meq/kg 10 -20 meq/day Phos 1 -2 mmol/kg 0. 5 -2 mmol/kg 10 -40 mmol/day Mg 0. 3 -0. 5 meq/kg 10 -30 meq/day Acetate As needed to maintain acid-base balance Chloride As needed to maintain acid-base balance ASPEN (2010)

PNALD § § § § Avoid macronutrient overfeeding in general Decrease lipids GIR ≤ 12. 5 mg/kg/min Cholestatic trace elements § § § Decreased Cu; no Mn Cycle TPN as able Initiate EN asap (even trophic feeds) Btaiche and Khalidi (2002), Kaufman (2002)

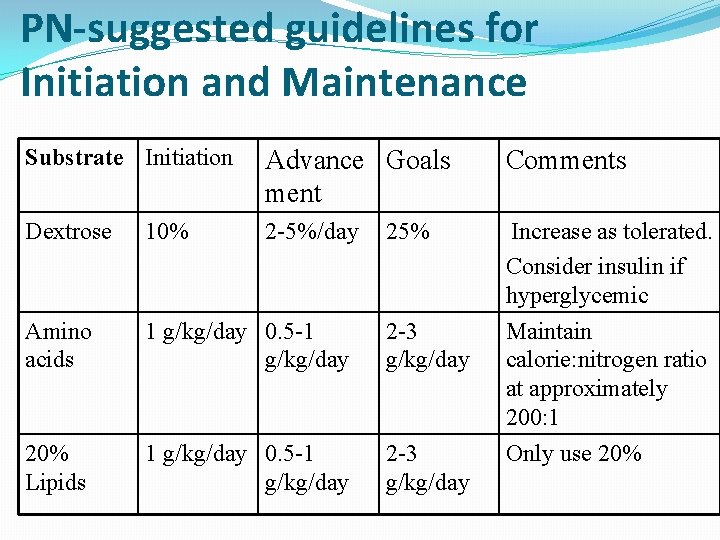
PN-suggested guidelines for Initiation and Maintenance Substrate Initiation Advance Goals ment Comments Dextrose 10% 2 -5%/day Amino acids 1 g/kg/day 0. 5 -1 g/kg/day 2 -3 g/kg/day 20% Lipids 1 g/kg/day 0. 5 -1 g/kg/day 2 -3 g/kg/day Increase as tolerated. Consider insulin if hyperglycemic Maintain calorie: nitrogen ratio at approximately 200: 1 Only use 20% 25%

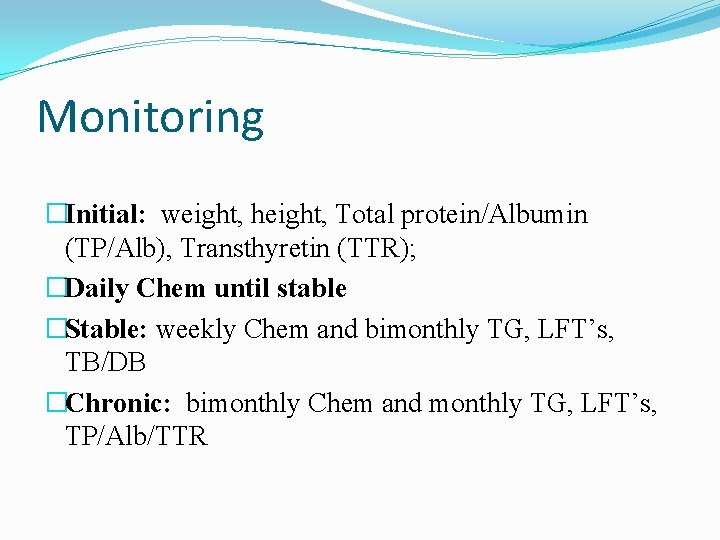
Monitoring �Initial: weight, height, Total protein/Albumin (TP/Alb), Transthyretin (TTR); �Daily Chem until stable �Stable: weekly Chem and bimonthly TG, LFT’s, TB/DB �Chronic: bimonthly Chem and monthly TG, LFT’s, TP/Alb/TTR

