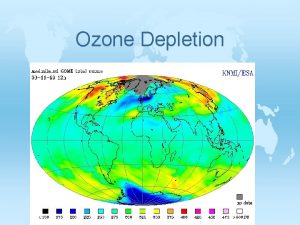
Ozone Depletion JHans van Leeuwen Stratospheric ozone and













- Slides: 45

Ozone Depletion J(Hans) van Leeuwen Stratospheric ozone and its importance – The hole story

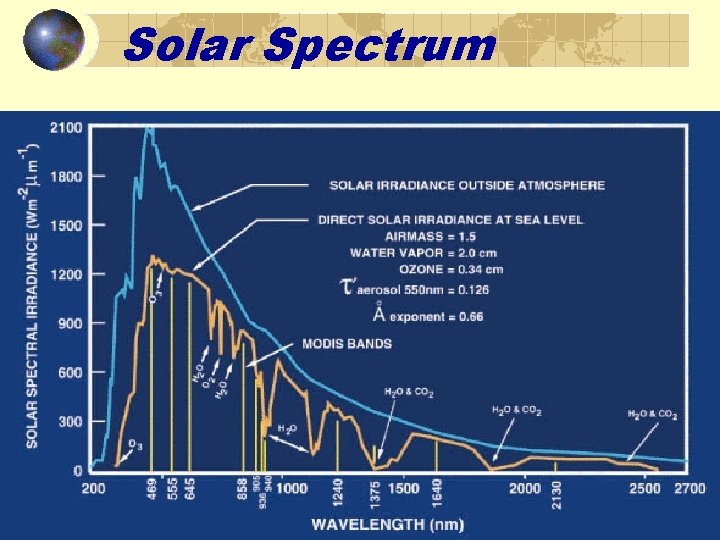
Solar Spectrum

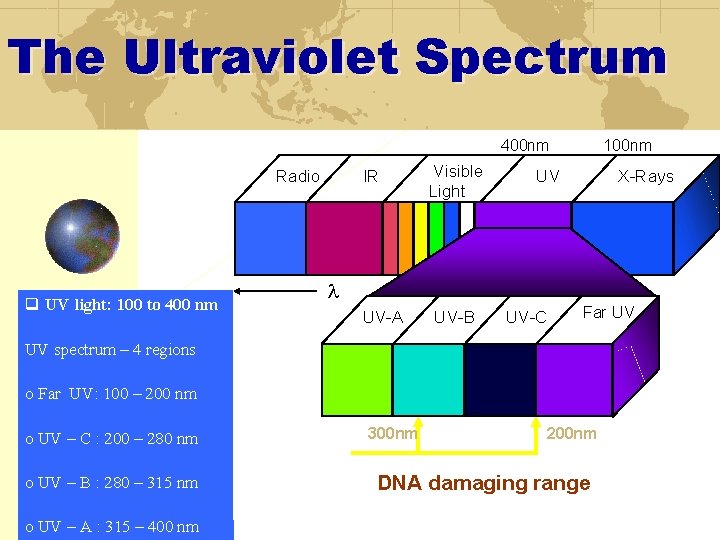
The Ultraviolet Spectrum 400 nm Radio q UV light: 100 to 400 nm IR Visible Light 100 nm UV X-Rays l UV-A UV-B UV-C Far UV UV spectrum – 4 regions o Far UV: 100 – 200 nm o UV – C : 200 – 280 nm o UV – B : 280 – 315 nm o UV – A : 315 – 400 nm 300 nm 200 nm DNA damaging range

