What is ozone depletion The Ozone layer The

























- Slides: 35

What is ozone depletion?

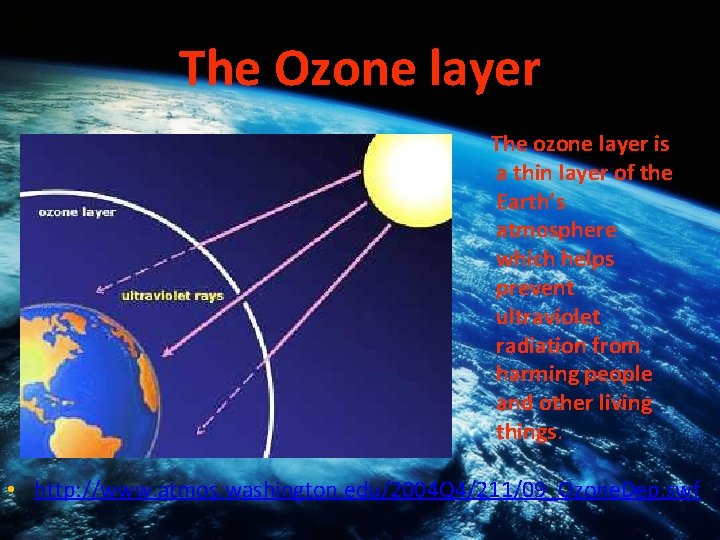
The Ozone layer The ozone layer is a thin layer of the Earth’s atmosphere which helps prevent ultraviolet radiation from harming people and other living things. • http: //www. atmos. washington. edu/2004 Q 4/211/09_Ozone. Dep. swf

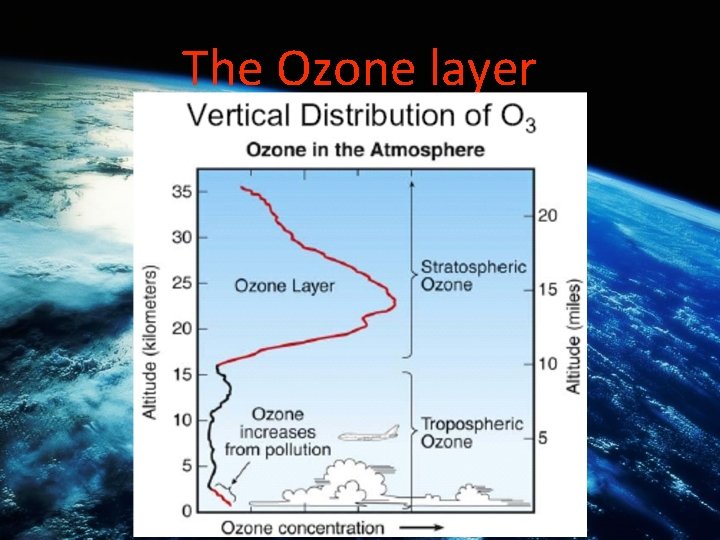
Ozone in the stratosphere • A thin layer of ozone gas (03) encircles the earth, in the stratosphere. • 03 is a natural occurring bluish gas which has an acrid odor. It has harmful effects on organisms however, it helps block out the harmful UV rays 15 km to 45 km up in the atmosphere.

The Ozone layer

FORMATION OF OZONE 1) O 2 2 O* (Oxygen free radical) The ozone is formed when the double bond in the oxygen is broken by very short wavelength UV-C light 2) O 2 + O * O 3 An oxygen free radical is therefore formed, this oxygen free radical further reacts with another oxygen atom to form ozone gas, O 3. • http: //www. atmos. washington. edu/2004 Q 4/211/09_Ozo ne. Dep. swf

OZONE DEPLETION The reverse process happens when O 3 absorbs longer wave length UV-B light. This is because two pi bonding electrons are shared between the entire structure therefore it has weaker bonds (longer bonds) compared to O 2. O 3 (g) O 2 (g) + ●O (g) The oxygen free radicals react with another resonance structure to form O 2. ●O (g) + O 3 (g) O 2 (g)