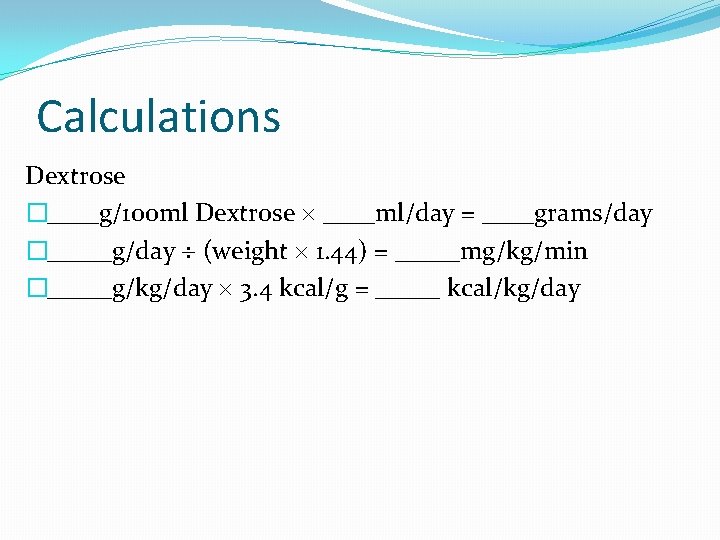
Calculations Dextrose �____g/100 ml Dextrose ____ml/day = ____grams/day �_____g/day (weight 1. 44) = _____mg/kg/min �_____g/kg/day 3. 4 kcal/g = _____ kcal/kg/day

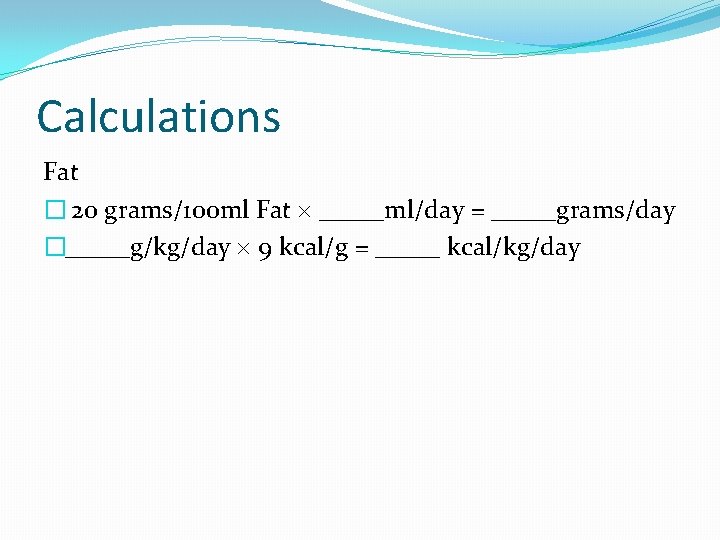
Calculations Fat � 20 grams/100 ml Fat _____ml/day = _____grams/day �_____g/kg/day 9 kcal/g = _____ kcal/kg/day

Calculations �grams Protein 6. 25 = _____ Nitrogen �Non-protein calories Nitrogen = Calorie: Nitrogen ratio

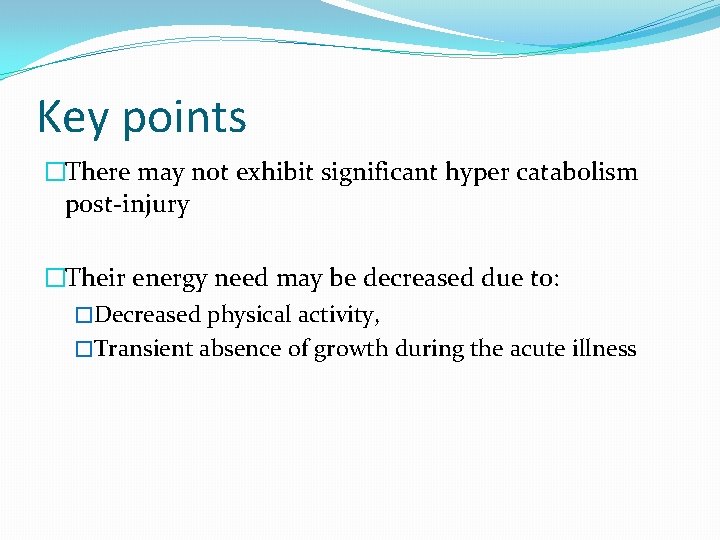
Key points �There may not exhibit significant hyper catabolism post-injury �Their energy need may be decreased due to: �Decreased physical activity, �Transient absence of growth during the acute illness

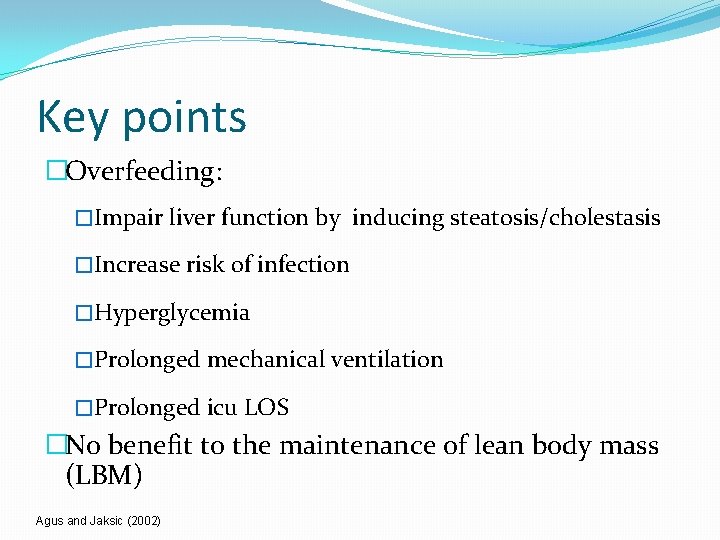
Key points �Overfeeding: �Impair liver function by inducing steatosis/cholestasis �Increase risk of infection �Hyperglycemia �Prolonged mechanical ventilation �Prolonged icu LOS �No benefit to the maintenance of lean body mass (LBM) Agus and Jaksic (2002)