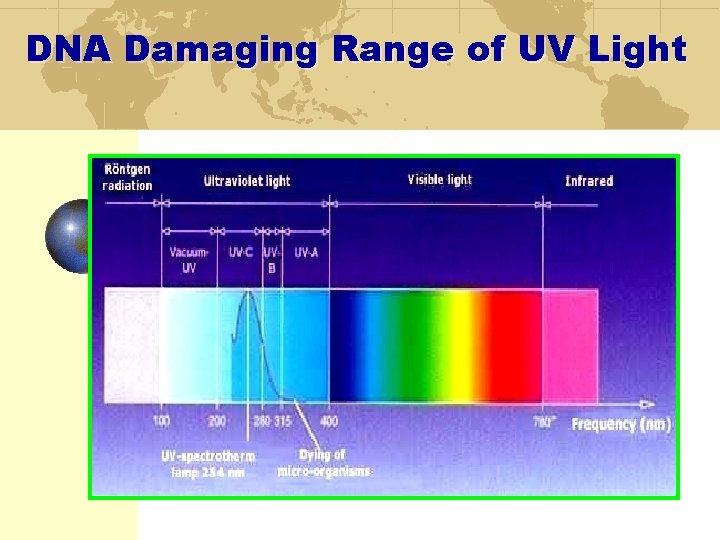
DNA Damaging Range of UV Light

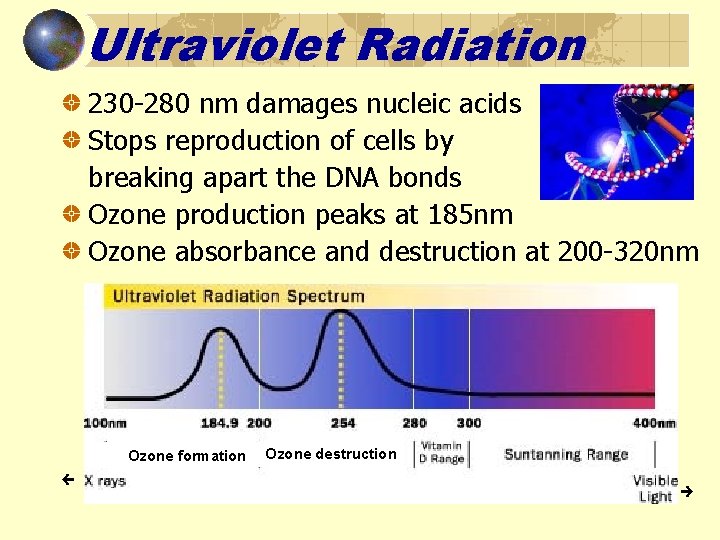
Ultraviolet Radiation 230 -280 nm damages nucleic acids Stops reproduction of cells by breaking apart the DNA bonds Ozone production peaks at 185 nm Ozone absorbance and destruction at 200 -320 nm Ozone formation Ozone destruction

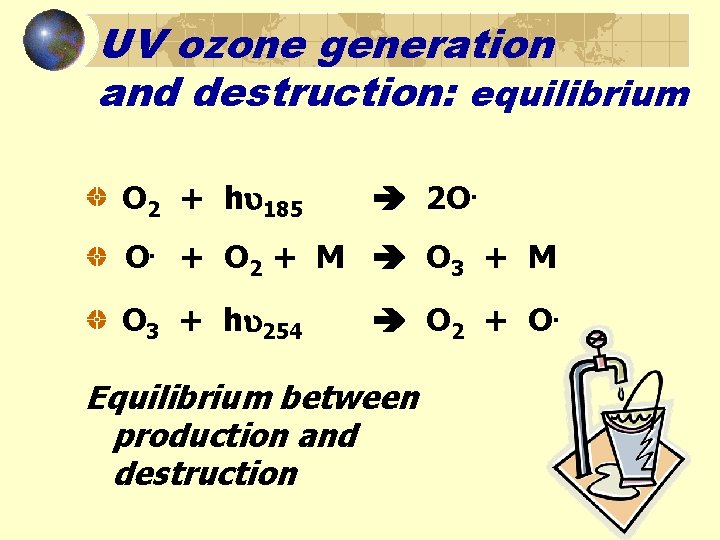
UV ozone generation and destruction: equilibrium O 2 + h 185 2 O. O. + O 2 + M O 3 + M O 3 + h 254 O 2 + O. Equilibrium between production and destruction

