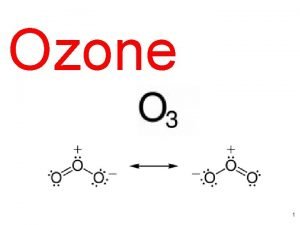
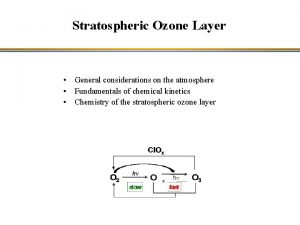
The Stratospheric Chemistry and The Ozone Layer Stratospheric














- Slides: 36

The Stratospheric Chemistry and The Ozone Layer *Stratospheric circulation *Stratospheric photochemistry *Gas phase reactions *Ozone and Catalytic cycles Dr Tony Cox ERCA 2004 -Lecture 2


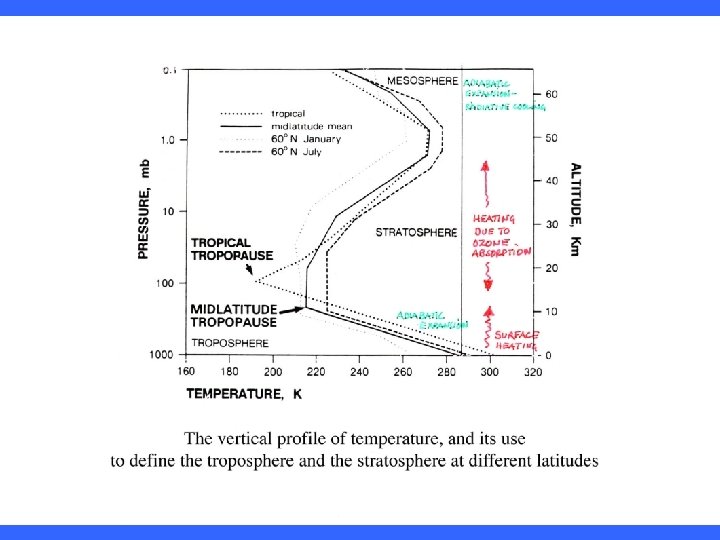
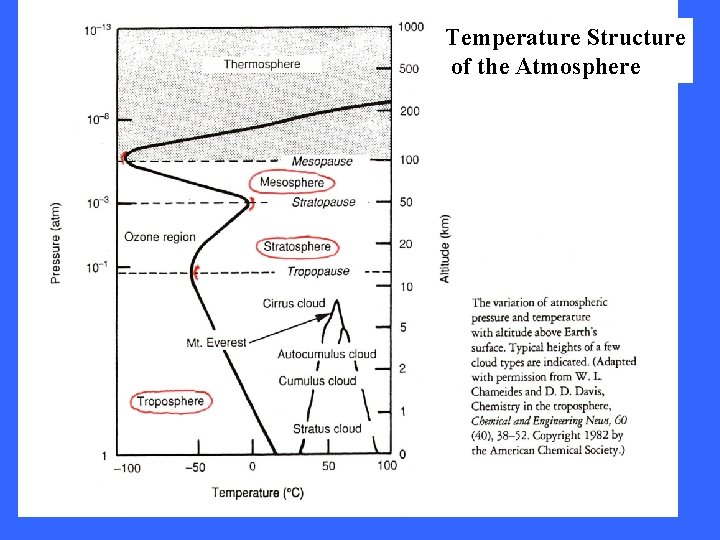
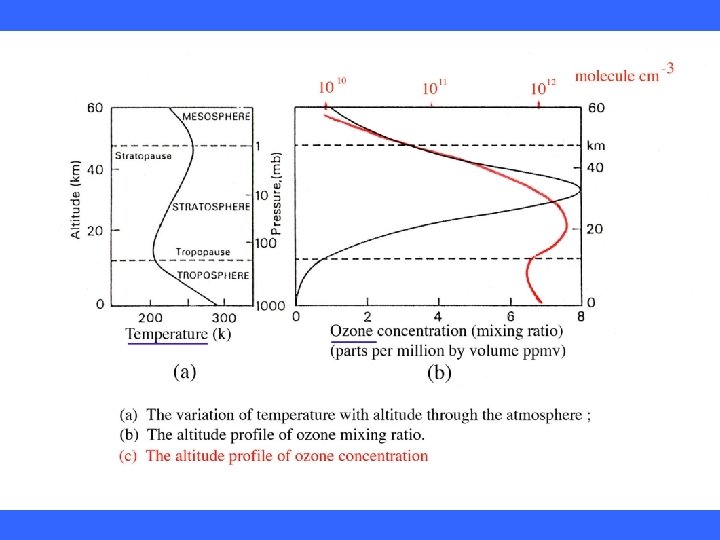
Temperature Structure of the Atmosphere

