Chapter 15 Air Pollution and Stratospheric Ozone Depletion





















- Slides: 32

Chapter 15 Air Pollution and Stratospheric Ozone Depletion

Air Pollution © Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems. This is at the level of the troposphere – ground level. There are natural and anthropogenic sources.

Major Air Pollutants © These are all criteria air pollutants because under the Clean Air Act 1970 the EPA must set allowable concentrations of each pollutant. © Sulfur Dioxide © Nitrogen Oxides © Carbon Oxides © Particulate Matter - haze © Volatiles Organic Compounds © Ozone - (oxidants + particulates = smog) (photochemical smog = brown/LA = dominated by ozone) (Sulfurous smog = London/gray smog) © Lead © Mercury




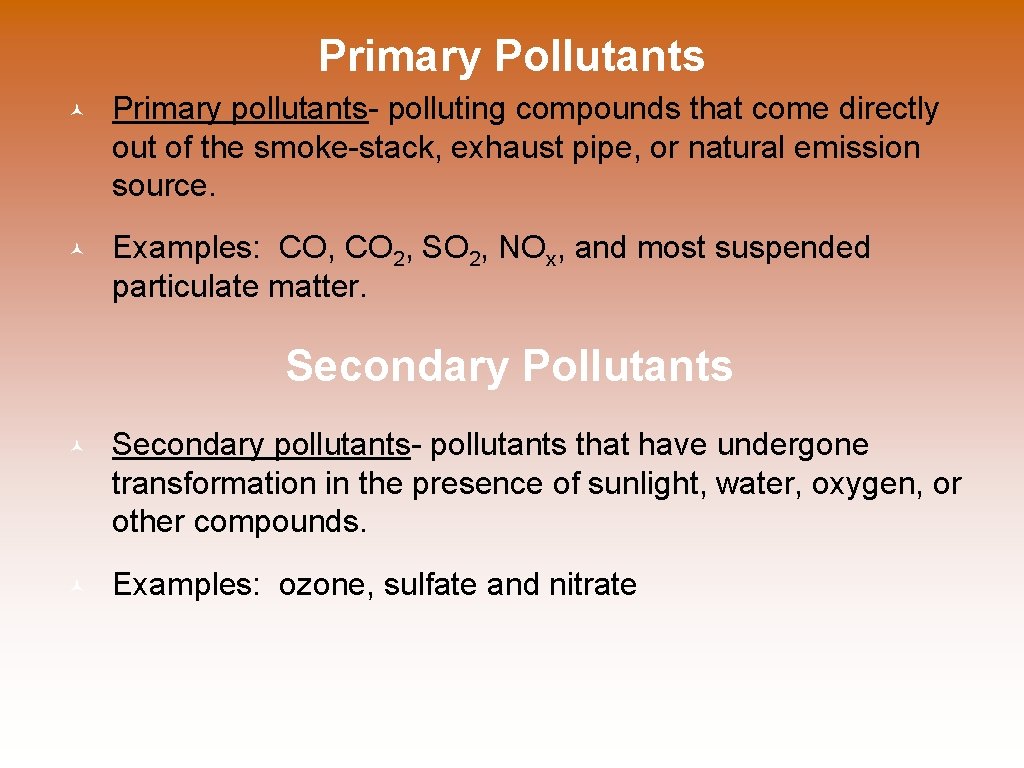
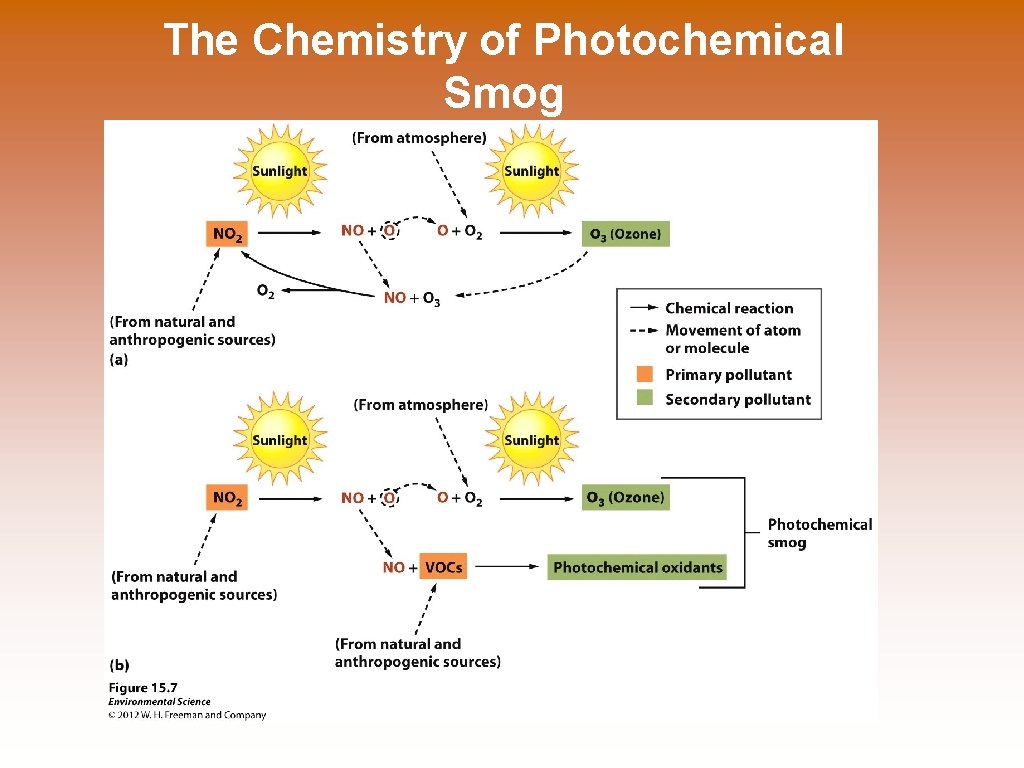
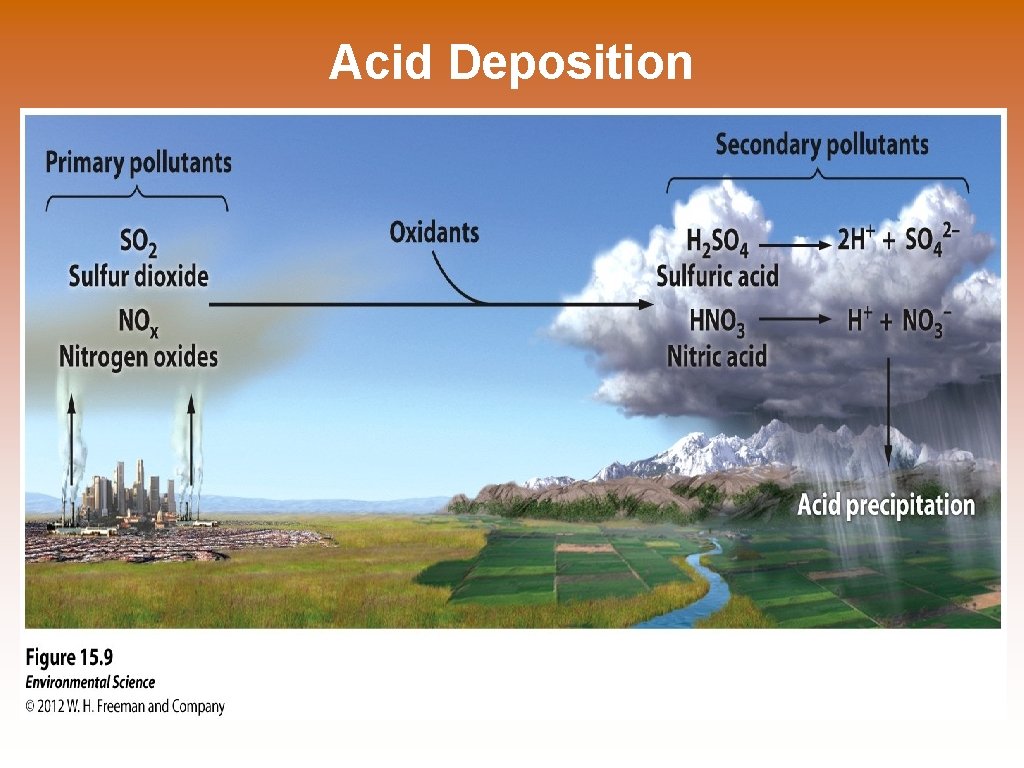
Primary Pollutants © Primary pollutants- polluting compounds that come directly out of the smoke-stack, exhaust pipe, or natural emission source. © Examples: CO, CO 2, SO 2, NOx, and most suspended particulate matter. Secondary Pollutants © Secondary pollutants- pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. © Examples: ozone, sulfate and nitrate


Natural Sources of Air Pollution © Volcanoes © Lightning © Forest fires © Plants

Anthropogenic Sources of Air Pollution © On-road vehicles © Power plants © Industrial processes © Waste disposal