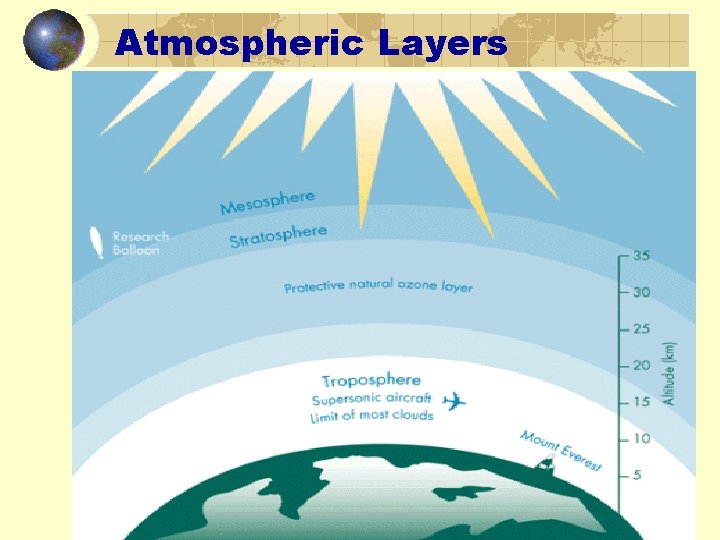
Atmospheric Layers

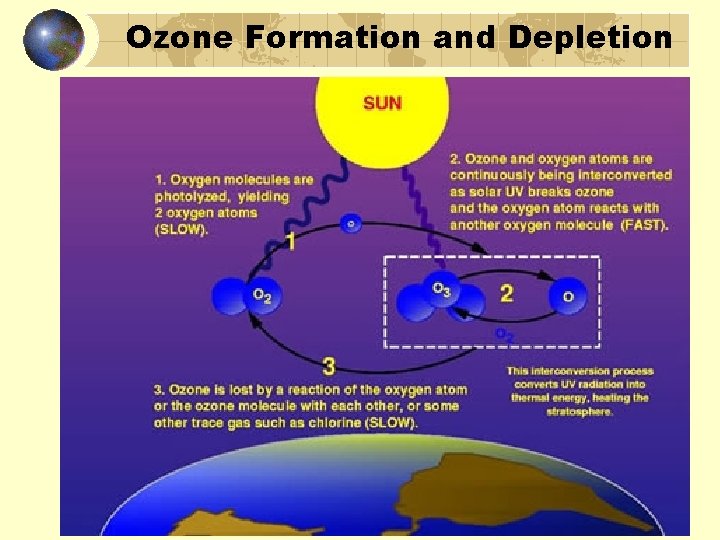
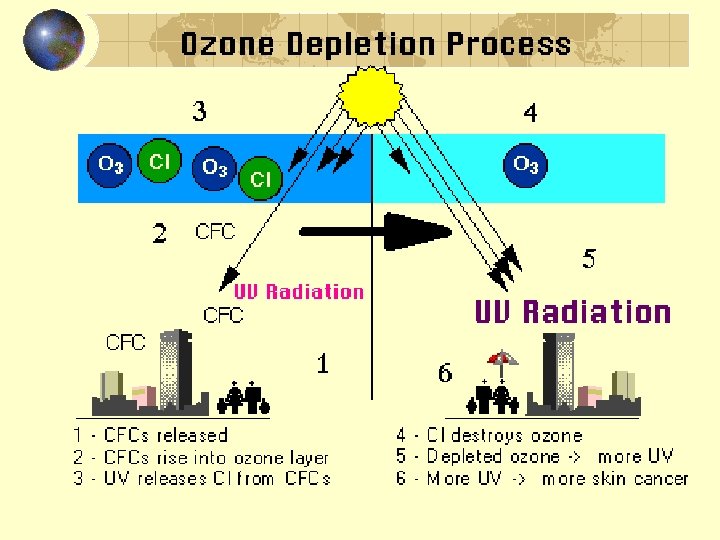
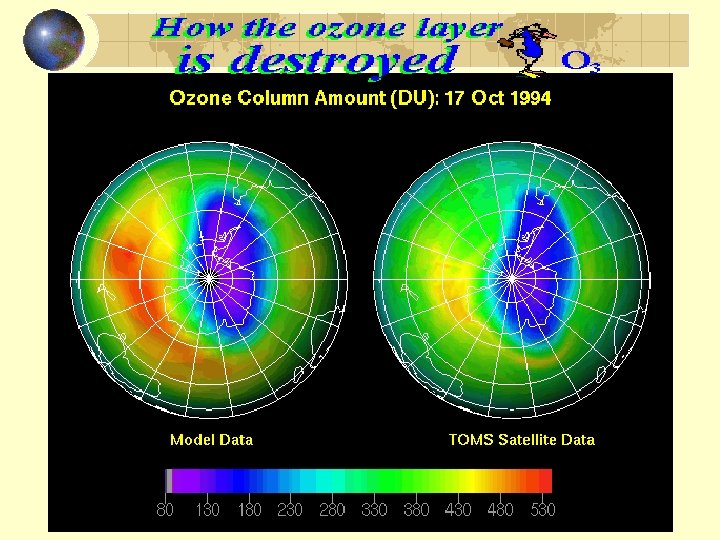
Ozone Formation and Depletion


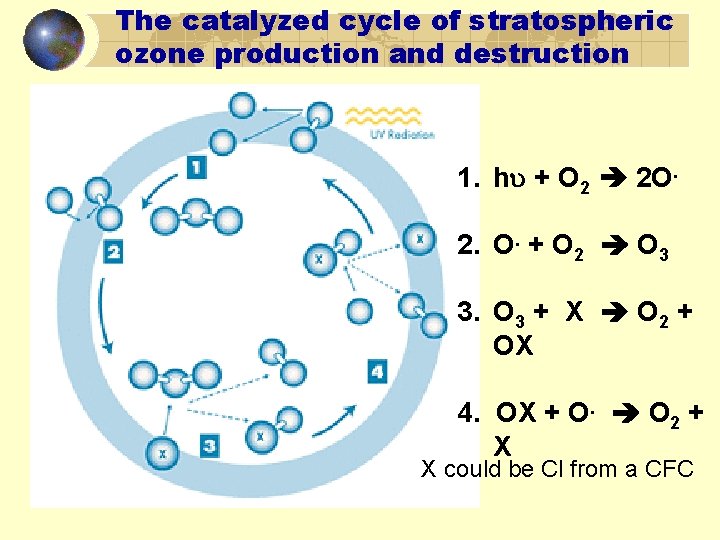
The catalyzed cycle of stratospheric ozone production and destruction 1. h + O 2 2 O. 2. O. + O 2 O 3 3. O 3 + X O 2 + OX 4. OX + O. O 2 + X X could be Cl from a CFC


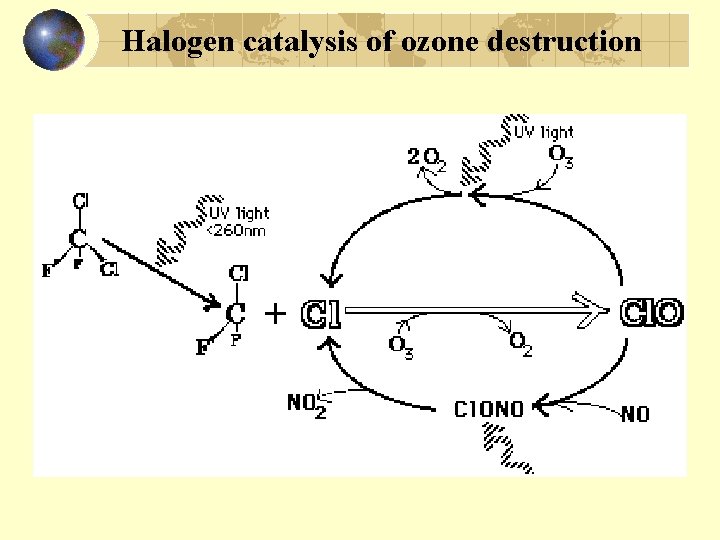
Halogen catalysis of ozone destruction

Halogen removal from atmosphere Cl + CH 4 HCl + CH 3. Cl. O + NO 2 Cl. ONO 2 Both HCl and Cl. ONO 2 inactive: rain out Br + O 3 Br. O + O 2 Br + CH 4 HBr + CH 3. HBr can photolytically provide Br again Halons and CH 3 Br provide Br

