Chapter 15 Air Pollution and Stratospheric Ozone Depletion












- Slides: 42

Chapter 15 Air Pollution and Stratospheric Ozone Depletion

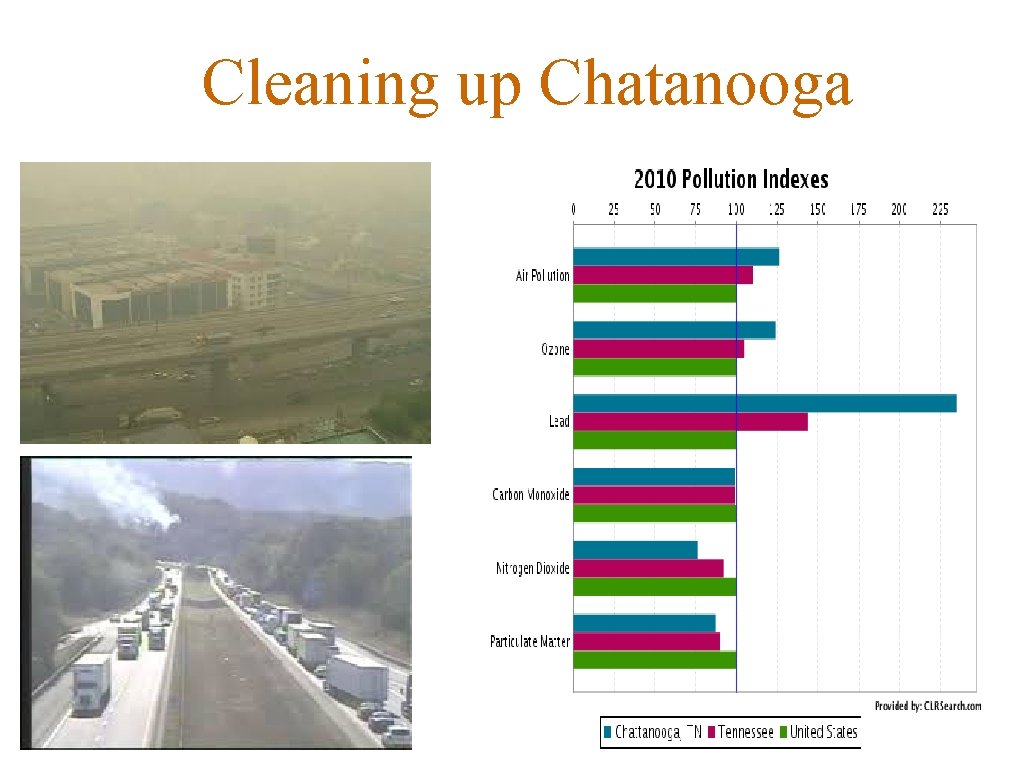
Cleaning up Chatanooga

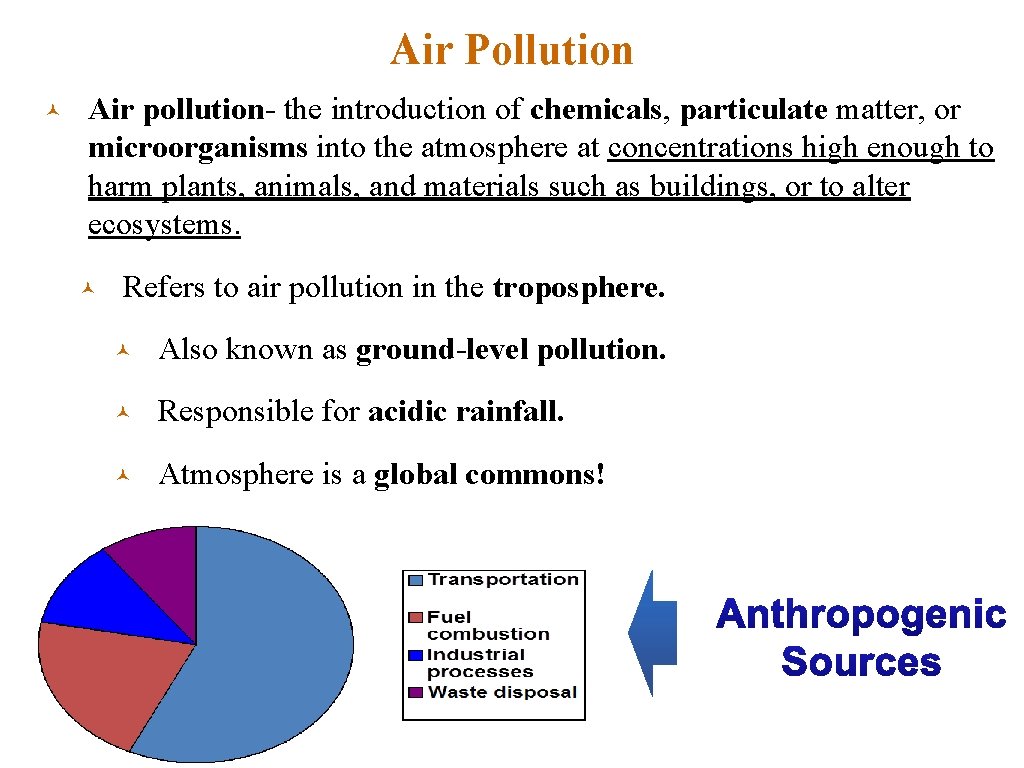
Air Pollution © Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems. © Refers to air pollution in the troposphere. © Also known as ground-level pollution. © Responsible for acidic rainfall. © Atmosphere is a global commons!

Natural Sources of Air Pollution © Volcanoes © Lightning © Forest fires © Plants

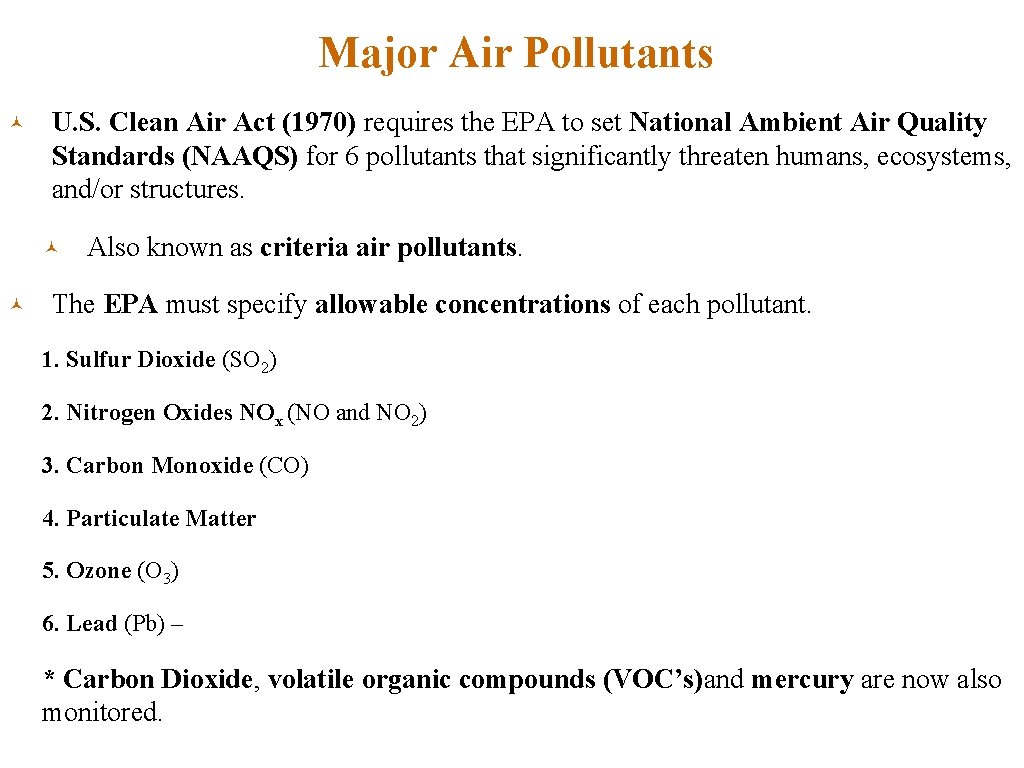
Major Air Pollutants © U. S. Clean Air Act (1970) requires the EPA to set National Ambient Air Quality Standards (NAAQS) for 6 pollutants that significantly threaten humans, ecosystems, and/or structures. © © Also known as criteria air pollutants. The EPA must specify allowable concentrations of each pollutant. 1. Sulfur Dioxide (SO 2) 2. Nitrogen Oxides NOx (NO and NO 2) 3. Carbon Monoxide (CO) 4. Particulate Matter 5. Ozone (O 3) 6. Lead (Pb) – * Carbon Dioxide, volatile organic compounds (VOC’s)and mercury are now also monitored.

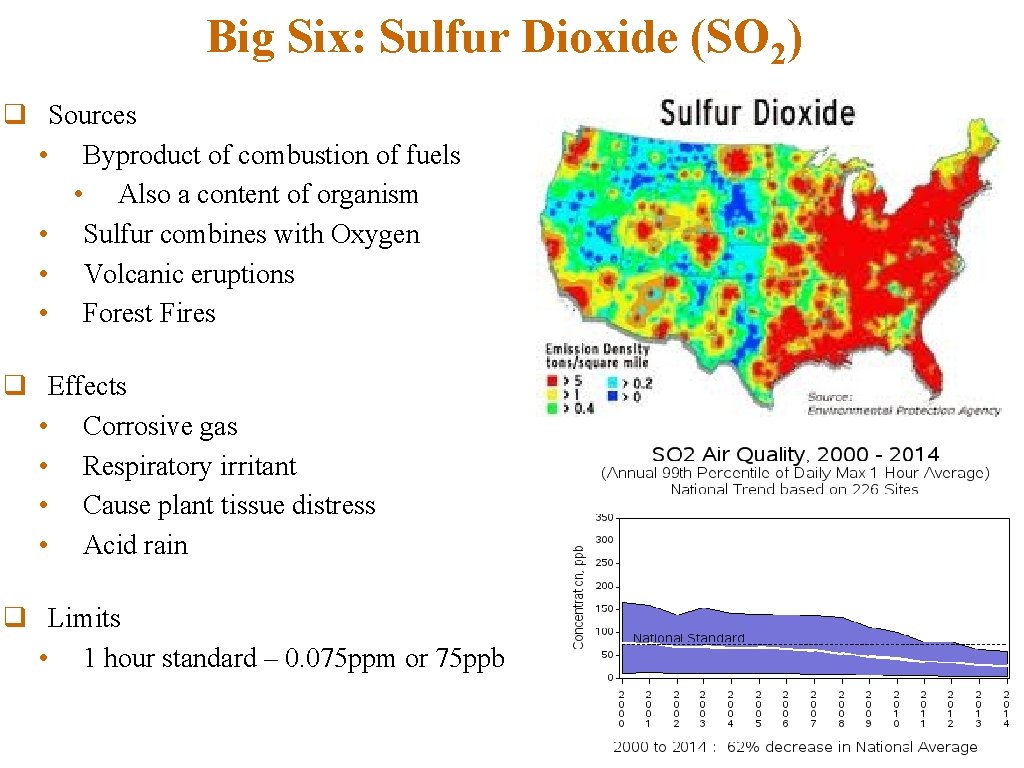
Big Six: Sulfur Dioxide (SO 2) q Sources • Byproduct of combustion of fuels • Also a content of organism • Sulfur combines with Oxygen • Volcanic eruptions • Forest Fires q Effects • Corrosive gas • Respiratory irritant • Cause plant tissue distress • Acid rain q Limits • 1 hour standard – 0. 075 ppm or 75 ppb