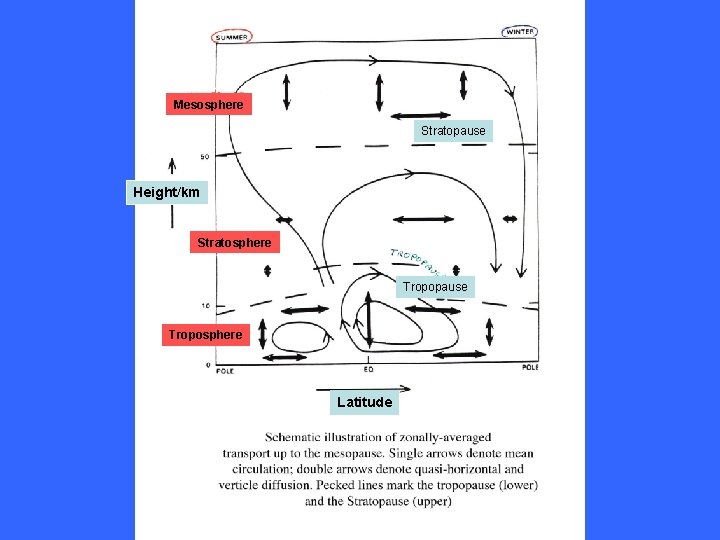
Mesosphere Stratopause Height/km Stratosphere Tropopause Troposphere Latitude

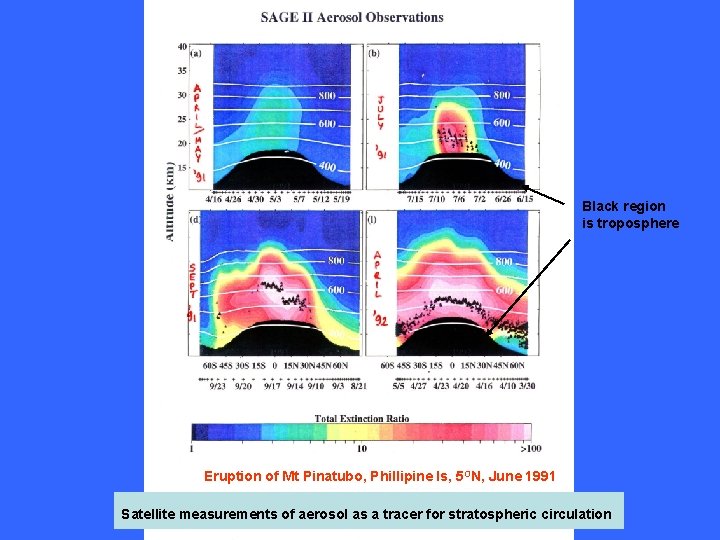
Black region is troposphere Eruption of Mt Pinatubo, Phillipine Is, 5 ON, June 1991 Satellite measurements of aerosol as a tracer for stratospheric circulation

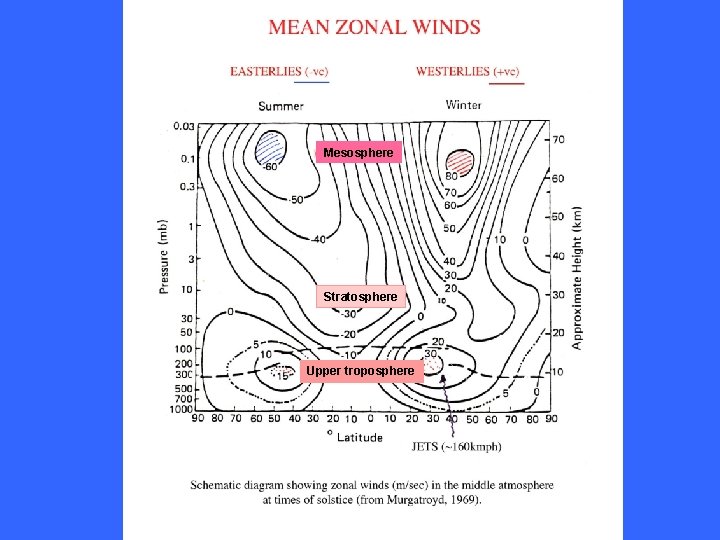
Mesosphere Stratosphere Upper troposphere