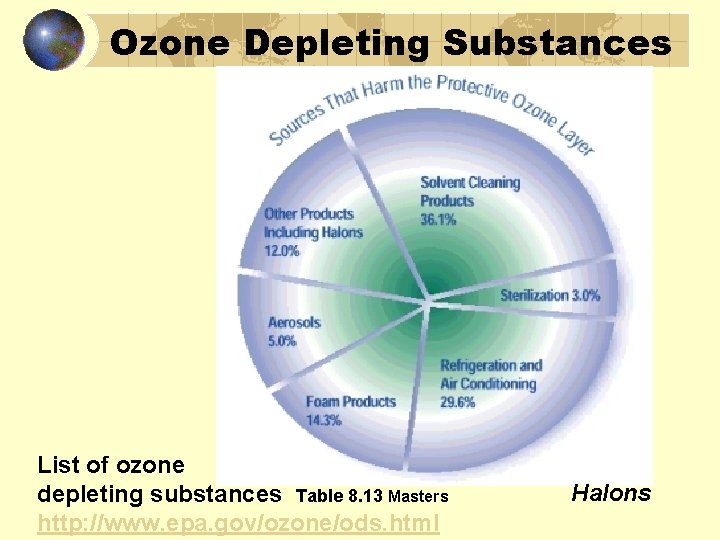
Ozone Depleting Substances List of ozone depleting substances Table 8. 13 Masters http: //www. epa. gov/ozone/ods. html Halons

Antarctic Ozone Problems

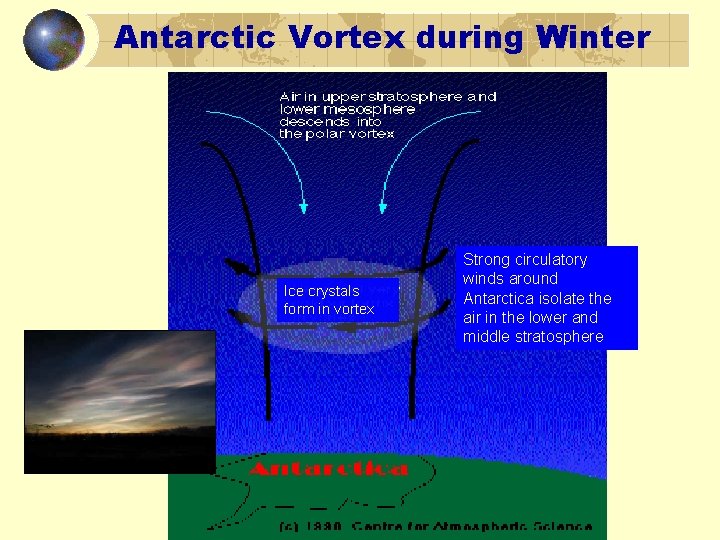
Antarctic Vortex during Winter Ice crystals form in vortex Strong circulatory winds around Antarctica isolate the air in the lower and middle stratosphere

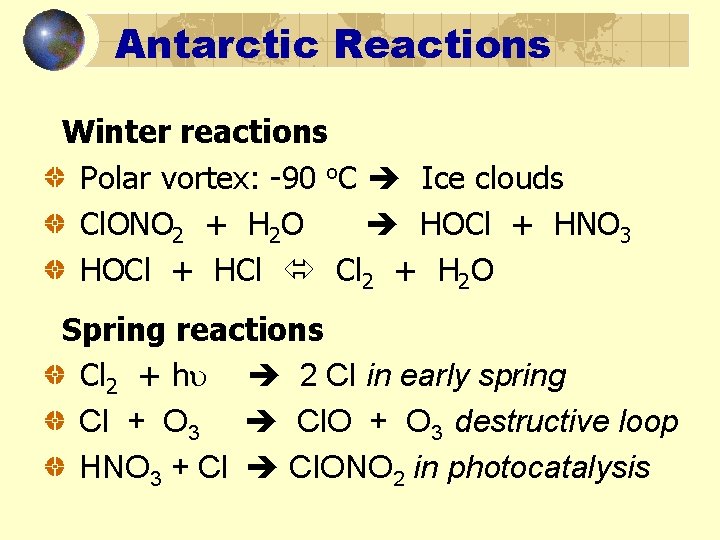
Antarctic Reactions Winter reactions Polar vortex: -90 o. C Ice clouds Cl. ONO 2 + H 2 O HOCl + HNO 3 HOCl + HCl Cl 2 + H 2 O Spring reactions Cl 2 + h 2 Cl in early spring Cl + O 3 Cl. O + O 3 destructive loop HNO 3 + Cl Cl. ONO 2 in photocatalysis

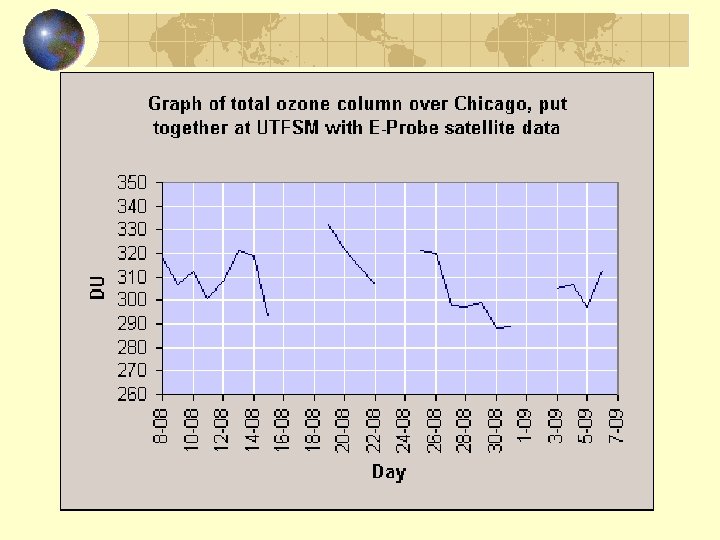
Ozone in Stratosphere: Dobson Units (DU) If 100 DU of ozone were brought to the Earth's surface, it would form a layer 1 millimeter thick. In the tropics, ozone levels are typically between 250 and 300 DU year-round. In temperate regions, seasonal variations can produce large swings in ozone levels. For instance, measurements in Leningrad have recorded ozone levels as high as 475 DU and as low as 300 DU.