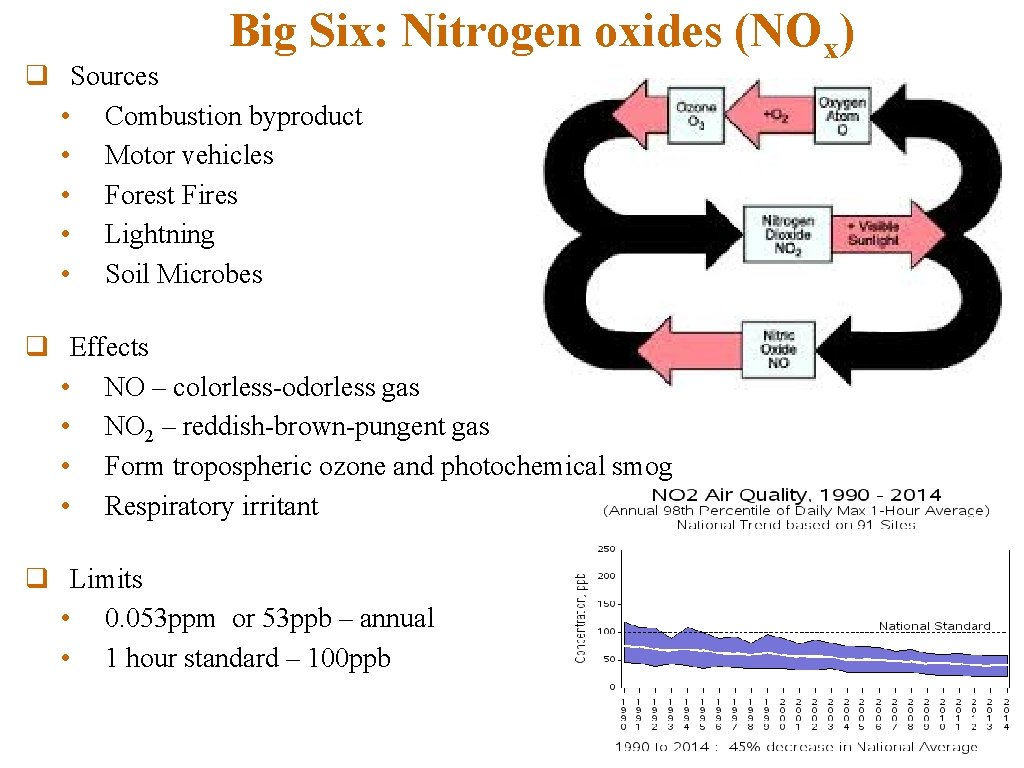
Big Six: Nitrogen oxides (NOx) q Sources • Combustion byproduct • Motor vehicles • Forest Fires • Lightning • Soil Microbes q Effects • NO – colorless-odorless gas • NO 2 – reddish-brown-pungent gas • Form tropospheric ozone and photochemical smog • Respiratory irritant q Limits • 0. 053 ppm or 53 ppb – annual • 1 hour standard – 100 ppb

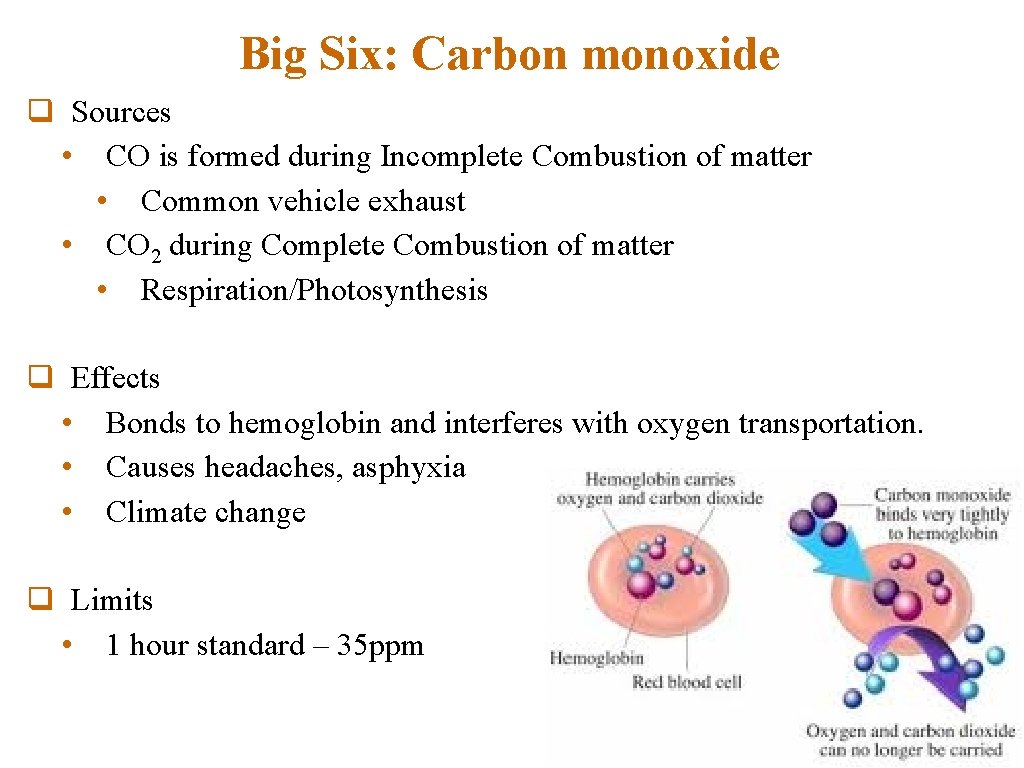
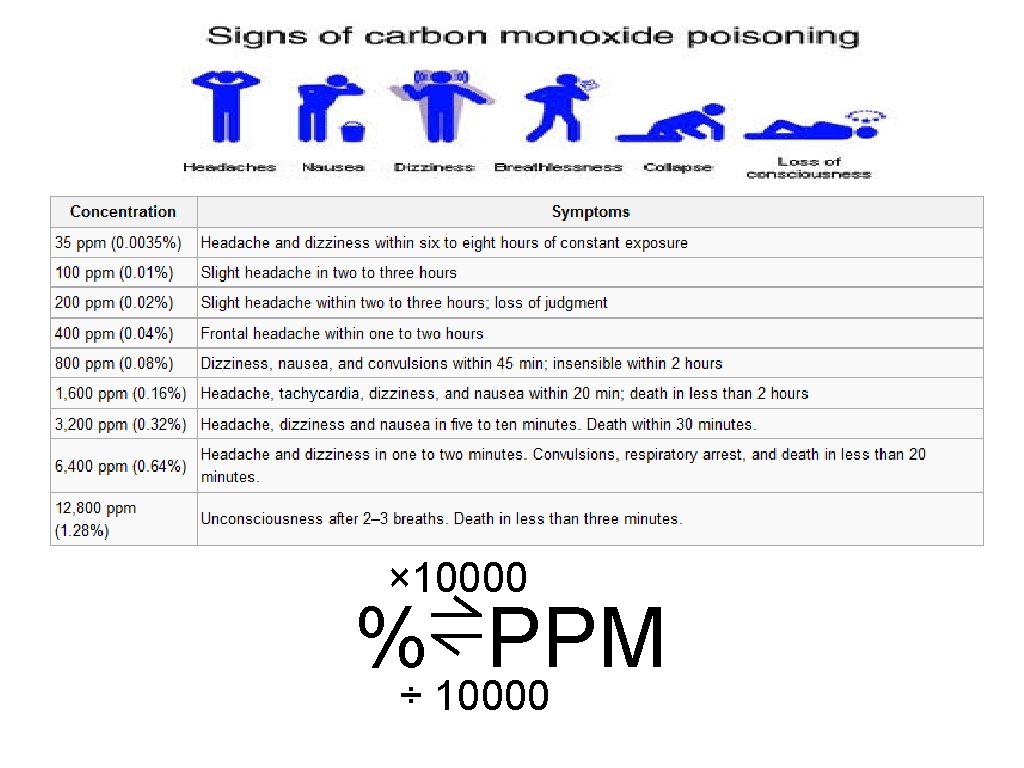
Big Six: Carbon monoxide q Sources • CO is formed during Incomplete Combustion of matter • Common vehicle exhaust • CO 2 during Complete Combustion of matter • Respiration/Photosynthesis q Effects • Bonds to hemoglobin and interferes with oxygen transportation. • Causes headaches, asphyxia • Climate change q Limits • 1 hour standard – 35 ppm