MEAN (11 yr) ATMOSPHERIC WINDS AT 20 km ALTITUDE (NOAA Analysis)

MEAN (11 yr) ATMOSPHERIC TEMPERATURE AT 20 km ALTITUDE (NOAA Analysis)

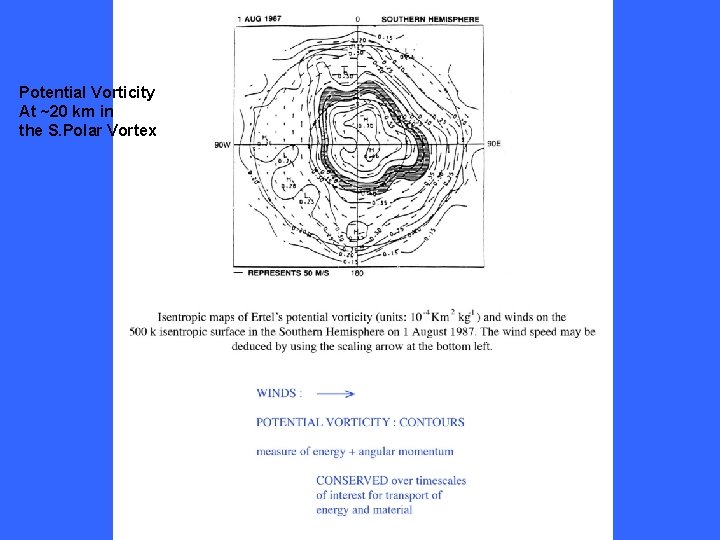
Potential Vorticity At ~20 km in the S. Polar Vortex

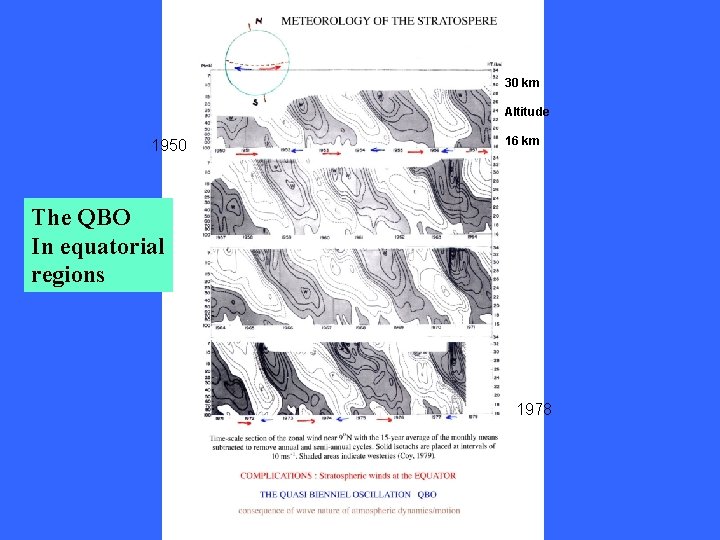
30 km Altitude 1950 16 km The QBO In equatorial regions 1978

Variations in mean O 3 column(DU) due to QBO (from satellite observations)

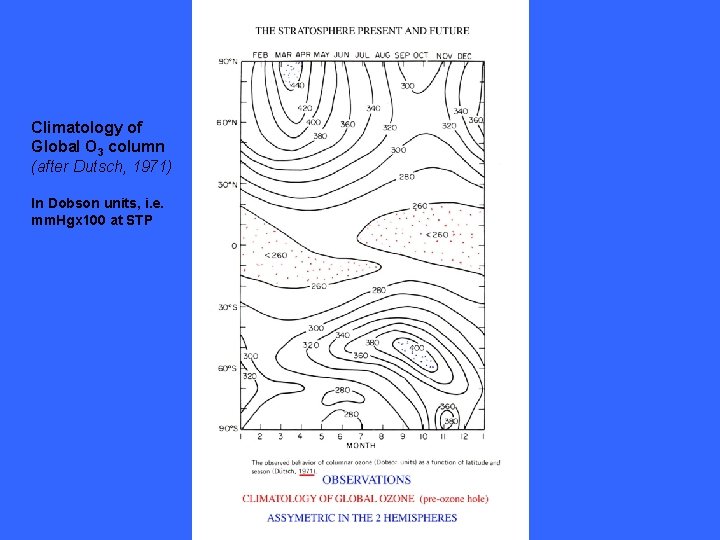
Climatology of Global O 3 column (after Dutsch, 1971) In Dobson units, i. e. mm. Hgx 100 at STP