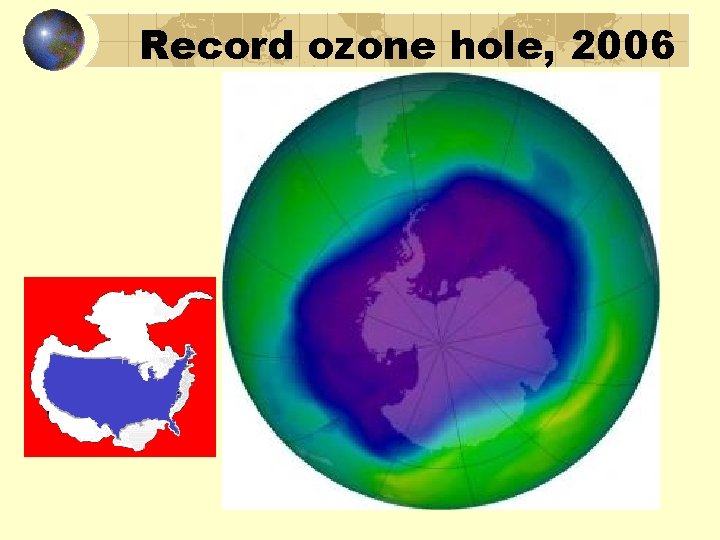
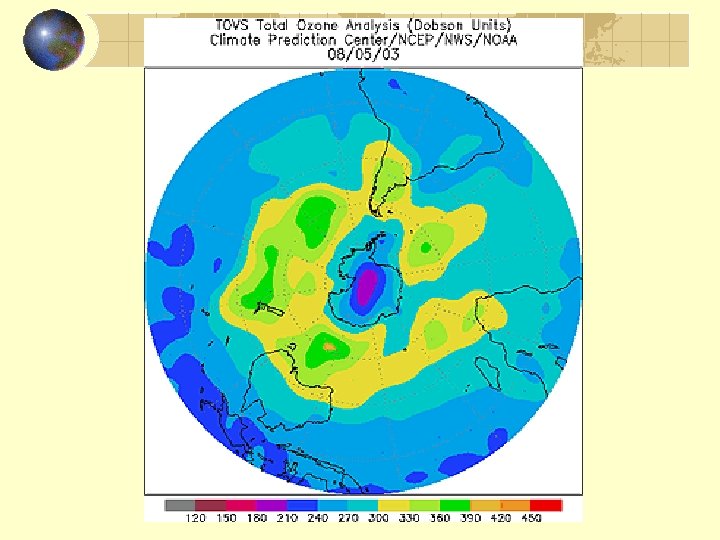
Record ozone hole, 2006