× 10000 %⇌PPM ÷ 10000

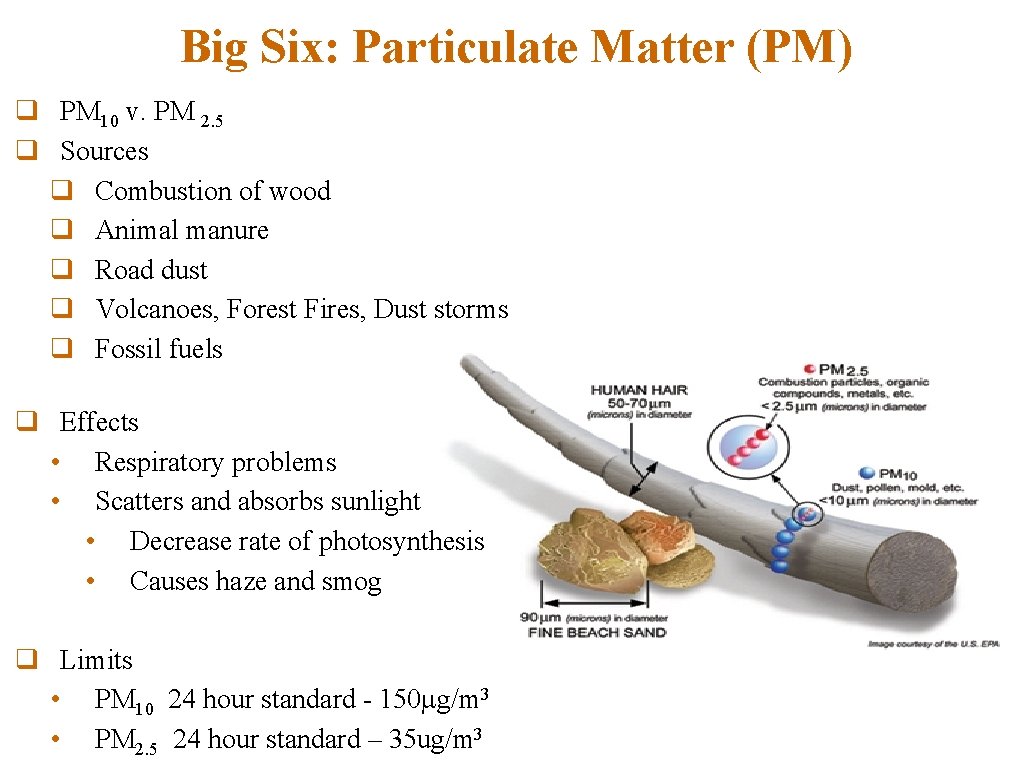
Big Six: Particulate Matter (PM) q PM 10 v. PM 2. 5 q Sources q Combustion of wood q Animal manure q Road dust q Volcanoes, Forest Fires, Dust storms q Fossil fuels q Effects • Respiratory problems • Scatters and absorbs sunlight • Decrease rate of photosynthesis • Causes haze and smog q Limits • PM 10 24 hour standard - 150 g/m 3 • PM 2. 5 24 hour standard – 35 ug/m 3


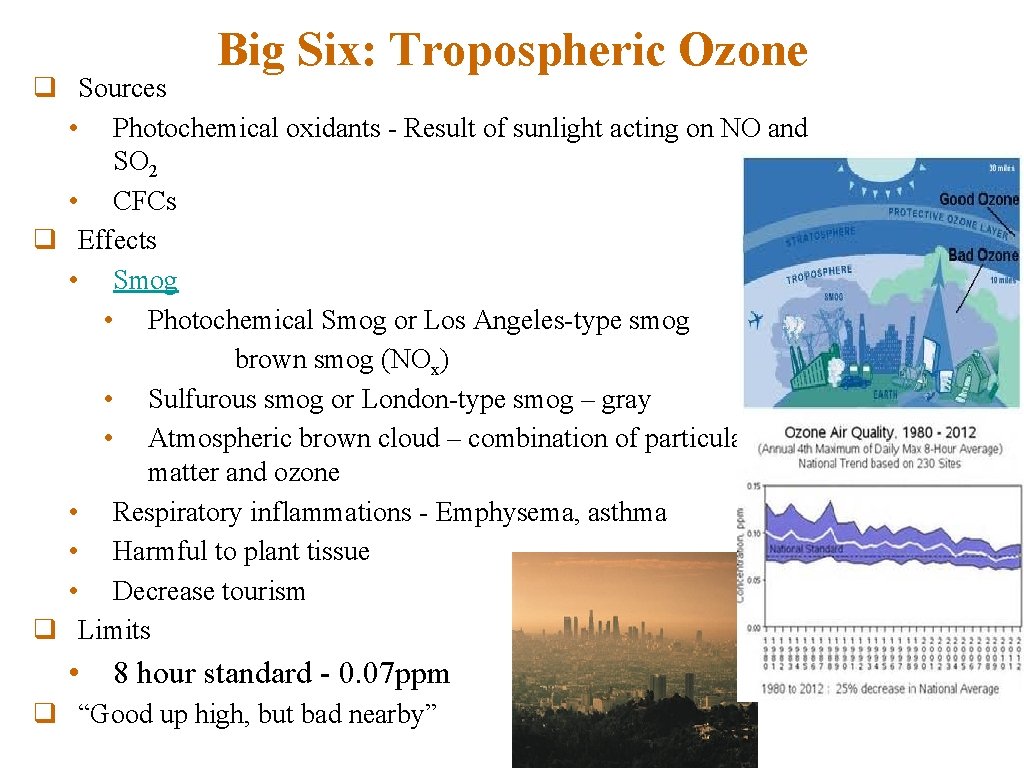
Big Six: Tropospheric Ozone q Sources • Photochemical oxidants - Result of sunlight acting on NO and SO 2 • CFCs q Effects • Smog • Photochemical Smog or Los Angeles-type smog brown smog (NOx) • Sulfurous smog or London-type smog – gray • Atmospheric brown cloud – combination of particulate matter and ozone • Respiratory inflammations - Emphysema, asthma • Harmful to plant tissue • Decrease tourism q Limits • 8 hour standard - 0. 07 ppm q “Good up high, but bad nearby”

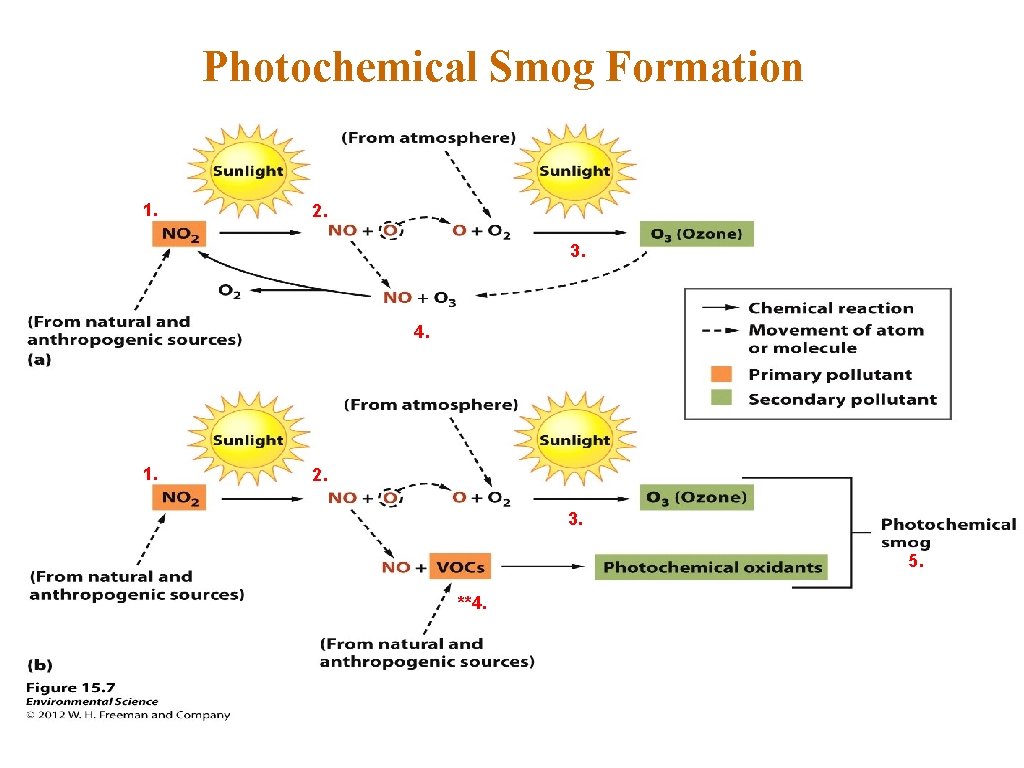
Photochemical Smog Formation 1. 2. 3. 4. 1. 2. 3. 5. **4.

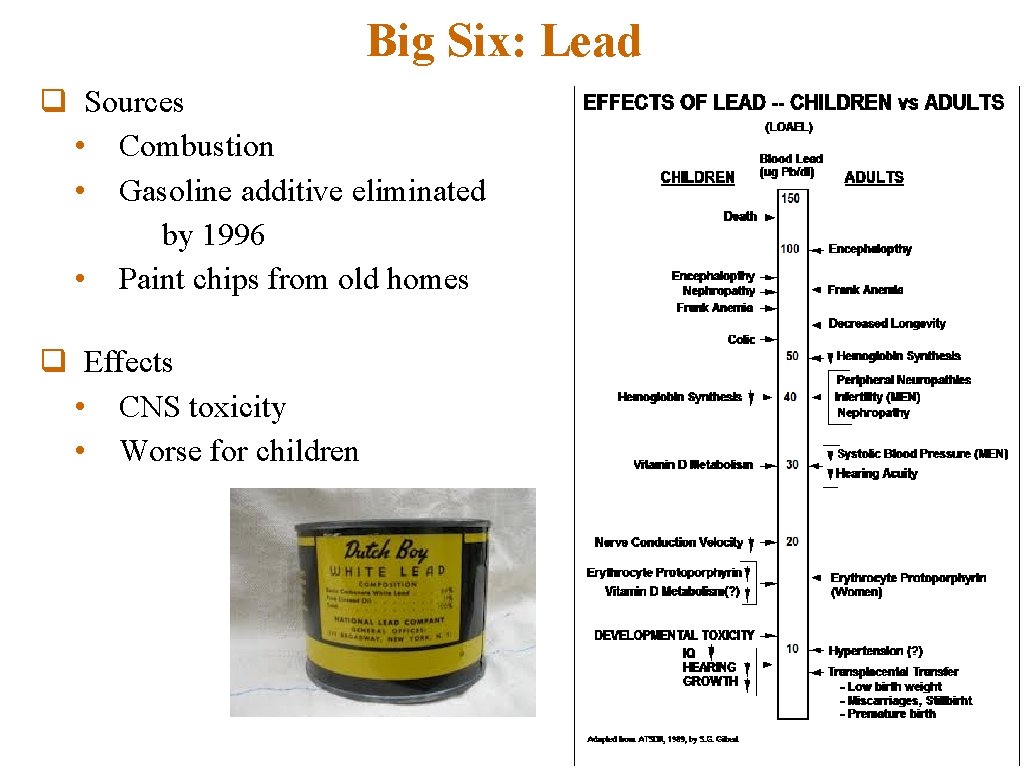
Big Six: Lead q Sources • Combustion • Gasoline additive eliminated by 1996 • Paint chips from old homes q Effects • CNS toxicity • Worse for children