Bejing

Bejing Air Pollution



The Chemistry of Photochemical Smog

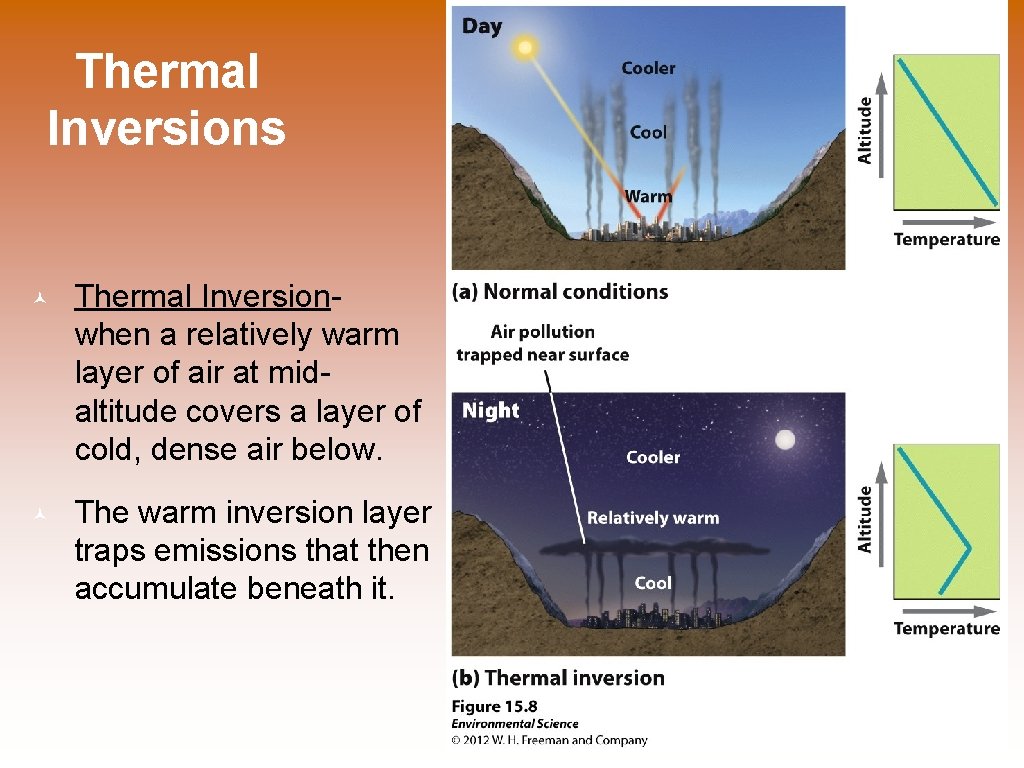
Thermal Inversions © Thermal Inversionwhen a relatively warm layer of air at midaltitude covers a layer of cold, dense air below. © The warm inversion layer traps emissions that then accumulate beneath it.

Acid Deposition

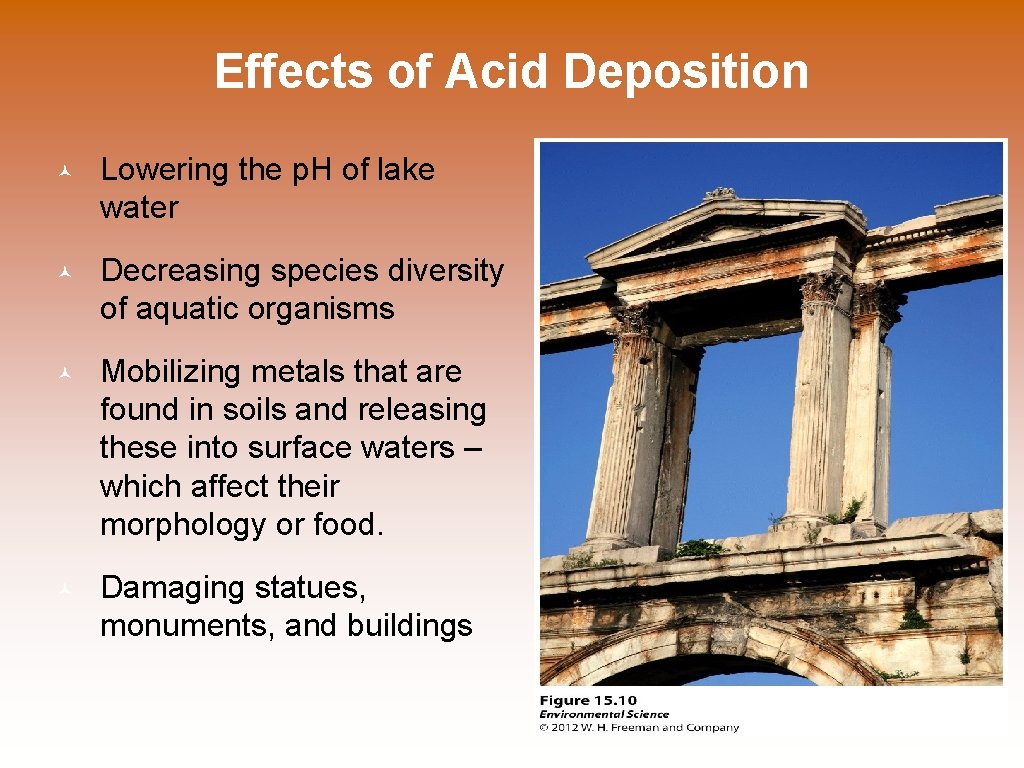
Effects of Acid Deposition © Lowering the p. H of lake water © Decreasing species diversity of aquatic organisms © Mobilizing metals that are found in soils and releasing these into surface waters – which affect their morphology or food. © Damaging statues, monuments, and buildings