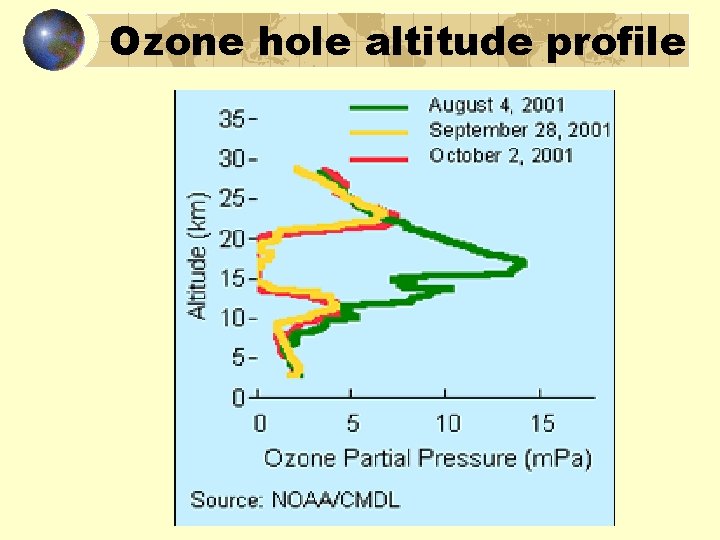
Ozone hole altitude profile

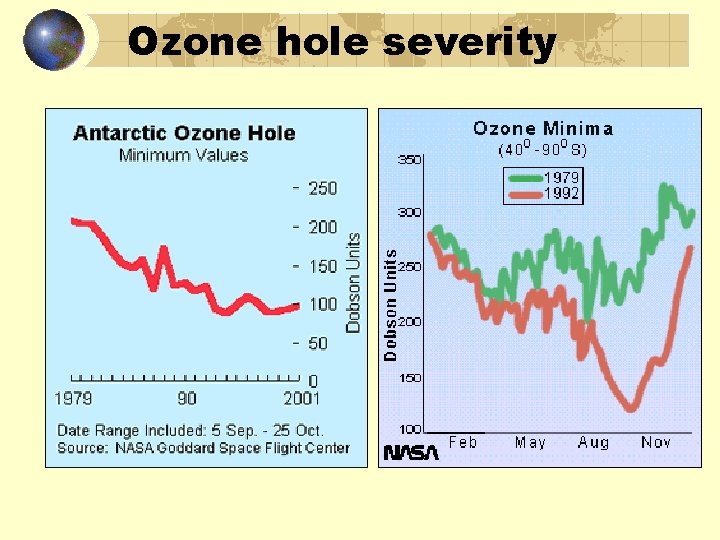
Ozone hole severity

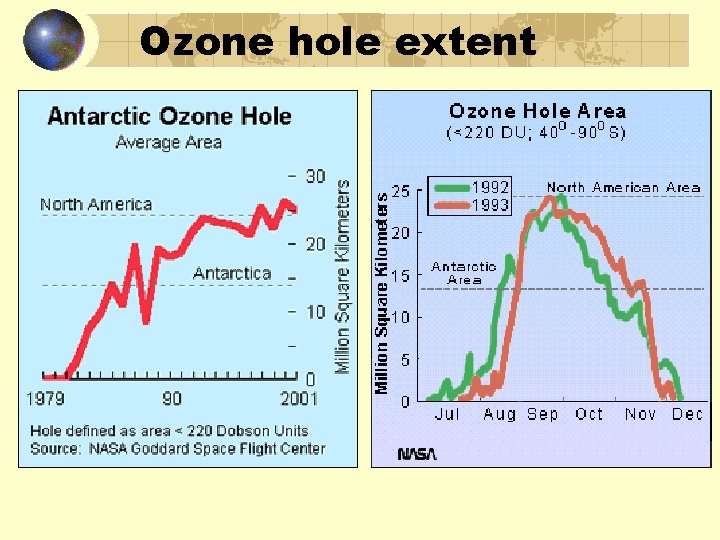
Ozone hole extent

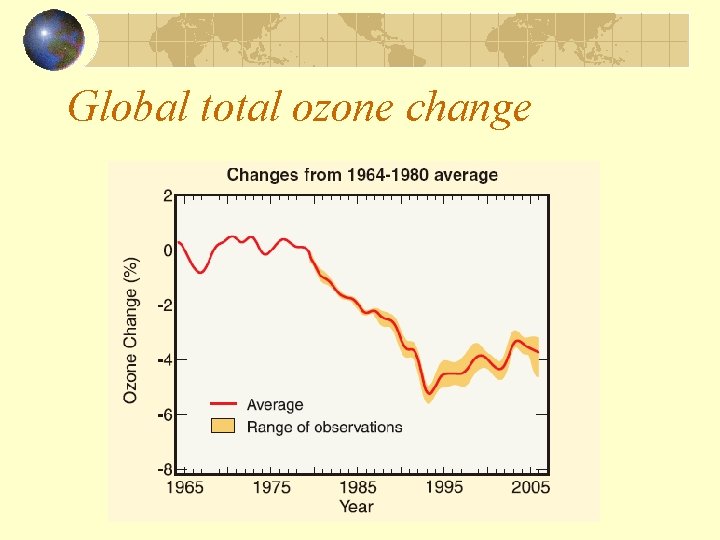
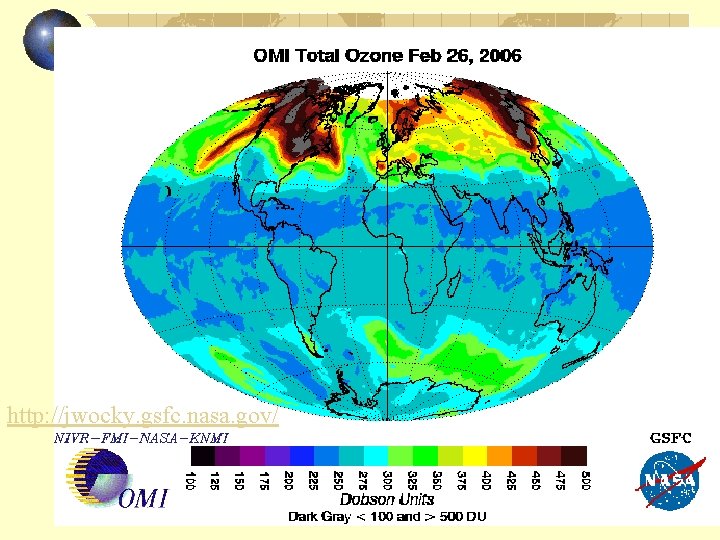
Global total ozone change



http: //jwocky. gsfc. nasa. gov/




The Nobel Prize in Chemistry 1995 Paul J. Crutzen Mario J. Molina F. Sherwood Rowland The Netherlands USA Max-Planck-Institute for Chemistry Mainz, Germany MIT, USA Cambridge, MA Department of Chemistry, University of California Irvine, CA, USA 1933 - 1943 - 1927 - "for their work in atmospheric chemistry, particularly concerning the formation and decomposition of ozone"