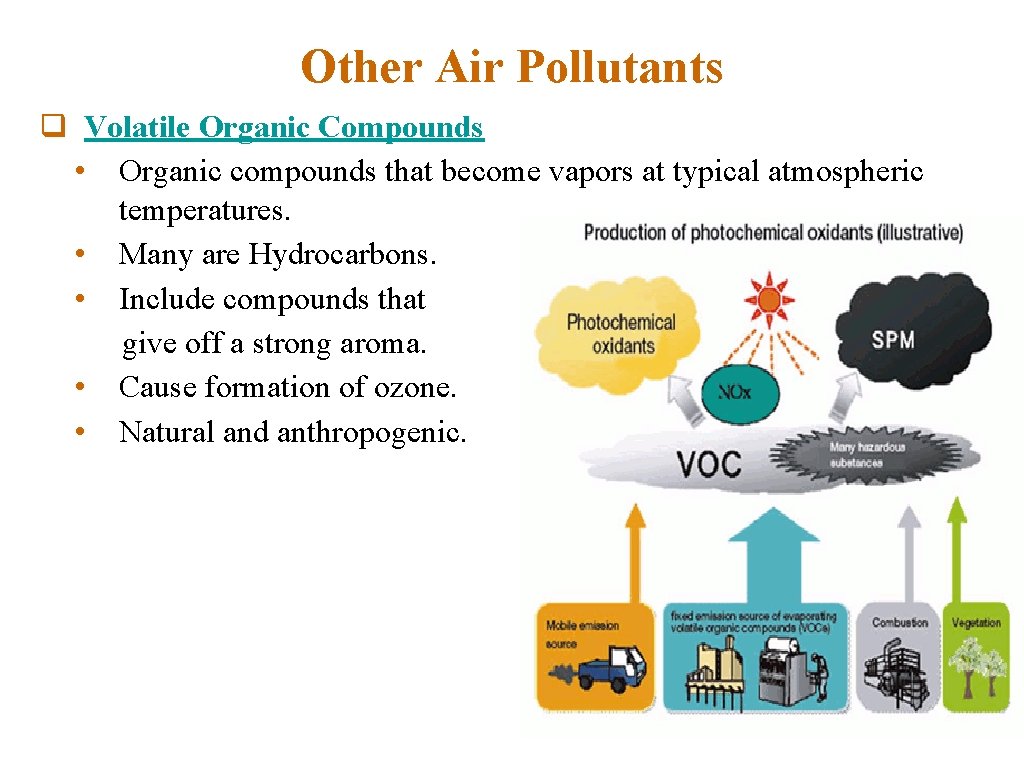
Other Air Pollutants q Volatile Organic Compounds • Organic compounds that become vapors at typical atmospheric temperatures. • Many are Hydrocarbons. • Include compounds that give off a strong aroma. • Cause formation of ozone. • Natural and anthropogenic.


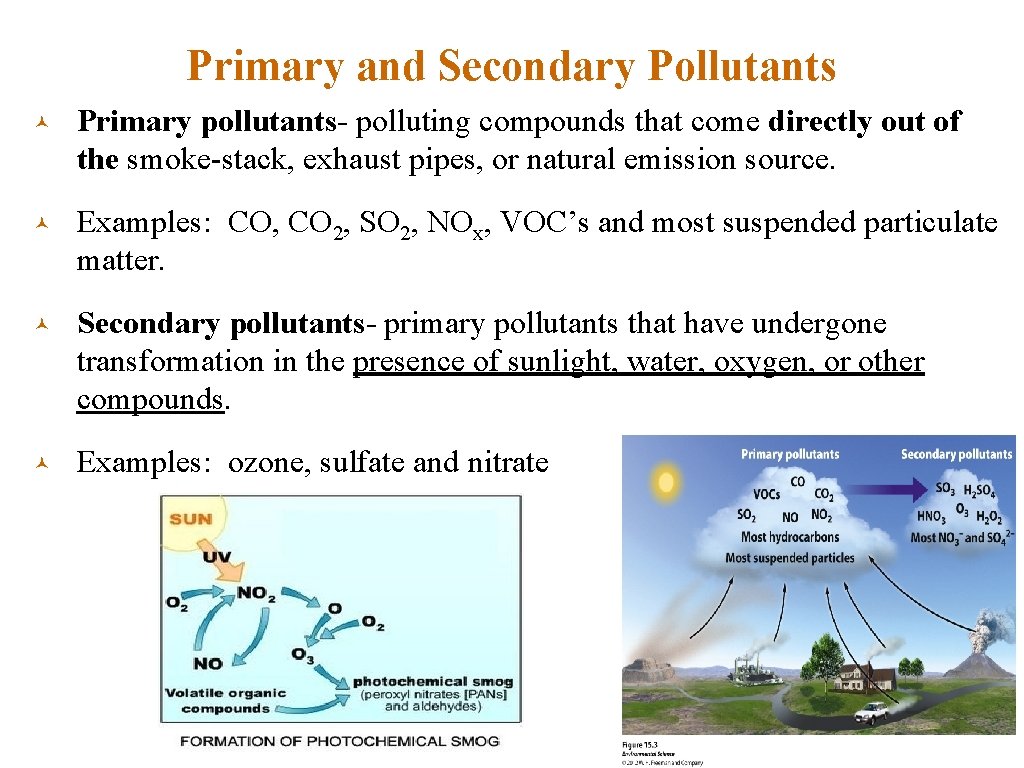
Primary and Secondary Pollutants © Primary pollutants- polluting compounds that come directly out of the smoke-stack, exhaust pipes, or natural emission source. © Examples: CO, CO 2, SO 2, NOx, VOC’s and most suspended particulate matter. © Secondary pollutants- primary pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. © Examples: ozone, sulfate and nitrate

Natural and Anthropogenic Sources of Air Pollution © Natural sources of air pollution lead to smog and photochemical oxidant pollution. © © The Blue Ridge Mts. and The Smokey Mts. Natural Emissions include:

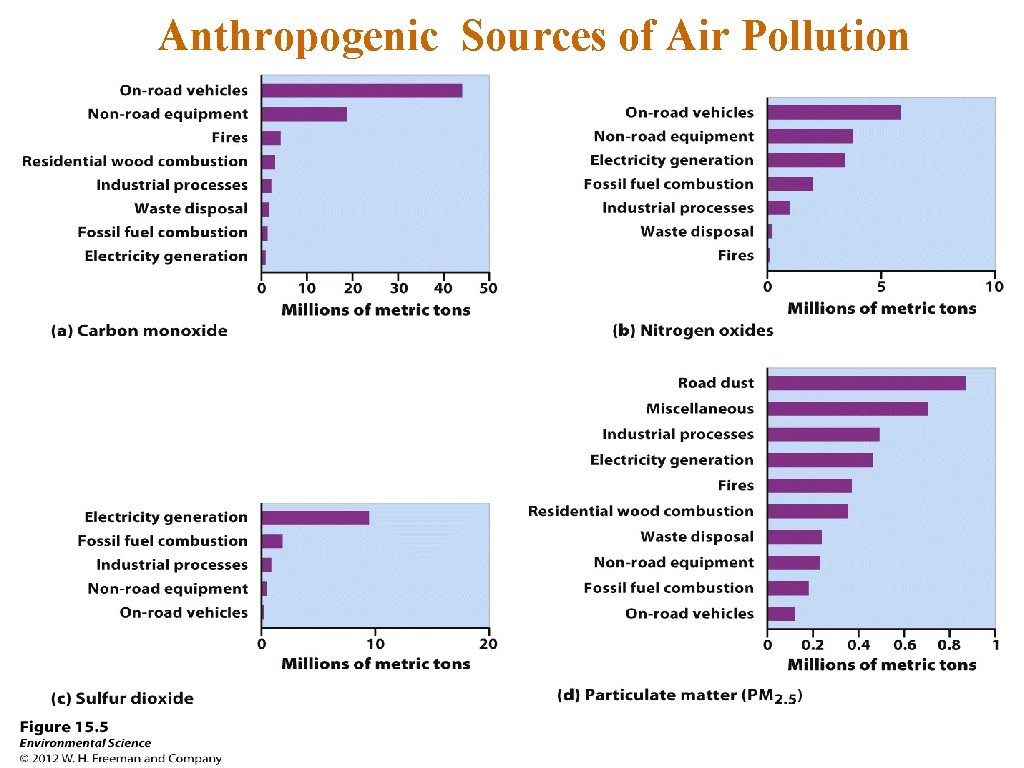
Anthropogenic Sources of Air Pollution


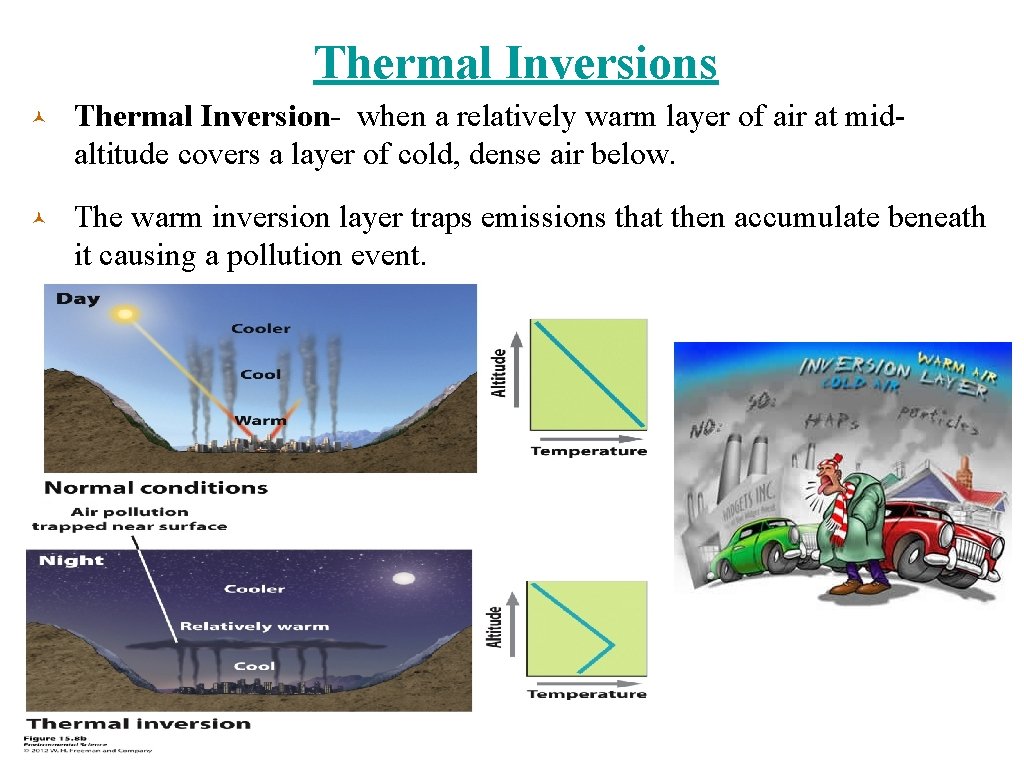
Thermal Inversions © Thermal Inversion- when a relatively warm layer of air at midaltitude covers a layer of cold, dense air below. © The warm inversion layer traps emissions that then accumulate beneath it causing a pollution event.