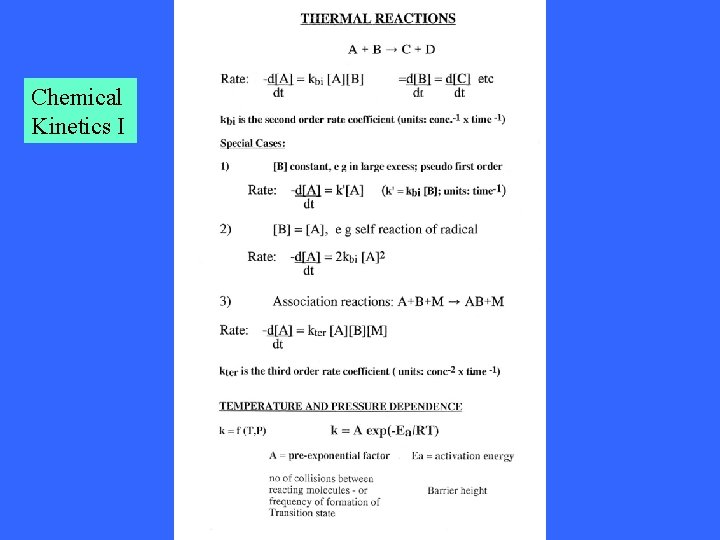
Chemical Kinetics I

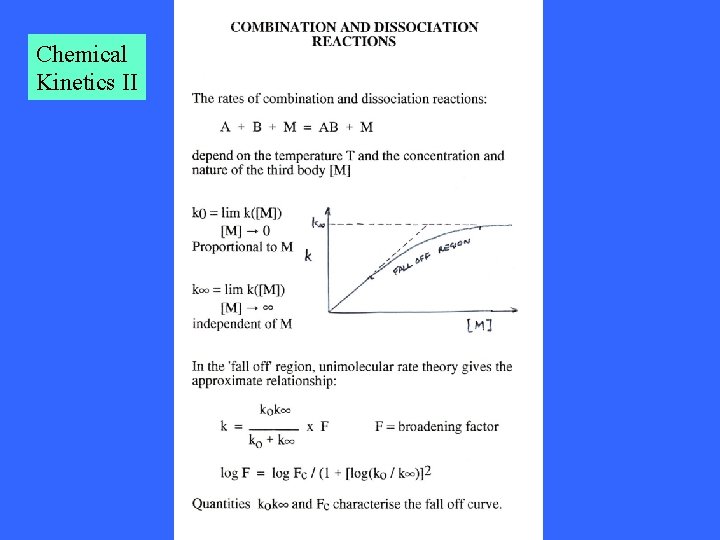
Chemical Kinetics II

Chemical Kinetics III

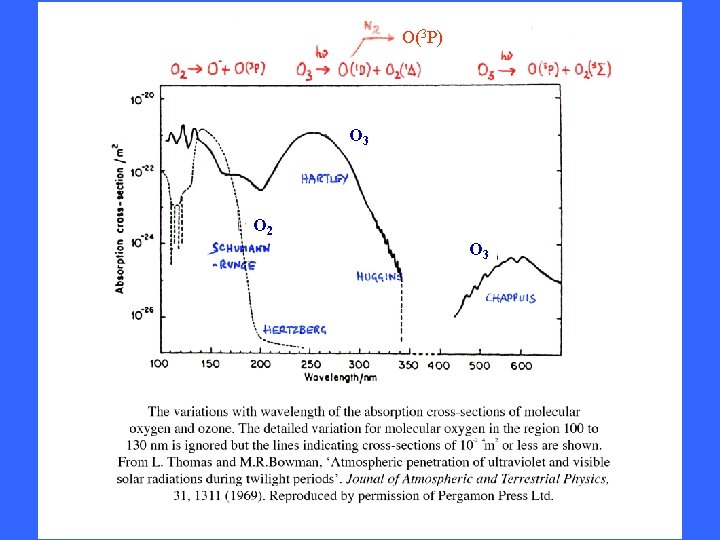
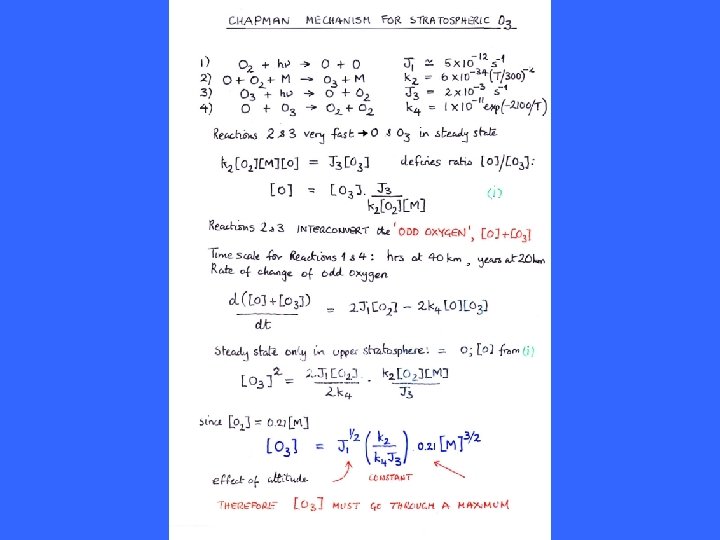
O(3 P) O 3 O 2 O 3