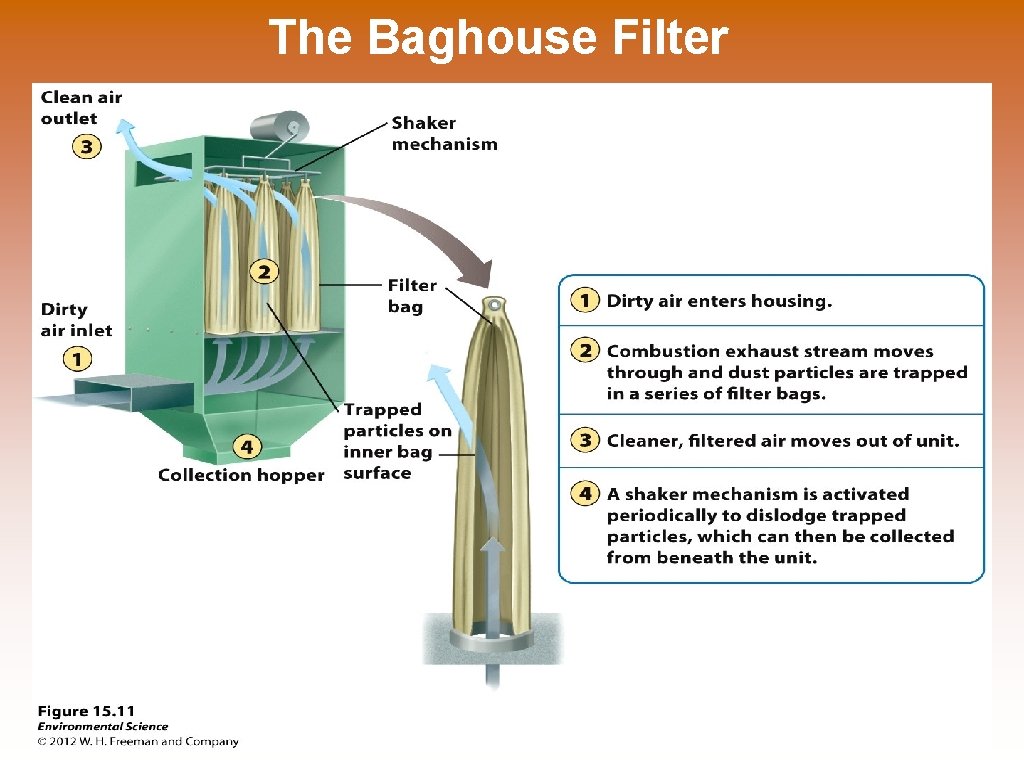
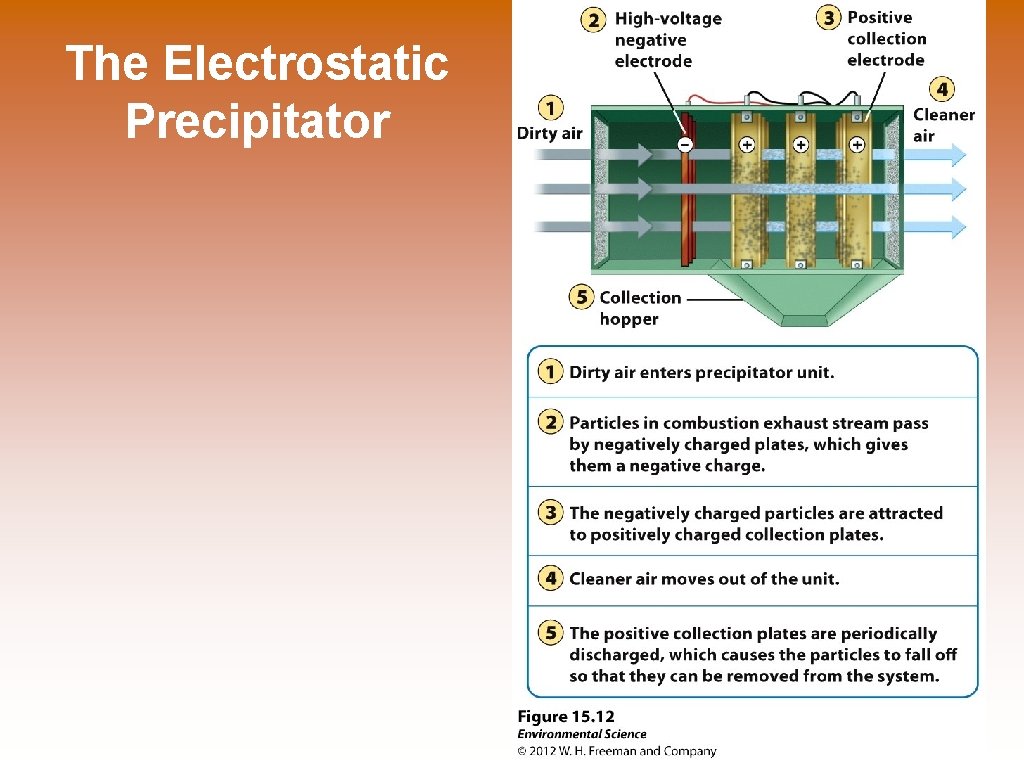
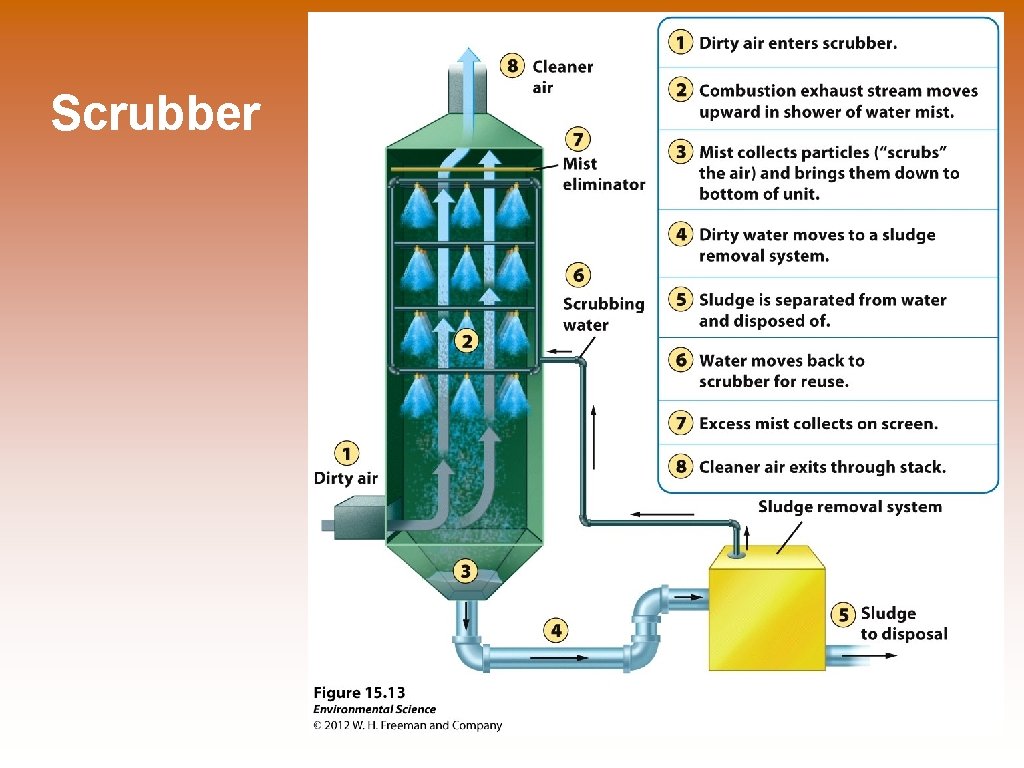
Ways to Prevent Air Pollution © Removing sulfur dioxide from coal by fluidized bed combustion – this occurs when granulated coal is burned in close proximity to calcium carbonate. The heated calcium carbonate absorbs sulfur dioxide to produce calcium sulfate, which is turned into gypsum for wallboard in homes. © Catalytic converters on cars © Scrubbers on smoke stacks © Baghouse filters © Electrostatic precipitators