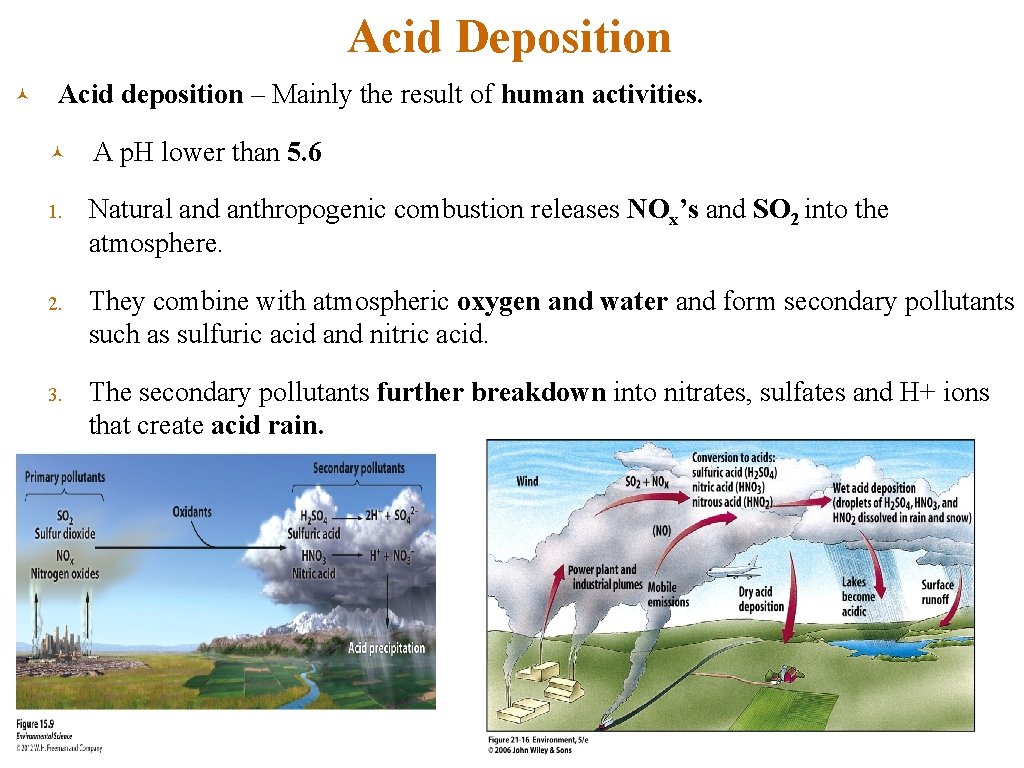
Acid Deposition © Acid deposition – Mainly the result of human activities. © A p. H lower than 5. 6 1. Natural and anthropogenic combustion releases NOx’s and SO 2 into the atmosphere. 2. They combine with atmospheric oxygen and water and form secondary pollutants such as sulfuric acid and nitric acid. 3. The secondary pollutants further breakdown into nitrates, sulfates and H+ ions that create acid rain.

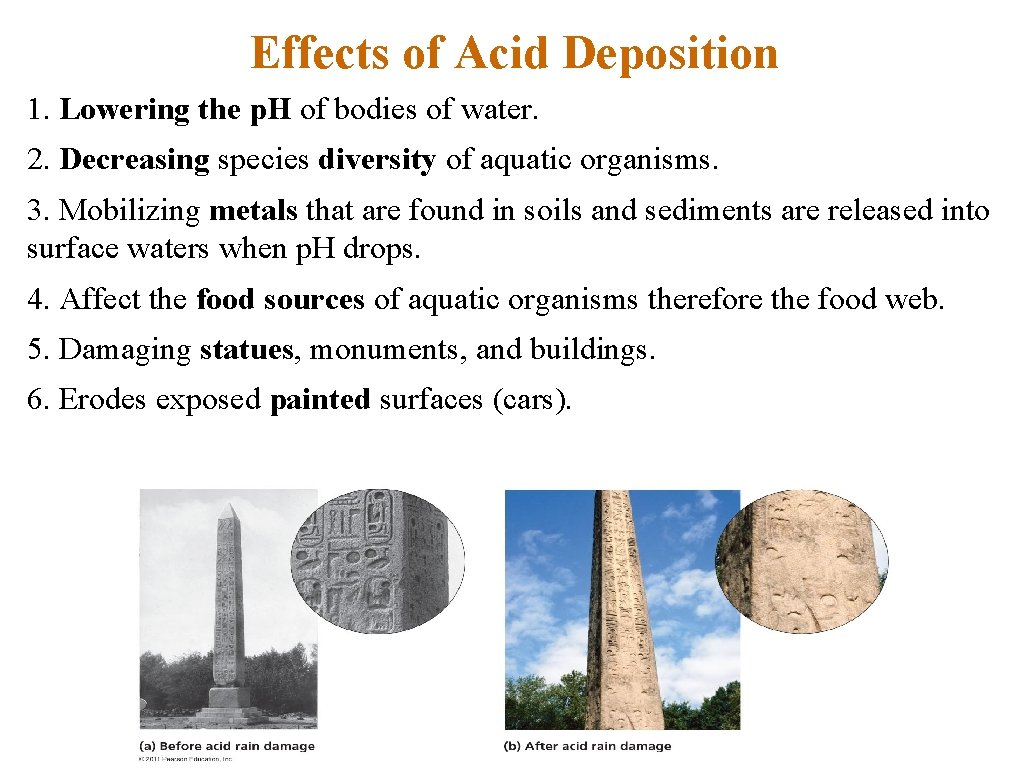
Effects of Acid Deposition 1. Lowering the p. H of bodies of water. 2. Decreasing species diversity of aquatic organisms. 3. Mobilizing metals that are found in soils and sediments are released into surface waters when p. H drops. 4. Affect the food sources of aquatic organisms therefore the food web. 5. Damaging statues, monuments, and buildings. 6. Erodes exposed painted surfaces (cars).

Ways to Prevent Air Pollution © 1. Use a low-sulfur coal or oil (cost more). © 2. Use less fuel. © 3. Alternative energy sources. © 4. Control of pollution after combustion. a. Control Sulfur and Nitrogen Oxide Emissions - Removing sulfur dioxide from coal exhaust during combustion by fluidized bed combustion. - Granulated coal burned in close proximity to calcium carbonate. - The heated Ca. CO 3 absorbs the SO 2 and produces calcium sulfate (used in production of sheetrock) - Ca. CO 3 + SO 2 Ca. SO 4

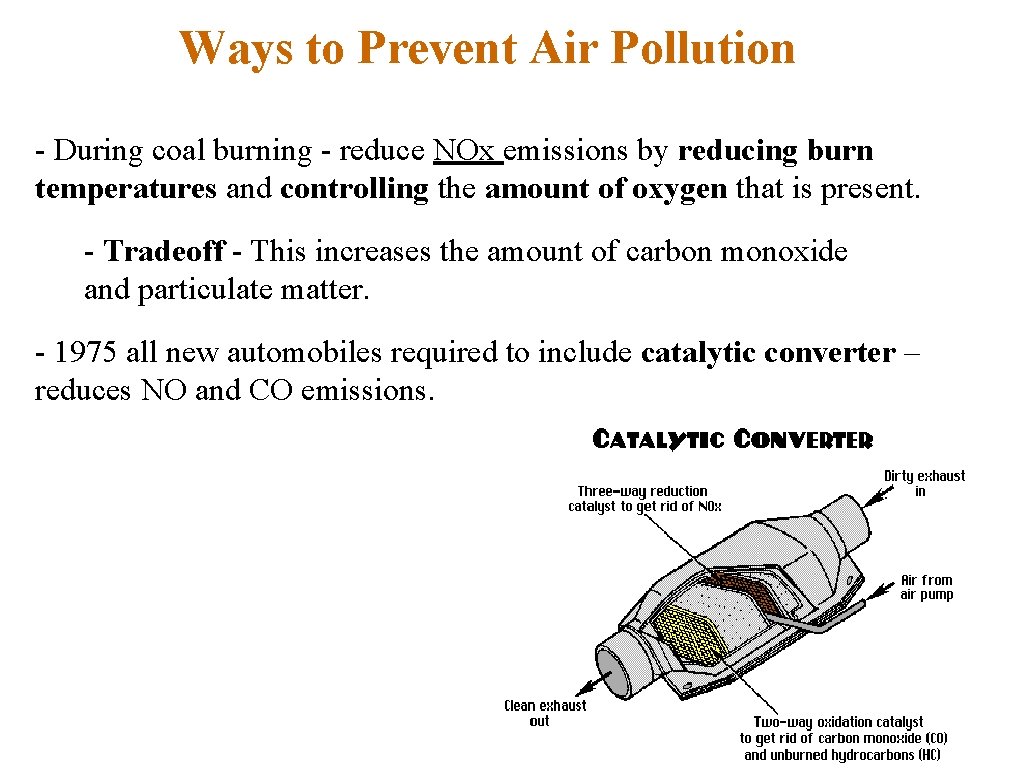
Ways to Prevent Air Pollution - During coal burning - reduce NOx emissions by reducing burn temperatures and controlling the amount of oxygen that is present. - Tradeoff - This increases the amount of carbon monoxide and particulate matter. - 1975 all new automobiles required to include catalytic converter – reduces NO and CO emissions.

Ways to Prevent Air Pollution b. Removal of Particulate Matter after combustion is the most common means of pollution control. 1. Gravitational settling – gravity removes some of the particles as the exhaust travels through the smokestack. © Disposal concerns – ash can contain sulfur and high conc. of metals. 2. Baghouse filters (fabric filters) –traps particulate matter through a series of filter bags as gas moves through it.