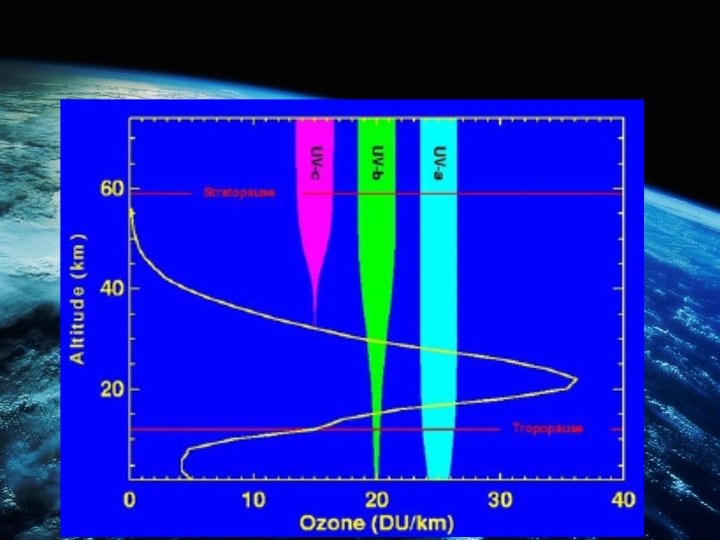
STEADY STATE • As a result the rate of which Ozone is produced and depleted would be equal, this is known as the steady state. • The ozone helps protect the earth from 99% of the dangerous UV light of longer wavelength (240 nm to 330 nm) than that absorbed by oxygen (less than 240 nm) and nitrogen by this process, however, this steady state is constantly being interfered with by ozonedepleting pollutants.


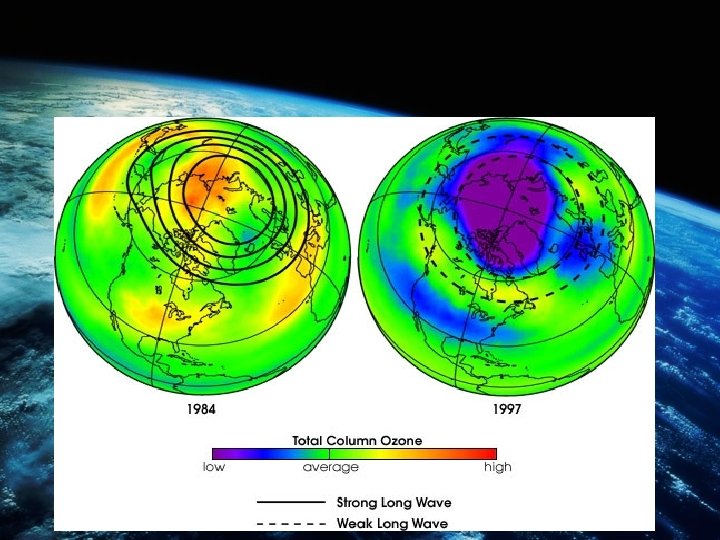
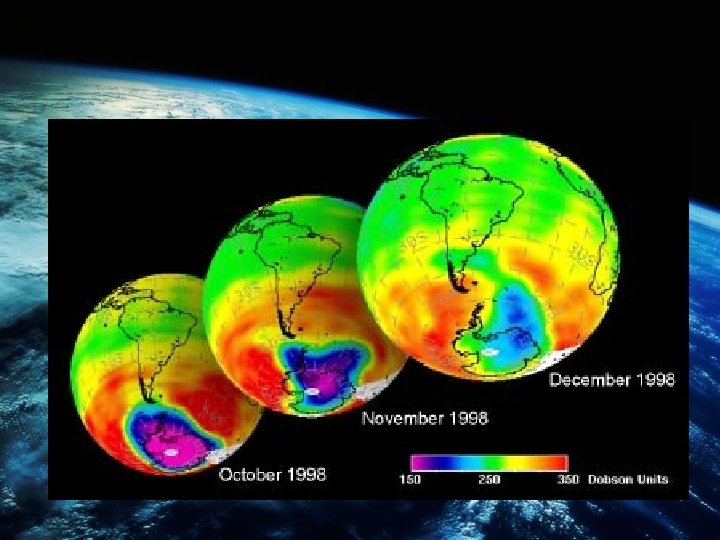
OZONE LAYER BEING REDUCED • There has been a clear decline over the last 30 years in ozone concentrations above the north and south poles, which is seasonal with the lowest levels being found in the Antarctic region during the spring (October). • Due to cold winter months and ice particles surface behaving as a catalyst for the photo – dissociation of chlorine molecules.




Ozone Hole Theories • Chemistry vs Meteorology • Human vs Natural • Solar Cycles

OZONE DEPLETING POLLUTANTS • The main pollutants includes: CFCs (Chlorofluorocarbons) and oxides of nitrogen. (NOx) Sources CFCs are mainly found in hair spray or deodorant cans as a propellant, old refrigerators, air conditioners e. t. c. Oxides of nitrogen are formed from high temperature reactions of N 2 and O 2 in supersonic aircraft engines and lightning.