The Baghouse Filter

The Electrostatic Precipitator

Scrubber

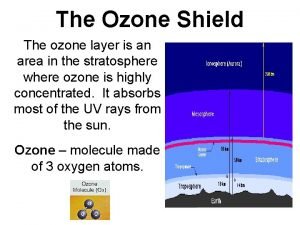
Stratospheric Ozone © The stratospheric ozone layer exists roughly 45 -60 kilometers above the Earth. © Ozone has the ability to absorb ultraviolet radiation and protect life on Earth.

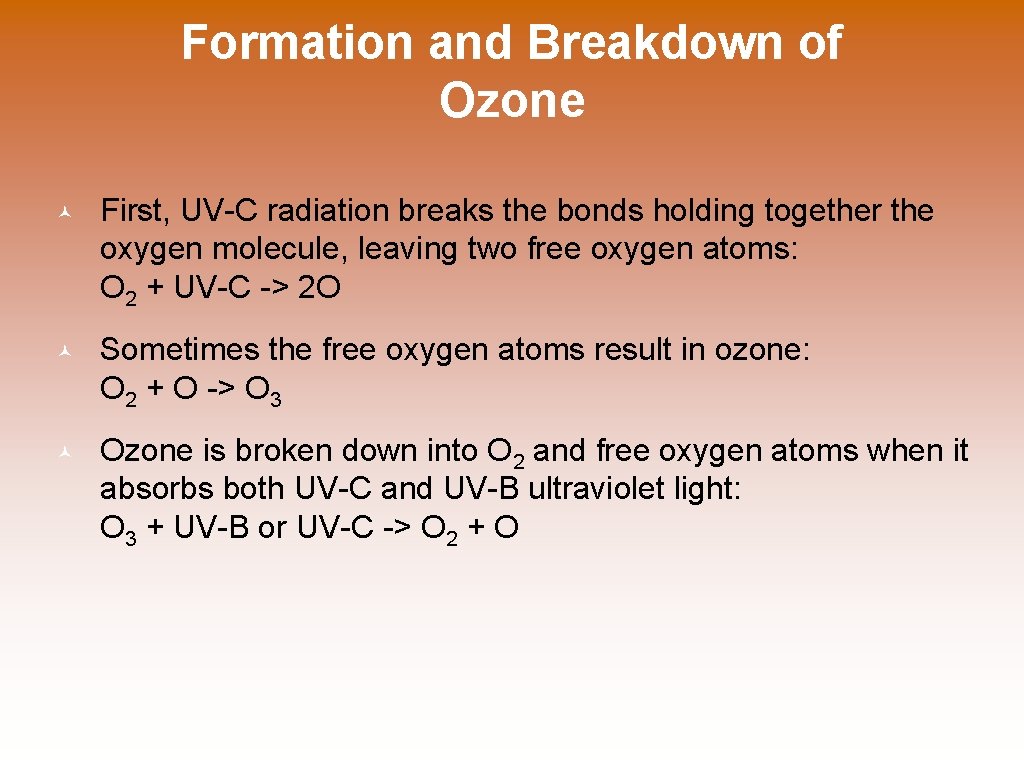
Formation and Breakdown of Ozone © First, UV-C radiation breaks the bonds holding together the oxygen molecule, leaving two free oxygen atoms: O 2 + UV-C -> 2 O © Sometimes the free oxygen atoms result in ozone: O 2 + O -> O 3 © Ozone is broken down into O 2 and free oxygen atoms when it absorbs both UV-C and UV-B ultraviolet light: O 3 + UV-B or UV-C -> O 2 + O

Anthropogenic Contributions to Ozone Destruction © Certain chemicals can break down ozone, particularly chlorine. © The major source of chlorine in the stratosphere is a compound known as chlorofluorocarbons (CFCs) © CFCs are used in refrigeration and air conditioning, as propellants in aerosol cans and as “blowing agents” to inject air into foam products like Styrofoam.

Anthropogenic Contributions to Ozone Destruction © When CFCs are released into the troposphere they don’t degrade, dissolve in water, or change chemically and make their way to the stratosphere. © The ultraviolet radiation present has enough energy to break the bond connecting chlorine to the CFC molecule. © First, chlorine breaks ozone’s bonds and pulls off one atom of oxygen, forming a chlorine monoxide molecule and O 2: O 3 + Cl -> Cl. O + O 2 © Next, a free oxygen atoms pulls the oxygen atom from Cl. O, liberating the chlorine and creating one oxygen molecule: Cl. O + O -> Cl + O 2 © One chlorine atom can catalyze the breakdown of as many as 100, 000 ozone molecules before it leaves the stratosphere.