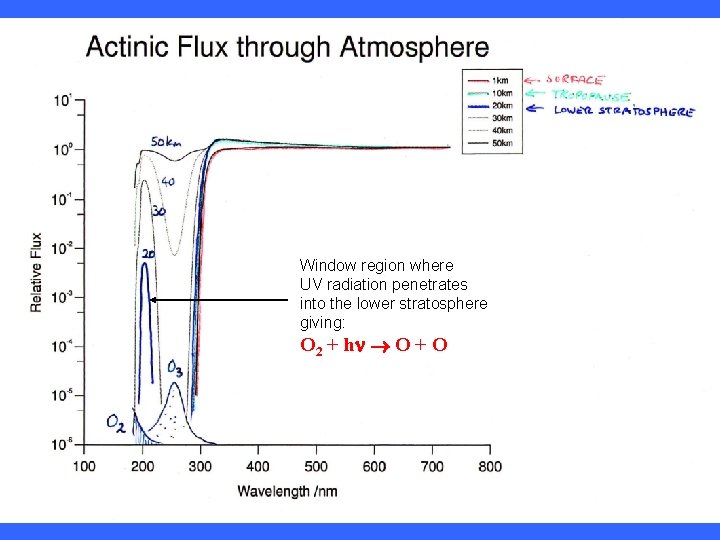
Window region where UV radiation penetrates into the lower stratosphere giving: O 2 + hn O + O

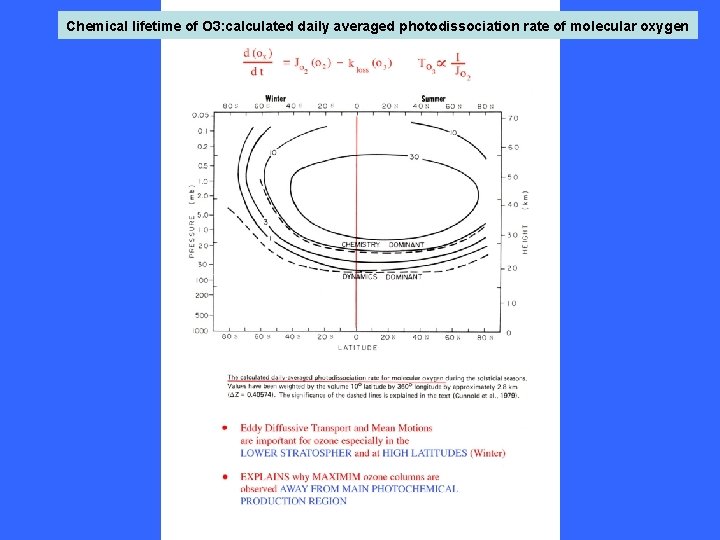
Chemical lifetime of O 3: calculated daily averaged photodissociation rate of molecular oxygen