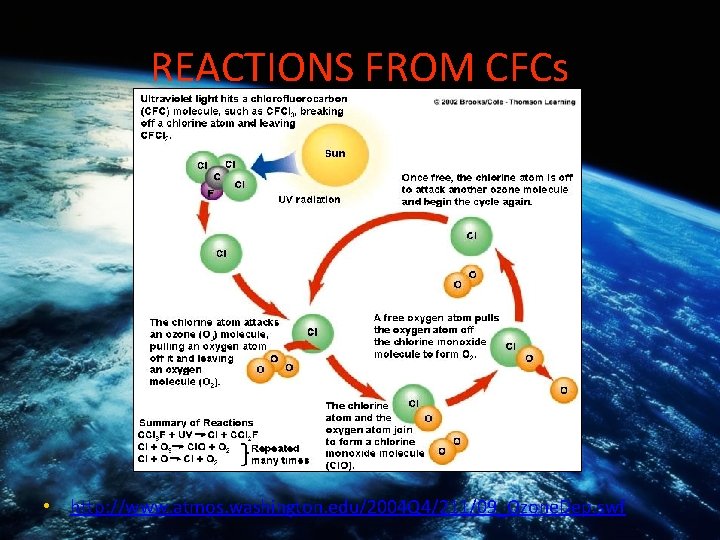
REACTIONS FROM CFCs • CFC’s contain bonds which are relatively strong (H-F 484 k. J/mol, H-Cl 384 k. J/mol) so they don’t react easily. But as they float up toward the unfiltered UV light, the H-Cl bond can break and then produce a highly reactive Cl free radical. . . CCl 2 F 2 + uv light CCl. F 2 + Cl + O 3 Cl. O. + O 2

REACTIONS FROM CFCs • http: //www. atmos. washington. edu/2004 Q 4/211/09_Ozone. Dep. swf

REACTIONS FROM NOX NO + O 3 NO 2 + O 2 NO 2 + . O NO + O 2 (what is the overall result) • Consequences are more UV light will reach the earth increasing the chances for skin cancer, sun burns, damage to animals and vegetation and even genetic mutations.

ALTERNATIVE TO CFCs • Properties required for substitutes are low reactivity, low toxicity, low flammability as well as no weak C-Cl bonds.

ALTERNATIVE TO CFCs 1. Hydrocarbons i. e. C 3 H 8 (flammable, GHG) 2. Hydrochlorofluorocarbons i. e. HCl. FCs (still reduce ozone layer, just less) 3. Fluorocarbons i. e. CF 4 (almost perfect except act like GHG) 4. Hydrofluorocarbons i. e. CF 3 CH 2 F (almost perfect except act like GHG)