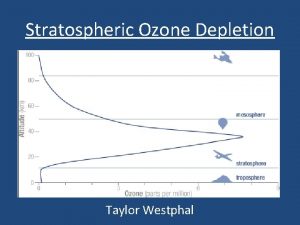
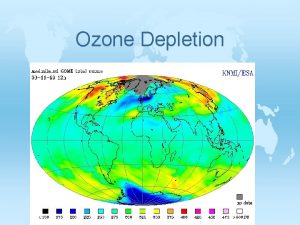
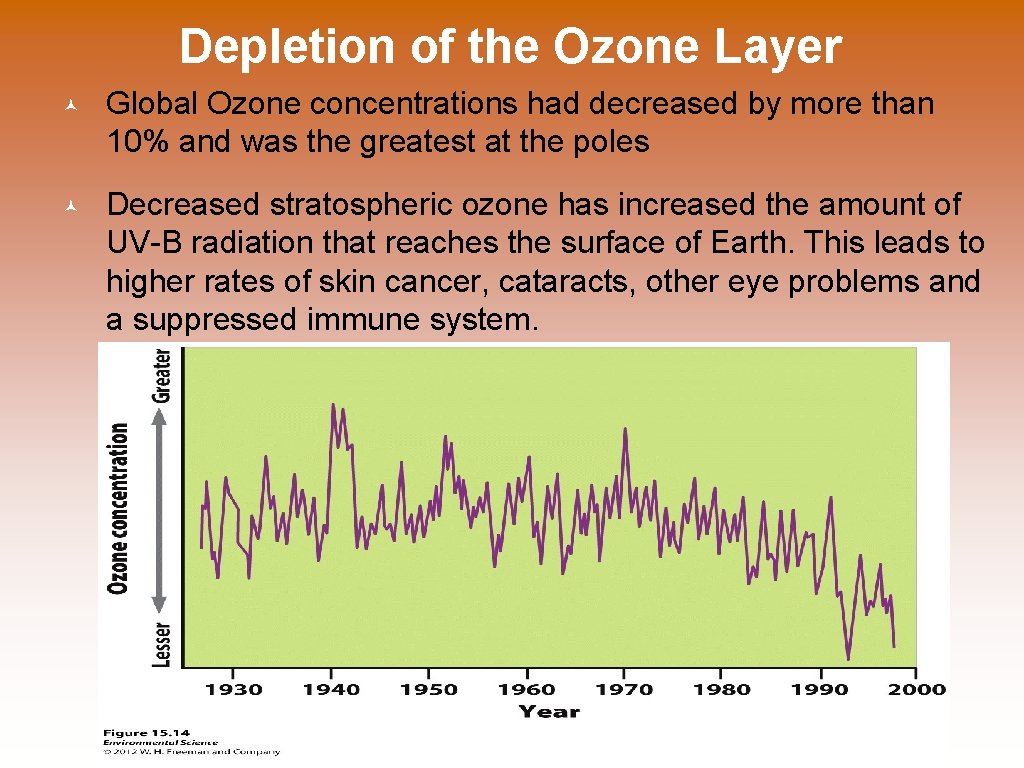
Depletion of the Ozone Layer © Global Ozone concentrations had decreased by more than 10% and was the greatest at the poles © Decreased stratospheric ozone has increased the amount of UV-B radiation that reaches the surface of Earth. This leads to higher rates of skin cancer, cataracts, other eye problems and a suppressed immune system.

Efforts to Reduce Ozone Depletion © Montreal Protocol on Substances that Deplete the Ozone Layer 1987 – Was signed by 27 countries to commit to a 50% reduction in CFC’s by 2000. © The US put the protection of the global biosphere ahead of its economic self interests. © Other more stringent ammendments were signed by 180 countries, including the elimination of CFC production for developing countries by 1996. © This stabilized the concentration of Cl in the stratosphere at 5 ppb and should reduce it to 1 ppb by 2100.