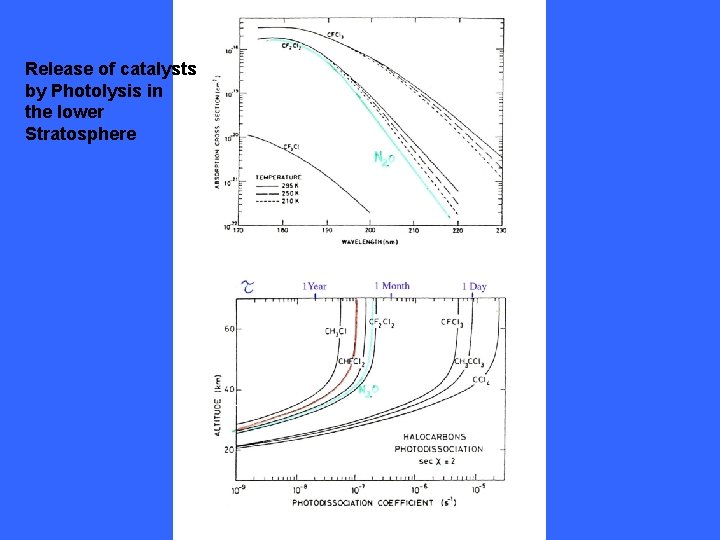
Release of catalysts by Photolysis in the lower Stratosphere

O 2 + hn(l<243 nm) O + O oxygen photolysis O + O 2 + M O 3 + M ozone formation O + O 3 O 2 + O 2 ozone destruction O 3 + hn(l<310 nm) O(1 D) + O 2 O 3 + hn(l<1180 nm) O + O 2 oxygen photolysis NO 2 + hn(l<243 nm) NO + O 2 + M O 3 + M Net X + O 3 XO + O 2 XO + O X + O 2 O + O 3 O 2 + O 2 [X = H, OH, NO, Cl, Br, etc] NO 2 photolysis ozone formation ozone destruction






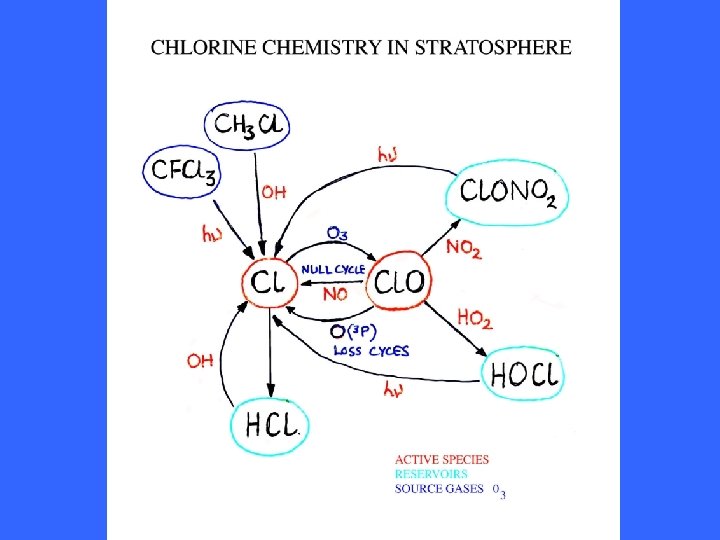
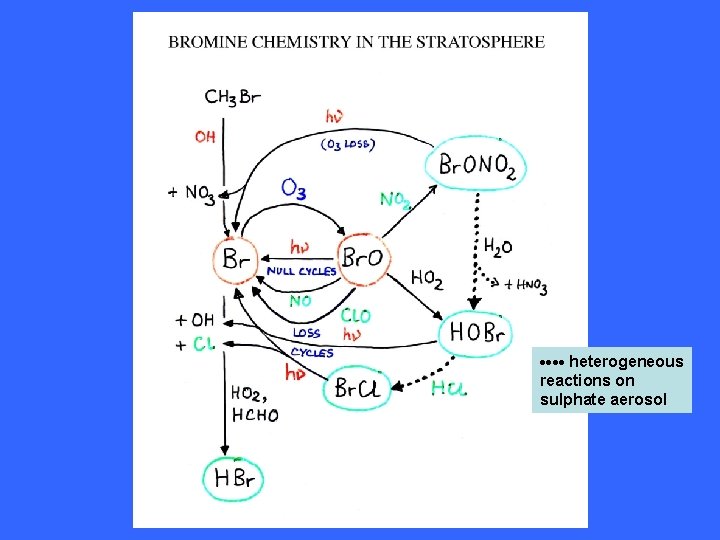
Interconversion of Cl & Cl. O Formation and release from reservoirs of Cl & Cl. O