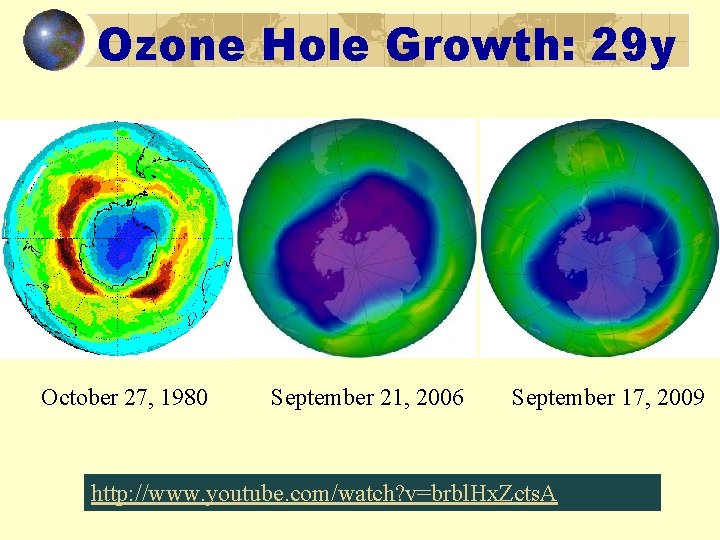
Ozone Hole Growth: 29 y October 27, 1980 September 21, 2006 September 17, 2009 http: //www. youtube. com/watch? v=brbl. Hx. Zcts. A