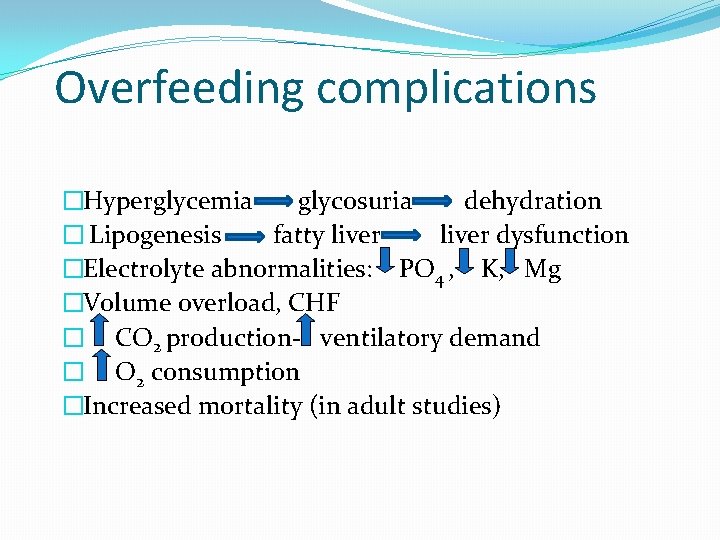
Overfeeding complications �Hyperglycemia glycosuria dehydration � Lipogenesis fatty liver dysfunction �Electrolyte abnormalities: PO 4 , K, Mg �Volume overload, CHF � CO 2 production- ventilatory demand � O 2 consumption �Increased mortality (in adult studies)

MONITORING Prevent Overfeeding �Carbohydrate: High RQ indicates CHO excess, stool reducing substances �Protein: Nitrogen balance �Fat: triglyceride �Visceral protein monitoring �Electrolytes, vitamin levels

Other complications �CHOLESTASIS �elevated conjugated bilirubin and other liver function tests. �Patients most at risk to develop cholestasis: � • overfeeding � • lack enteral nutrition � • long-term parenteral nutrition � • gastrointestinal surgery � • were preterm � • a history of recurrent sepsis � • peak conjugated bilirubin may occur up to one month after cessation of PN

Other complications �Chylothorax �Elevated serum urea �Hyperglycemia �Glycosuria �Hyperbilirobinemia �Hyperlipidemia �Hypoglycemia


41 Thank you
 Tpn cycling
Tpn cycling Complication of parenteral nutrition
Complication of parenteral nutrition Complication of parenteral nutrition
Complication of parenteral nutrition Cost of tpn per day
Cost of tpn per day Types of parenteral nutrition
Types of parenteral nutrition Small bowel obstruction parenteral nutrition
Small bowel obstruction parenteral nutrition Tpn calculations
Tpn calculations Rxkinetics tpn
Rxkinetics tpn Milan miljevic
Milan miljevic Amu msc
Amu msc Msc in construction law
Msc in construction law Msc i
Msc i Tpc.msc
Tpc.msc Trakcing msc
Trakcing msc Blackboard qu
Blackboard qu Msc que significa
Msc que significa Msc bis
Msc bis Tscc.msc
Tscc.msc Msc nerissa current position
Msc nerissa current position Telecommunication systems book
Telecommunication systems book Almacenes msc
Almacenes msc University of birmingham msc international business
University of birmingham msc international business Prof msc
Prof msc Matthias zabel
Matthias zabel Msc gsm
Msc gsm Msc olga
Msc olga 2vision msc
2vision msc Msc acs
Msc acs Msc sirkka
Msc sirkka Vdoe msc
Vdoe msc Msc joanna droga
Msc joanna droga Msc marianna
Msc marianna Nazvn
Nazvn Marc ruttenberg
Marc ruttenberg Diverkasi
Diverkasi Local loop
Local loop Msc finance and banking tor vergata
Msc finance and banking tor vergata Msc eir
Msc eir Scan barcode msc
Scan barcode msc Grenoble ecole de management msc finance
Grenoble ecole de management msc finance