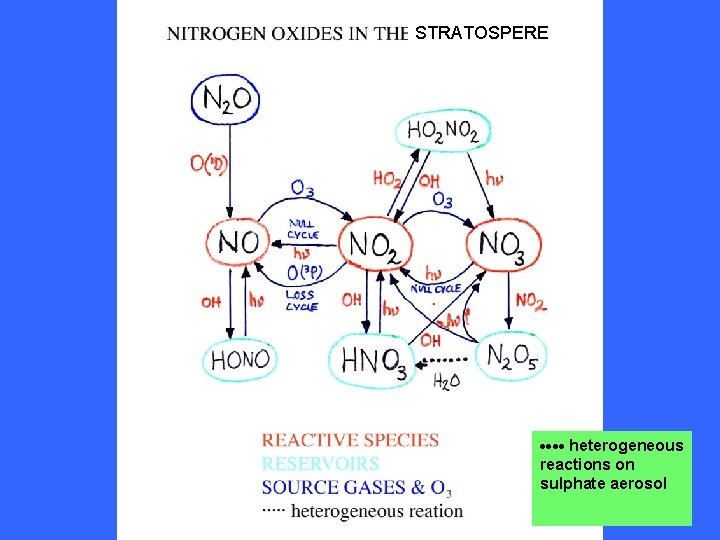
STRATOSPERE heterogeneous reactions on sulphate aerosol





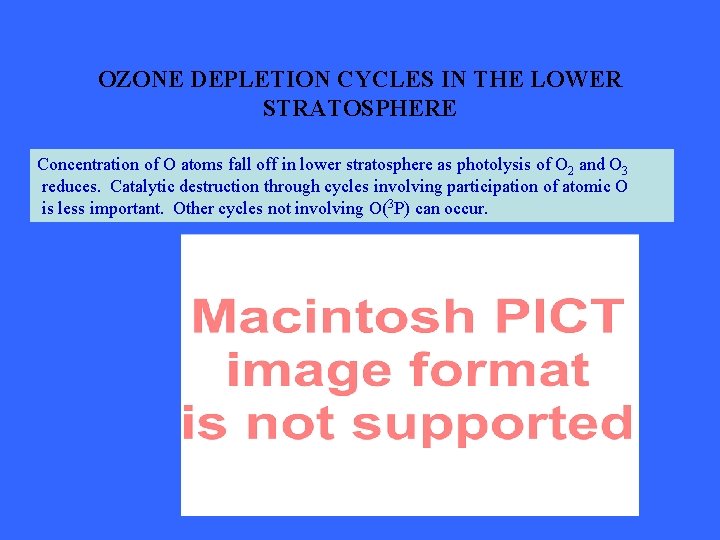
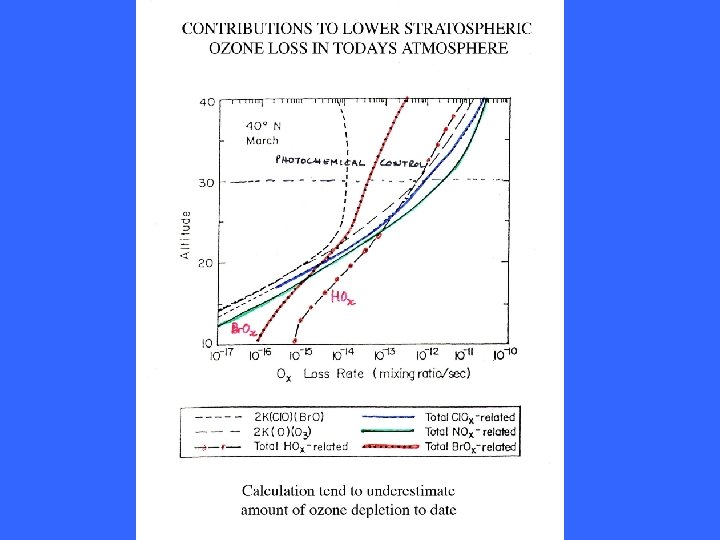
OZONE DEPLETION CYCLES IN THE LOWER STRATOSPHERE Concentration of O atoms fall off in lower stratosphere as photolysis of O 2 and O 3 reduces. Catalytic destruction through cycles involving participation of atomic O is less important. Other cycles not involving O(3 P) can occur.