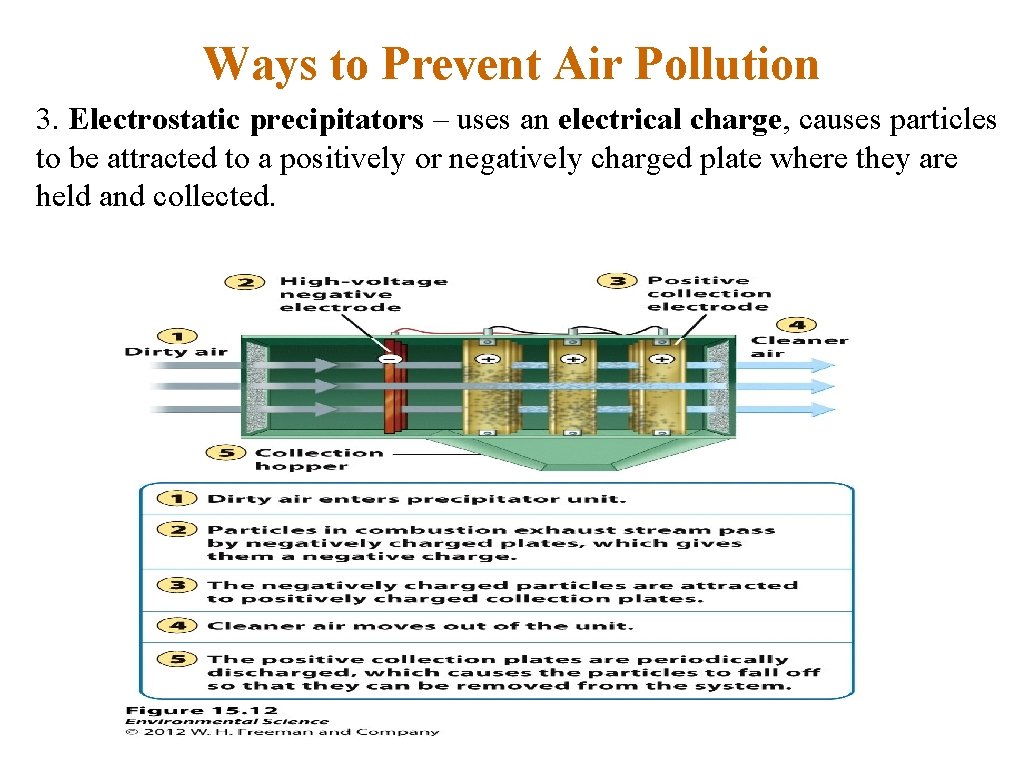
Ways to Prevent Air Pollution 3. Electrostatic precipitators – uses an electrical charge, causes particles to be attracted to a positively or negatively charged plate where they are held and collected.

Ways to Prevent Air Pollution 4. Scrubbers – uses a combination of water and air that separates and removes the particles in a sludge form allowing the clean gas to exit. - Also used to reduce the emissions of sulfur dioxide. *Downsides to all forms of pollution control devices:

Ways to Prevent Air Pollution © 1967 Air Quality Act - no standards © 1970 Clean Air Act (standards) / Earth Day © © 1980 Lead drops 50% in environment & drops 37% in blood 2000 Milestones: © Carbon Monoxide: 31% decrease © Sulfur Dioxide: 27% decrease © VOC’s down 42% © Particulate Matter* (PM-10): 71% decrease © Lead: 98% decrease

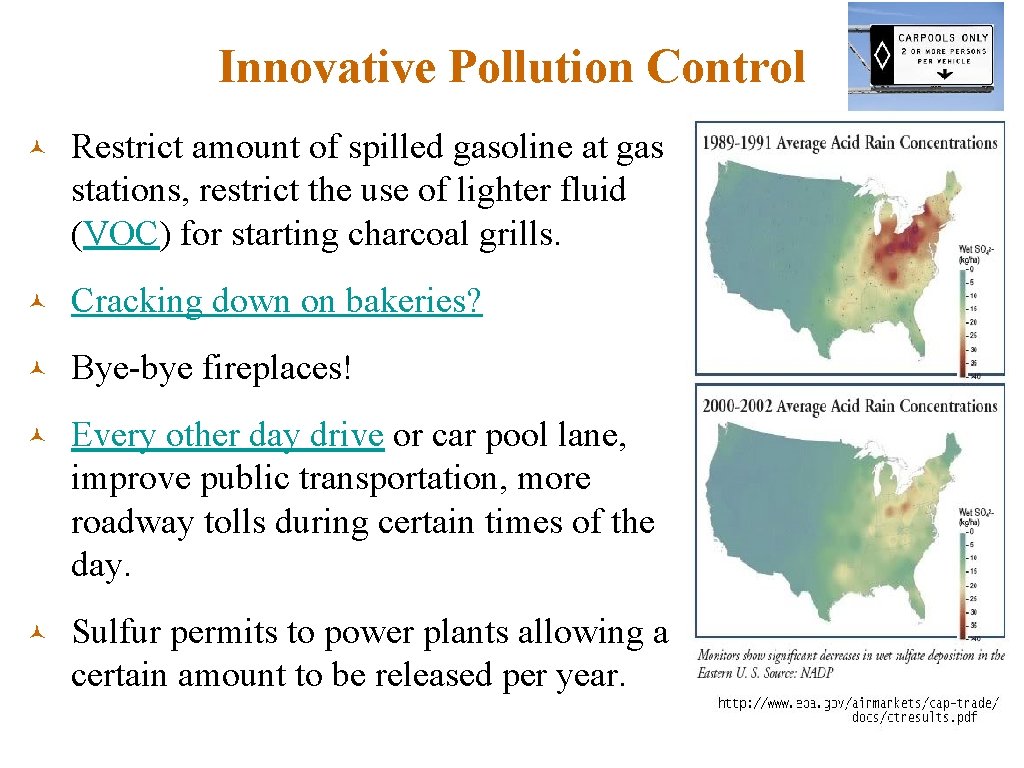
Innovative Pollution Control © Restrict amount of spilled gasoline at gas stations, restrict the use of lighter fluid (VOC) for starting charcoal grills. © Cracking down on bakeries? © Bye-bye fireplaces! © Every other day drive or car pool lane, improve public transportation, more roadway tolls during certain times of the day. © Sulfur permits to power plants allowing a certain amount to be released per year.

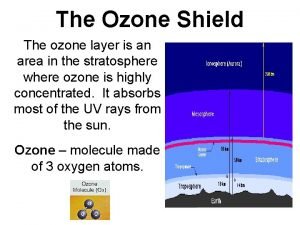
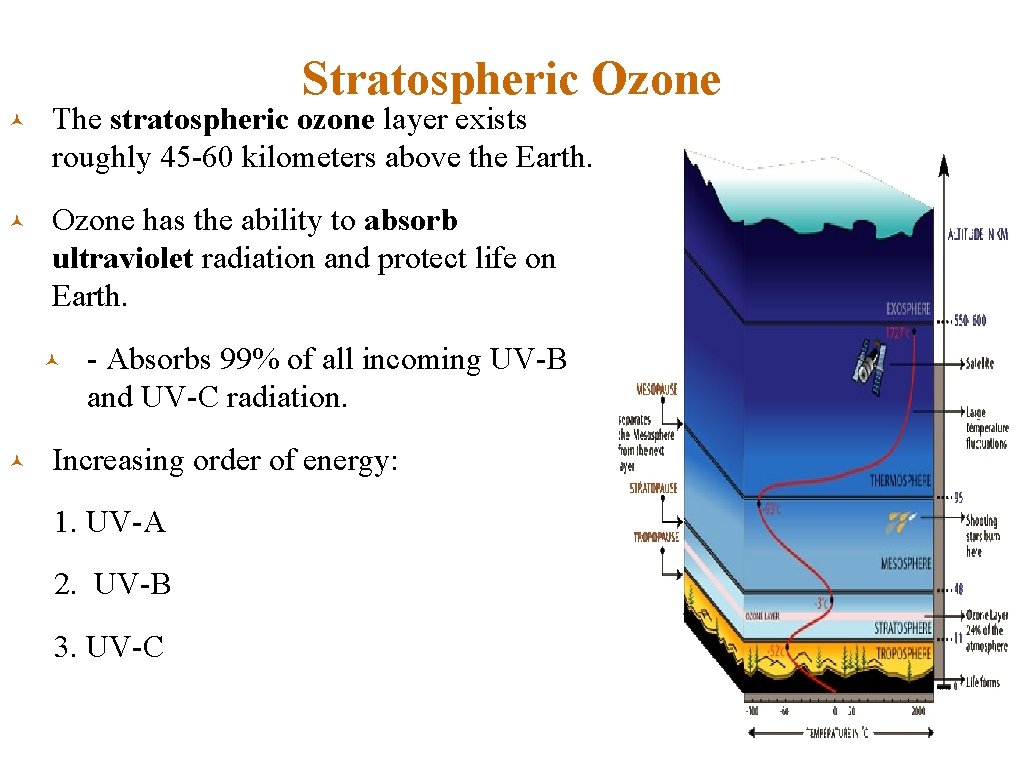
Stratospheric Ozone © The stratospheric ozone layer exists roughly 45 -60 kilometers above the Earth. © Ozone has the ability to absorb ultraviolet radiation and protect life on Earth. © © - Absorbs 99% of all incoming UV-B and UV-C radiation. Increasing order of energy: 1. UV-A 2. UV-B 3. UV-C

Formation and Breakdown of Ozone in the Stratospheric ozone forms and breaks down naturally in a closed-loop system in the presence of sunlight maintaining a steady state of ozone concentration. © First, UV-C radiation breaks the bonds holding together O 2 and leaves 2 free oxygen atoms. * only happens to a small fraction, most O 2 remains unaffected. O 2 + UV-C 2 O © Sometimes the remaining O 2 in the atmosphere reacts with the free Oxygen atoms and this results in ozone: O 2 + O O 3 © Ozone is broken down into O 2 and free oxygen atoms when it absorbs both UV-C and UV-B ultraviolet light: O 3 + UV-B or UV-C O 2 + O