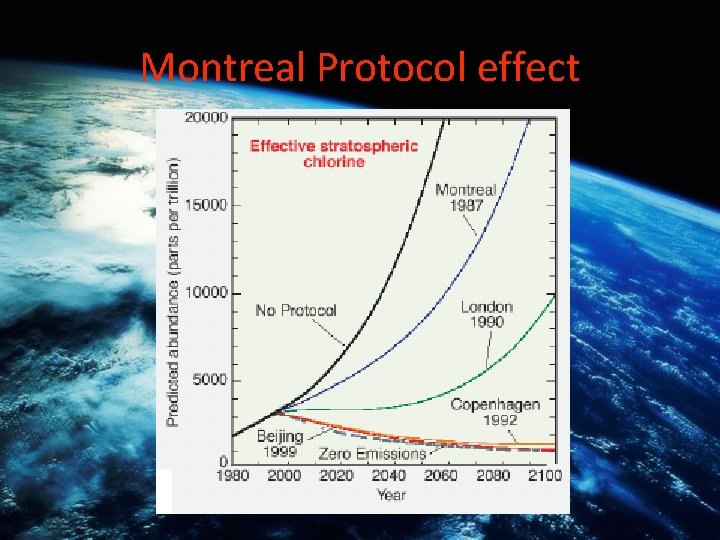
Montreal Protocol effect

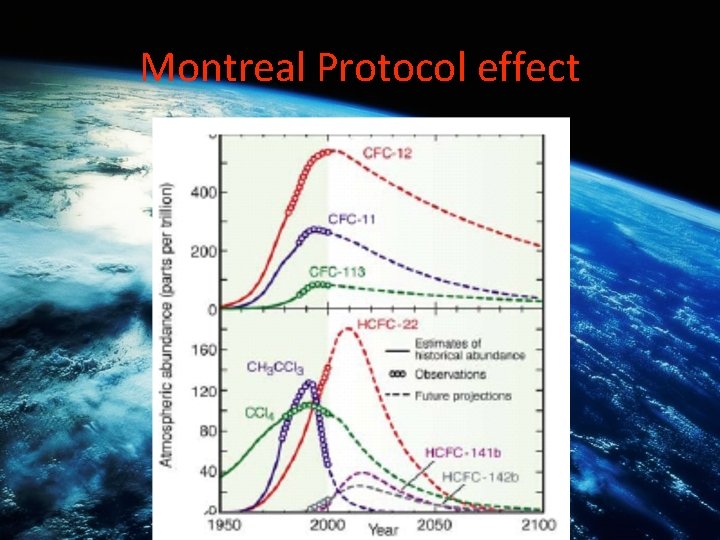
Montreal Protocol effect


QUESTIONS?





Marketing of CFCs 1958: Du. Pont releases CFCs on the market commercially 1971: James Lovelock speculates that CFCs put into the atmosphere may still be present 1973: Mario Molina and F. Sherry Roland start to investigate

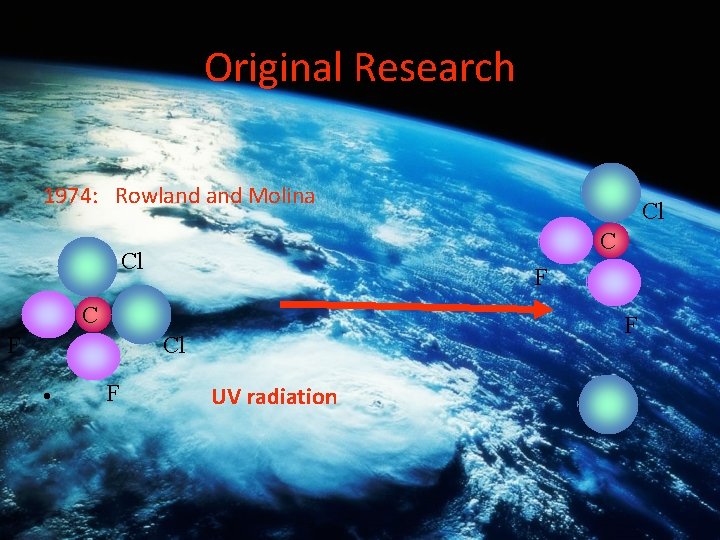
Original Research 1974: Rowland Molina C Cl F C F F Cl • F Cl UV radiation

In the news… 1974: Molina and Rowland publish their hypothesis in Nature. New York Times runs front page Du. Pont responds with study showing that CFCs in troposphere are benign

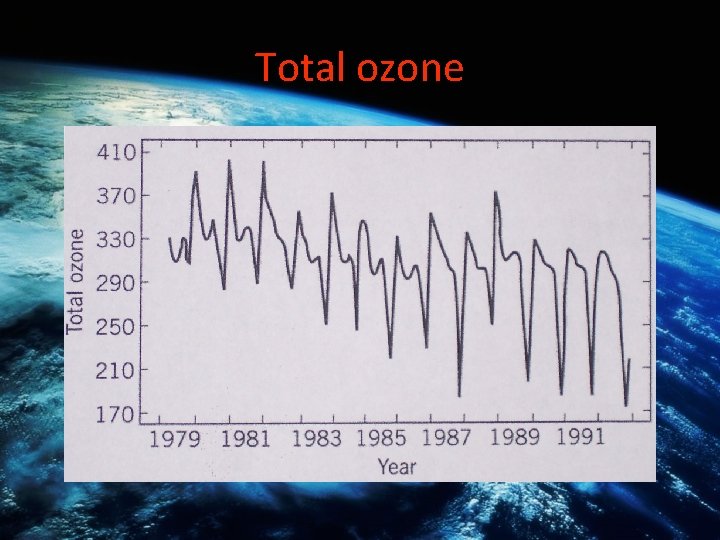
Scientific Controversies 1982: British science teams in Antarctica observe 20% decline in O 3 layer US scientists relying on TOMS (Total Ozone Mapping Spectrometer) measurements from space claim to observe nothing

Scientific Evidence 1983: British scientists observe 30% reduction in ozone layer. US scientists claims no reduction. 1985: British observe 50% reduction. US claims no reduction. US re-tests and confirms. WHY THE SCIENTIFIC CONTRDICTION? ?

Total ozone

One step back… 1995: Congress challenges ozone science: Junk science gains credibility despite scientific consensus of anthropogenic causes of O 3 depletion 1996: Ban begins but black market for CFCs appear