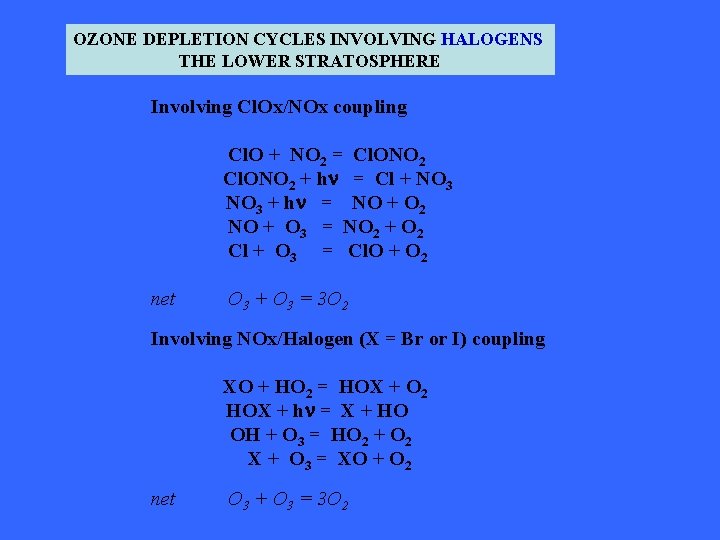
OZONE DEPLETION CYCLES INVOLVING HALOGENS THE LOWER STRATOSPHERE Involving Cl. Ox/NOx coupling Cl. O + NO 2 = Cl. ONO 2 + hn = Cl + NO 3 + hn = NO + O 2 NO + O 3 = NO 2 + O 2 Cl + O 3 = Cl. O + O 2 net O 3 + O 3 = 3 O 2 Involving NOx/Halogen (X = Br or I) coupling XO + HO 2 = HOX + O 2 HOX + hn = X + HO OH + O 3 = HO 2 + O 2 X + O 3 = XO + O 2 net O 3 + O 3 = 3 O 2

heterogeneous reactions on sulphate aerosol

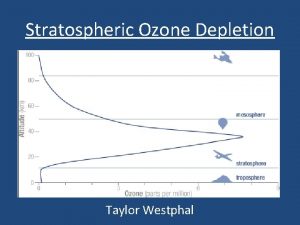
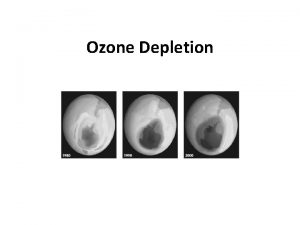
 Stratospheric ozone depletion
Stratospheric ozone depletion Negative effects of ozone depletion
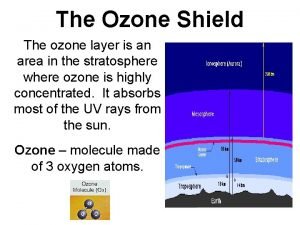
Negative effects of ozone depletion Protective ozone layer
Protective ozone layer Protective ozone layer
Protective ozone layer Ozone layer depletion
Ozone layer depletion Ozone layer simple definition
Ozone layer simple definition Ozone depletion effect on humans
Ozone depletion effect on humans Ozone layer depletion introduction
Ozone layer depletion introduction Ozone layer made up of
Ozone layer made up of Protection of ozone layer
Protection of ozone layer Ozone layer
Ozone layer Ozone layer levels
Ozone layer levels Benjamin cummings
Benjamin cummings Atmosphere unit
Atmosphere unit Ozone layer
Ozone layer Herbicides definition ap human geography
Herbicides definition ap human geography Wheres the ozone layer
Wheres the ozone layer Polar stratospheric clouds
Polar stratospheric clouds Polar stratospheric clouds
Polar stratospheric clouds Polar stratospheric clouds
Polar stratospheric clouds Stratospheric balloon
Stratospheric balloon Pigmented layer and neural layer
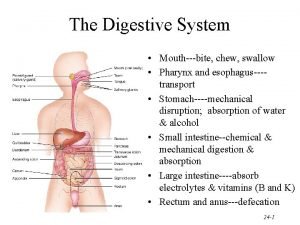
Pigmented layer and neural layer Chemical digestion
Chemical digestion Secure socket layer and transport layer security
Secure socket layer and transport layer security Secure socket layer and transport layer security
Secure socket layer and transport layer security Secure socket layer and transport layer security
Secure socket layer and transport layer security Secure socket layer and transport layer security
Secure socket layer and transport layer security Layer 6 presentation layer
Layer 6 presentation layer Layer 2 e layer 3
Layer 2 e layer 3 Layer-by-layer assembly
Layer-by-layer assembly Layer 2 vs layer 3 bitstream
Layer 2 vs layer 3 bitstream Semi auto analyser
Semi auto analyser How do cfcs destroy ozone
How do cfcs destroy ozone Sulphur oxide
Sulphur oxide Photonic medicine
Photonic medicine Microplasma ozone
Microplasma ozone The ozone blanket blocks
The ozone blanket blocks