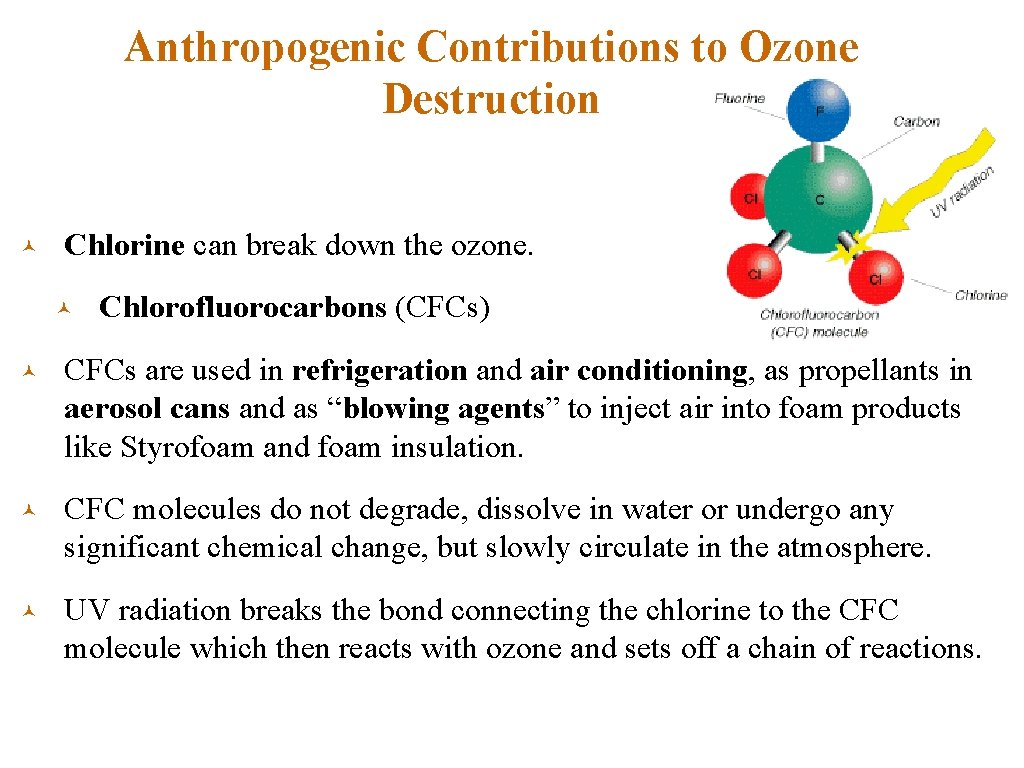
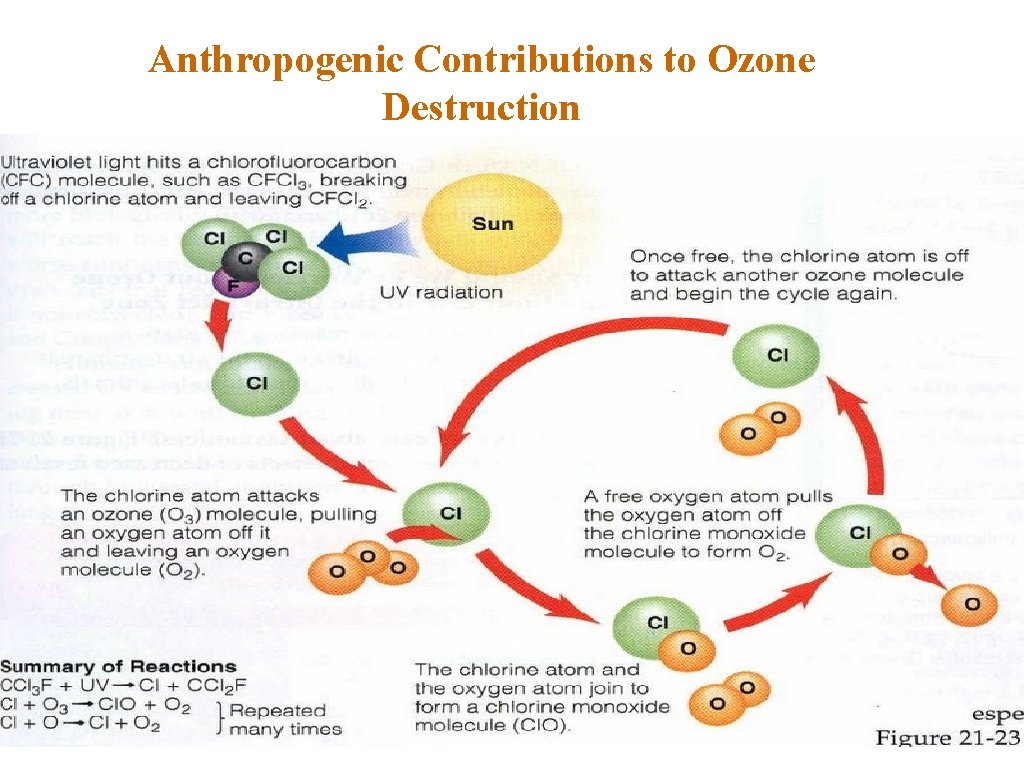
Anthropogenic Contributions to Ozone Destruction © Chlorine can break down the ozone. © Chlorofluorocarbons (CFCs) © CFCs are used in refrigeration and air conditioning, as propellants in aerosol cans and as “blowing agents” to inject air into foam products like Styrofoam and foam insulation. © CFC molecules do not degrade, dissolve in water or undergo any significant chemical change, but slowly circulate in the atmosphere. © UV radiation breaks the bond connecting the chlorine to the CFC molecule which then reacts with ozone and sets off a chain of reactions.

Anthropogenic Contributions to Ozone Destruction 1. Chlorine breaks ozone’s bonds and pulls off one atom of oxygen, forming a chlorine monoxide molecule and O 2: O 3 + Cl Cl. O + O 2 2. A free oxygen atoms pulls the oxygen atom from Cl. O, liberating the chlorine and creating one oxygen molecule: Cl. O + O Cl + O 2 3. This free Cl atom can catalyze the breakdown of as many as 100, 000 ozone molecules before it leaves the stratosphere. 4. The ozone that Cl breaks down is no longer available to absorb incoming UV-B radiation.

Anthropogenic Contributions to Ozone Destruction

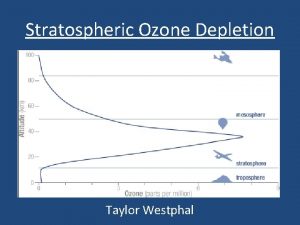
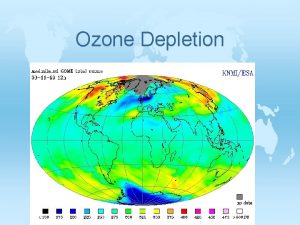
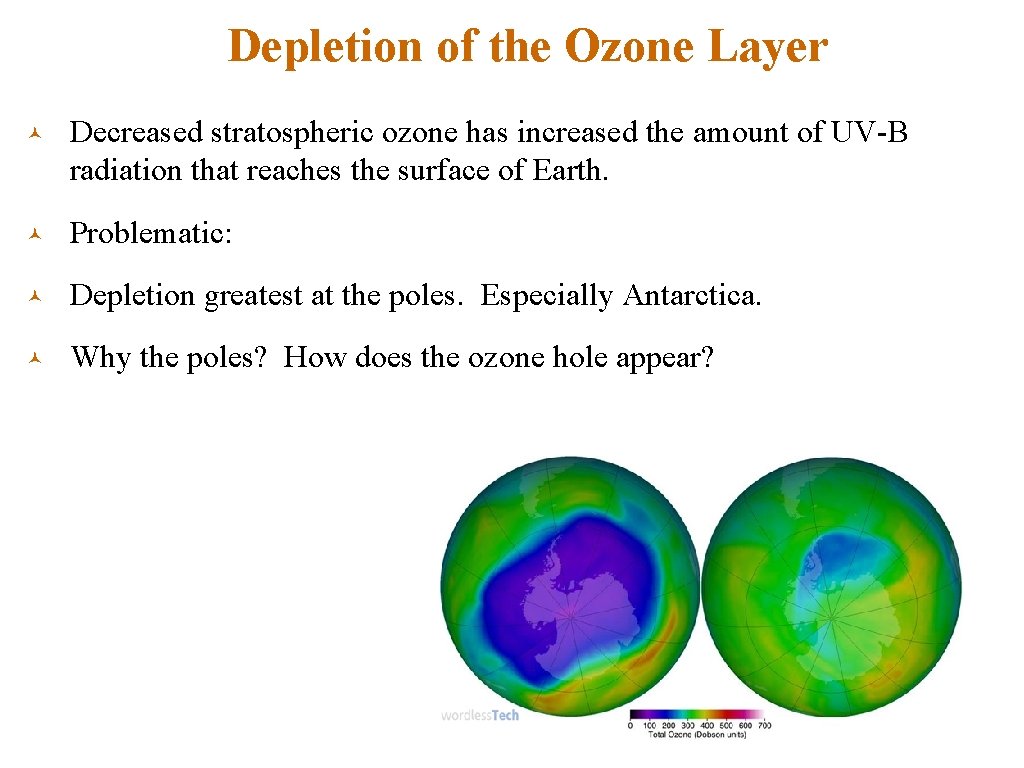
Depletion of the Ozone Layer © Decreased stratospheric ozone has increased the amount of UV-B radiation that reaches the surface of Earth. © Problematic: © Depletion greatest at the poles. Especially Antarctica. © Why the poles? How does the ozone hole appear?

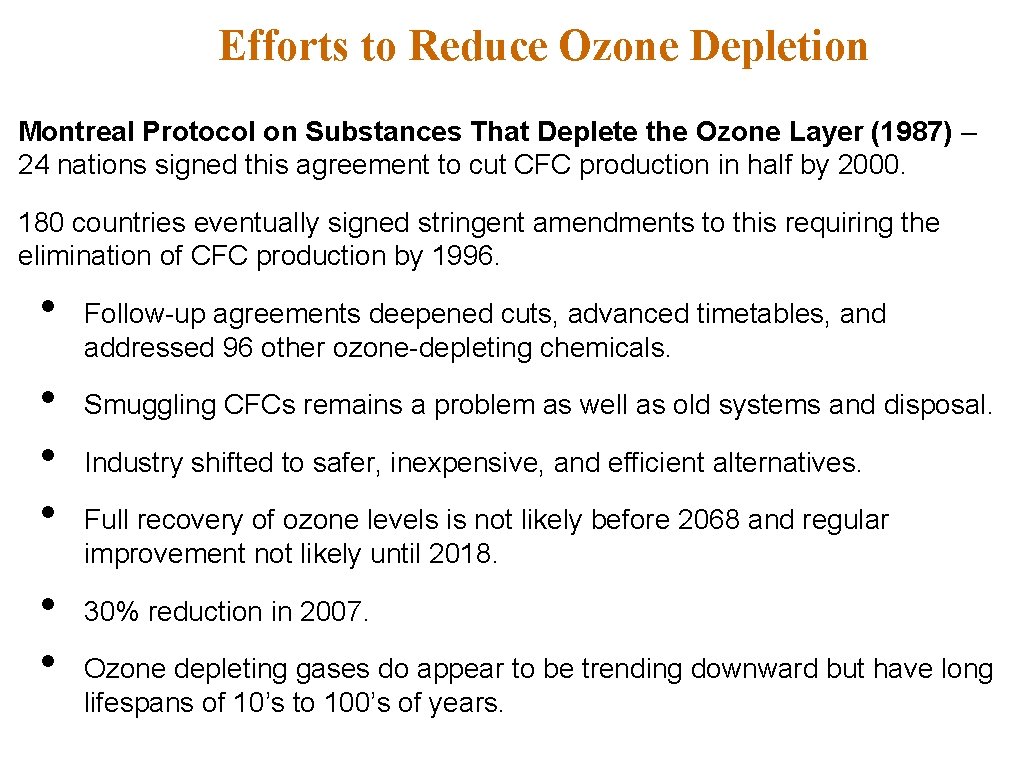
Efforts to Reduce Ozone Depletion Montreal Protocol on Substances That Deplete the Ozone Layer (1987) – 24 nations signed this agreement to cut CFC production in half by 2000. 180 countries eventually signed stringent amendments to this requiring the elimination of CFC production by 1996. • • • Follow-up agreements deepened cuts, advanced timetables, and addressed 96 other ozone-depleting chemicals. Smuggling CFCs remains a problem as well as old systems and disposal. Industry shifted to safer, inexpensive, and efficient alternatives. Full recovery of ozone levels is not likely before 2068 and regular improvement not likely until 2018. 30% reduction in 2007. Ozone depleting gases do appear to be trending downward but have long lifespans of 10’s to 100’s of years.