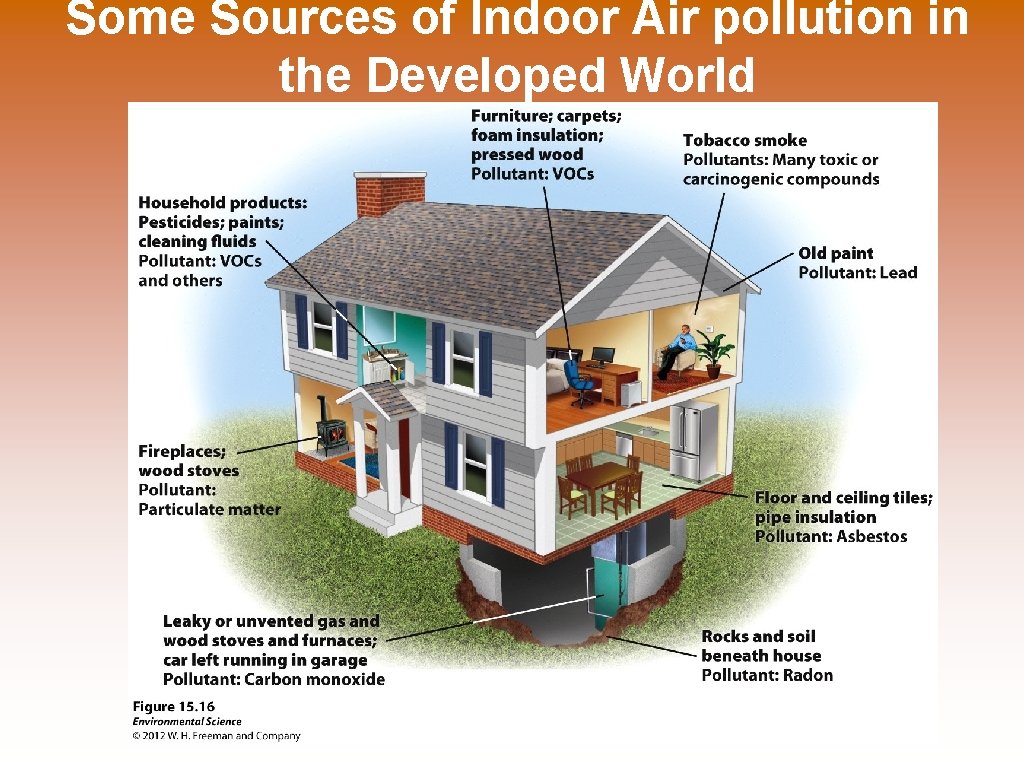
Indoor Air pollution © Indoor Air Pollution leads to more deaths each year than outdoor air pollution. © Many developing households use wood, coal, or manure to heat and cook in their home with no exhaust or ventilation. This leads to acute respiratory infections, pneumonia, bronchitis, and cancer. © WHO estimates that indoor air pollution is responsible for 1. 6 million deaths a year worldwide. 56% are children under 5 years old. 90% of these deaths are in developing countries.

Some Sources of Indoor Air pollution in the Developed World


Sick Building Syndrome © In the interest of saving energy and making a new building air tight to reduce heating and cooling may cause the buildup of toxic pollutants in this air tight space. © 4 reasons for sick building syndrome. © Inadequate ventilation © Chemical contamination from indoor sources (glues, carpets, furniture, and cleaning agents) © Chemical contamination from an outdoor source © Biological contamination from in or outdoors (mold or pollen)
 Stratospheric ozone depletion
Stratospheric ozone depletion Ozone layer definition
Ozone layer definition Ozone depletion effect on humans
Ozone depletion effect on humans Ozone layer
Ozone layer Ozone layer depletion introduction
Ozone layer depletion introduction Ozone depletion diagram
Ozone depletion diagram Cause of ozone depletion
Cause of ozone depletion Ozone layer depletion
Ozone layer depletion Copyright
Copyright Ozone depletion pictures
Ozone depletion pictures Chapter 12 section 3 acid precipitation
Chapter 12 section 3 acid precipitation Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Polar stratospheric clouds
Polar stratospheric clouds Polar stratospheric clouds
Polar stratospheric clouds Frequency factor units
Frequency factor units Stratospheric balloon
Stratospheric balloon Chapter 11 depreciation
Chapter 11 depreciation Which method is used to compute depletion?
Which method is used to compute depletion? Chapter 11 depreciation impairments and depletion
Chapter 11 depreciation impairments and depletion Hubungan air tanah dan tanaman
Hubungan air tanah dan tanaman Soil contamination effects on human health
Soil contamination effects on human health Soil pollution images diagram
Soil pollution images diagram Objective of pollution
Objective of pollution Examples of primary pollutants and secondary pollutants
Examples of primary pollutants and secondary pollutants Stationary and mobile sources of air pollution
Stationary and mobile sources of air pollution Baghouse filter definition apes
Baghouse filter definition apes Air noise and light pollution
Air noise and light pollution Section 2 air noise and light pollution
Section 2 air noise and light pollution 8 effects of water pollution
8 effects of water pollution Introduction about air pollution
Introduction about air pollution Effect of air pollution on plant
Effect of air pollution on plant Contents of air pollution
Contents of air pollution Main cause of air pollution
Main cause of air pollution