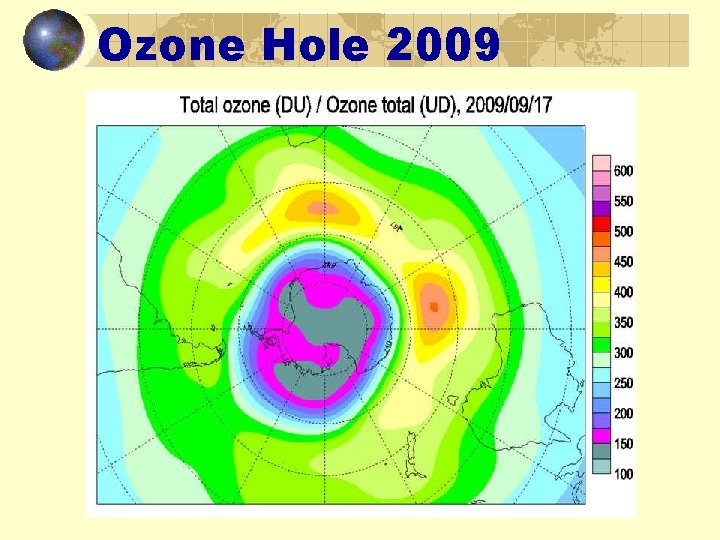
Ozone Hole 2009

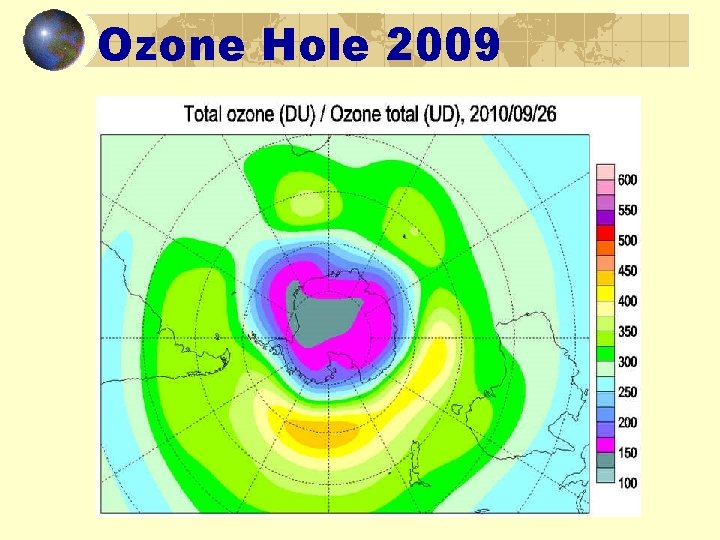
Ozone Hole 2009

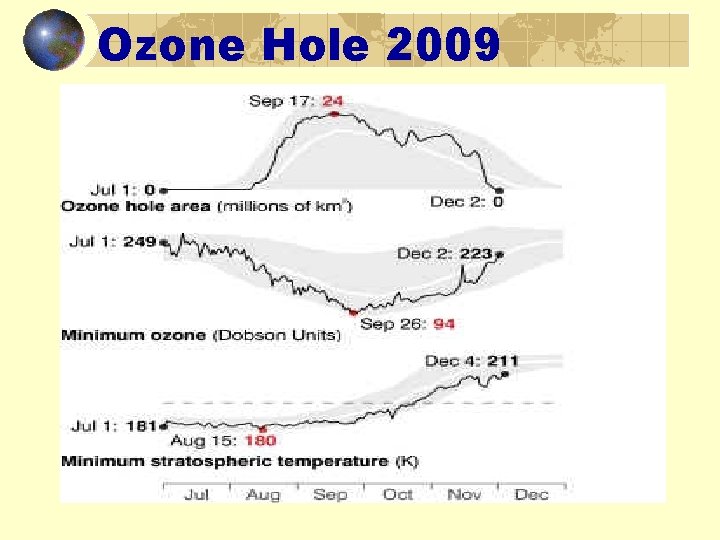
Ozone Hole 2009


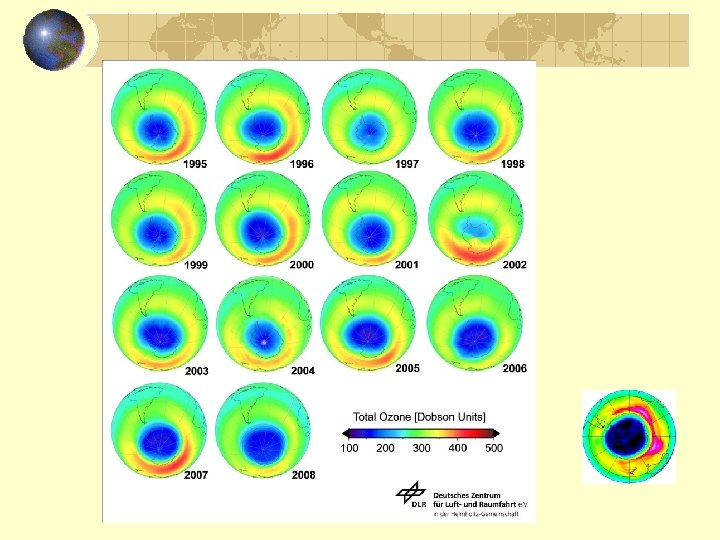
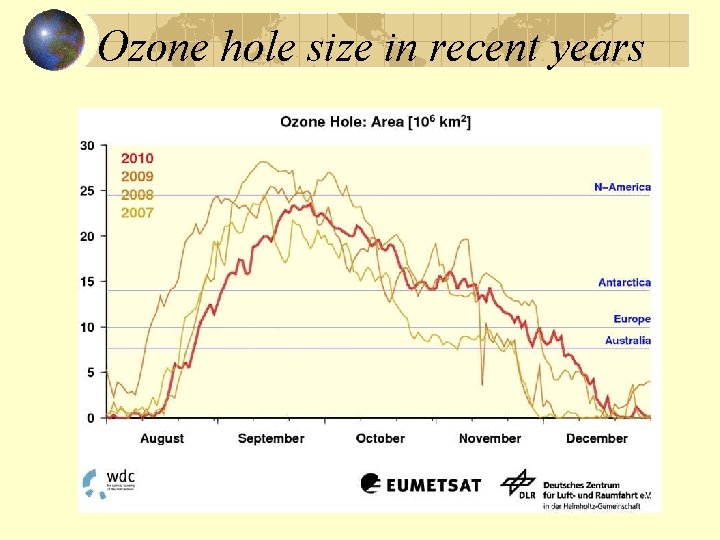
Ozone hole size in recent years

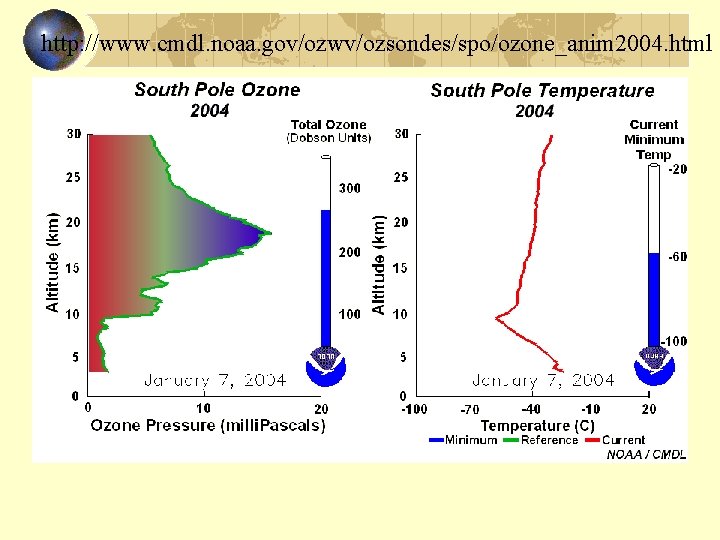
http: //www. cmdl. noaa. gov/ozwv/ozsondes/spo/ozone_anim 2004. html
 Stratospheric ozone depletion
Stratospheric ozone depletion Negative effects of ozone depletion
Negative effects of ozone depletion Ozone depletion effect on humans
Ozone depletion effect on humans Causes of the ozone depletion
Causes of the ozone depletion Ozone layer depletion introduction
Ozone layer depletion introduction Ozone depletion diagram
Ozone depletion diagram Cause of ozone depletion
Cause of ozone depletion Protective ozone layer
Protective ozone layer Benjamin cummings
Benjamin cummings Ozone depletion pictures
Ozone depletion pictures Bohr-van leeuwen theorem
Bohr-van leeuwen theorem Darmlavage definition
Darmlavage definition Flex college delft
Flex college delft Milou van leeuwen
Milou van leeuwen Peter jan van leeuwen
Peter jan van leeuwen Alice van leeuwen
Alice van leeuwen Marco van leeuwen overleden
Marco van leeuwen overleden Polar stratospheric clouds
Polar stratospheric clouds Polar stratospheric clouds
Polar stratospheric clouds Polar stratospheric clouds
Polar stratospheric clouds Stratospheric balloon
Stratospheric balloon Chapter 11 depreciation impairments and depletion
Chapter 11 depreciation impairments and depletion Composite depreciation
Composite depreciation Chapter 11 intermediate accounting
Chapter 11 intermediate accounting Ego depletion
Ego depletion Management allowed depletion
Management allowed depletion Depletion formula
Depletion formula Objectives of irrigation scheduling
Objectives of irrigation scheduling Ece 424
Ece 424 Ece 271
Ece 271 Animal depletion
Animal depletion Ch14 twitch
Ch14 twitch N+ polysilicon
N+ polysilicon Depletion
Depletion Depletion
Depletion Tamoxifen nutrient depletion
Tamoxifen nutrient depletion Resource depletion
Resource depletion Depletion
Depletion Determining the cost of plant assets
Determining the cost of plant assets P
P How do cfcs destroy ozone
How do cfcs destroy ozone Protective ozone layer
Protective ozone layer Ozone composition
Ozone composition Sopmed
Sopmed Microplasma ozone
Microplasma ozone Protective ozone layer
Protective ozone layer