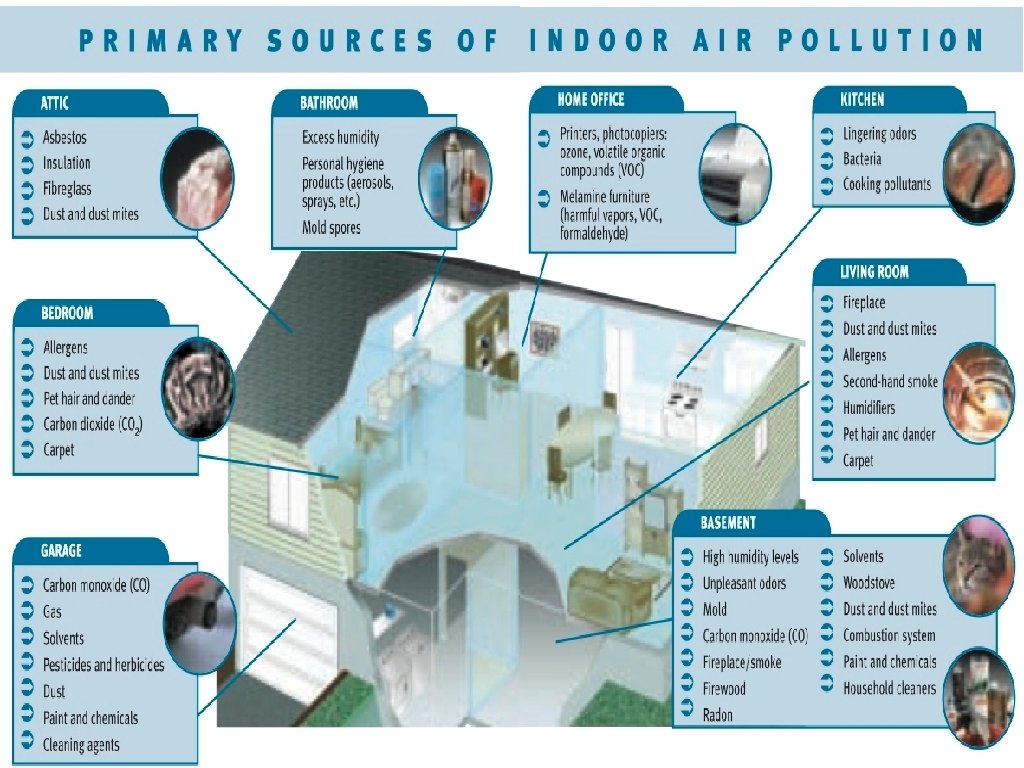
Indoor Air Pollutants © Indoor air pollutants causes more deaths than outdoor pollutants © More than 3 billion people worldwide use wood, animal manure or coal indoors for cooking and heating in developing countries. © Increases the risk of: © 90% of deaths attributable to indoor air pollution are in developing countries © Asbestos © Carbon Monoxide © Radon-222 © VOCs in home products © Tobacco smoke © Sick building syndrome

Indoor air pollution

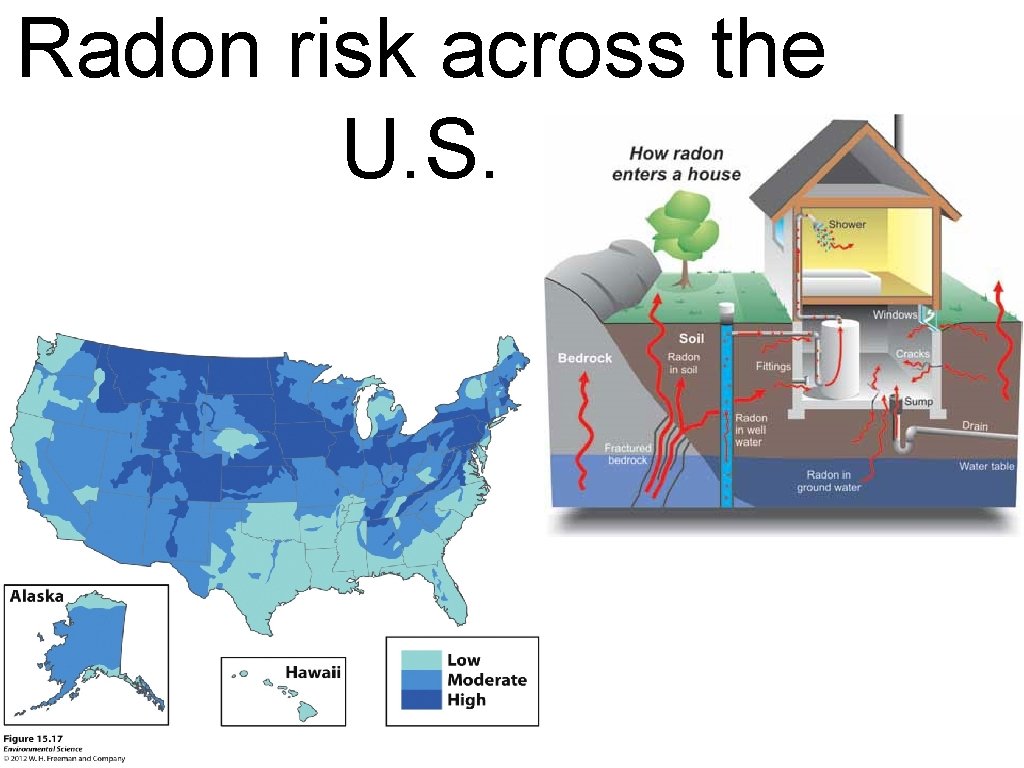
Radon risk across the U. S.

VOC’s • The most diverse group of indoor air pollutants • Released by everything from plastics and oils to perfumes and paints • Most VOCs are released in very small amounts • Unclear health implications due to low concentrations • Formaldehyde leaking from pressed wood and insulation irritates mucous membranes and induces skin allergies • Pesticides seep through floors and walls, also brought in on shoe soles In developed countries: • Use low-toxicity materials, limit use of plastics and treated wood, monitor air quality, keep rooms clean • Provide adequate ventilation • Limit exposure to known toxicants In developing countries: • Dry wood before burning • Cook outside • Use less-polluting fuels (natural gas)

Five Easy Ways to Improve Your Indoor Air Quality Open a window. Even if it’s chilly outside, you should open a window for even five minutes a day to significantly decrease the concentrations of indoor air pollutants in your home. Buy safer products. Everything you bring into your home impacts your indoor air quality. Choose unscented products as much as possible. Let anything with a “new smell” air out in a well-ventilated space or outdoors. Maintain humidity below 50 percent. Dehumidifying is enormously important, many individuals (particularly asthmatics) are highly allergic to mildew and molds. Droplets of water on windows, walls, or pipes are a sign of humidity. Minimize pet dander. Vacuum using a machine with a HEPA filter, dust frequently, wash drapes and rugs regularly, wash bedding weekly. Don’t harbor dust mites. Microscopic dust mites and their droppings are a potent allergen and asthma trigger. One of the best ways to limit the amount of dust mites in your homes are to encase mattresses with impermeable covers (just be sure they’re PVC-free).
 Stratospheric ozone depletion
Stratospheric ozone depletion Negative effects of ozone layer depletion
Negative effects of ozone layer depletion Ozone depletion effects on humans
Ozone depletion effects on humans Causes of the ozone depletion
Causes of the ozone depletion Ozone layer depletion introduction
Ozone layer depletion introduction Ozone depletion diagram
Ozone depletion diagram Global warming vs ozone depletion
Global warming vs ozone depletion Protective ozone layer
Protective ozone layer Ozone layer levels
Ozone layer levels How is smog formed
How is smog formed Chapter 12 section 1 what causes air pollution answers key
Chapter 12 section 1 what causes air pollution answers key Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Polar stratospheric clouds
Polar stratospheric clouds Polar stratospheric clouds
Polar stratospheric clouds Polar stratospheric clouds
Polar stratospheric clouds Stratospheric balloon
Stratospheric balloon Chapter 11 depreciation
Chapter 11 depreciation Journal entry for revaluation of assets
Journal entry for revaluation of assets Chapter 11 intermediate accounting
Chapter 11 intermediate accounting Air higroskopis air kapiler dan air gravitasi
Air higroskopis air kapiler dan air gravitasi Effects of land pollution on human health
Effects of land pollution on human health Land water and air pollution
Land water and air pollution Air pollution aims and objectives
Air pollution aims and objectives What are primary and secondary air pollutants
What are primary and secondary air pollutants Ari rokeach
Ari rokeach Baghouse filter definition apes
Baghouse filter definition apes Air noise and light pollution
Air noise and light pollution Section 2 air noise and light pollution
Section 2 air noise and light pollution Controlling measures of noise pollution
Controlling measures of noise pollution Introduction of pollution
Introduction of pollution Effect of air pollution in plants
Effect of air pollution in plants Contents of air pollution
Contents of air pollution Main cause of air pollution
Main cause of air pollution Air pollution box model example
Air pollution box model example General effects of air pollution
General effects of air pollution Air pollution consequences
Air pollution consequences Erg air pollution control
Erg air pollution control Air pollution
Air pollution Objectives of air pollution
Objectives of air pollution Pollution control laws in india
Pollution control laws in india Air pollution
Air pollution What is inorganic pollution
What is inorganic pollution Nultify
Nultify