Oxidant chemistry in the tropical troposphere role of

![Global tropospheric Bry budget in GEOS-Chem (Gg Br a-1) Liang et al. [2010] stratospheric Global tropospheric Bry budget in GEOS-Chem (Gg Br a-1) Liang et al. [2010] stratospheric](https://slidetodoc.com/presentation_image_h/243c10838855251c7c1785c117f0f1d5/image-15.jpg)
![Comparison to seasonal satellite data for tropospheric Br. O [Theys et al. , 2011] Comparison to seasonal satellite data for tropospheric Br. O [Theys et al. , 2011]](https://slidetodoc.com/presentation_image_h/243c10838855251c7c1785c117f0f1d5/image-18.jpg)
- Slides: 26

Oxidant chemistry in the tropical troposphere: role of oxygenated VOCs and halogens, and implications for mercury Daniel J. Jacob with Kevin J. Wecht, Lee T. Murray, Emily V. Fischer, Justin P. Parrella, Anne L. Soerensen, Helen M. Amos

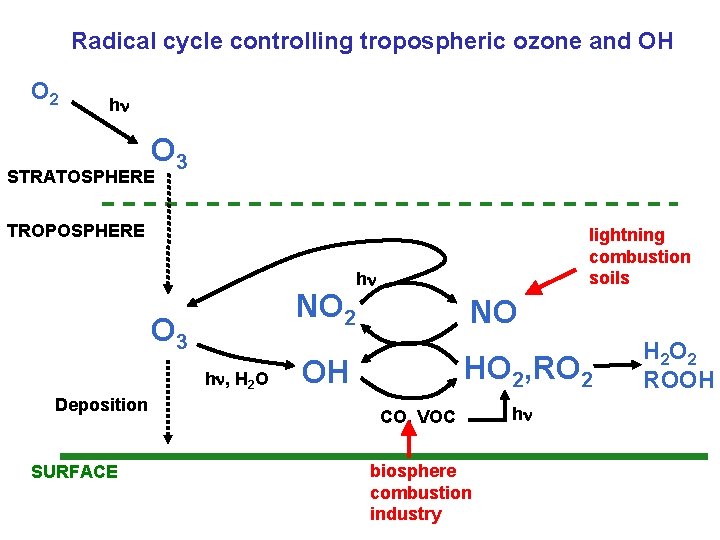
Radical cycle controlling tropospheric ozone and OH O 2 h O 3 STRATOSPHERE TROPOSPHERE NO 2 O 3 h , H 2 O Deposition SURFACE lightning combustion soils h NO HO 2, RO 2 OH CO, VOC biosphere combustion industry h H 2 O 2 ROOH

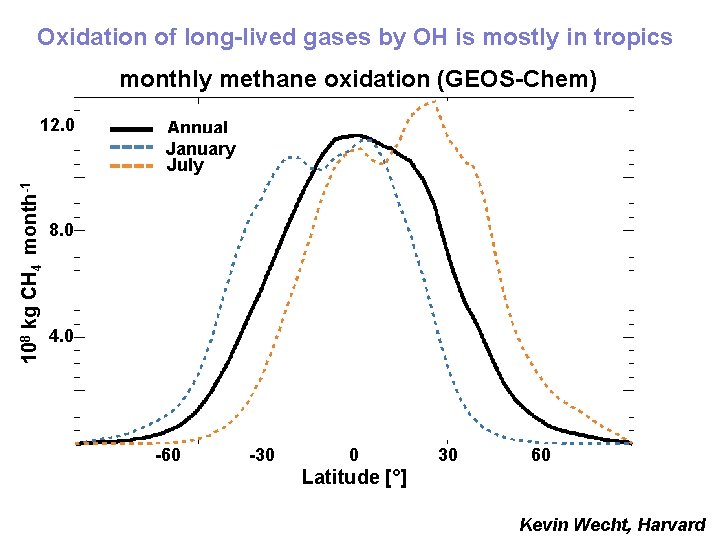
Oxidation of long-lived gases by OH is mostly in tropics monthly methane oxidation (GEOS-Chem) 108 kg CH 4 month-1 12. 0 Annual January Average July 8. 0 4. 0 -60 -30 0 30 60 Latitude [°] Kevin Wecht, Harvard

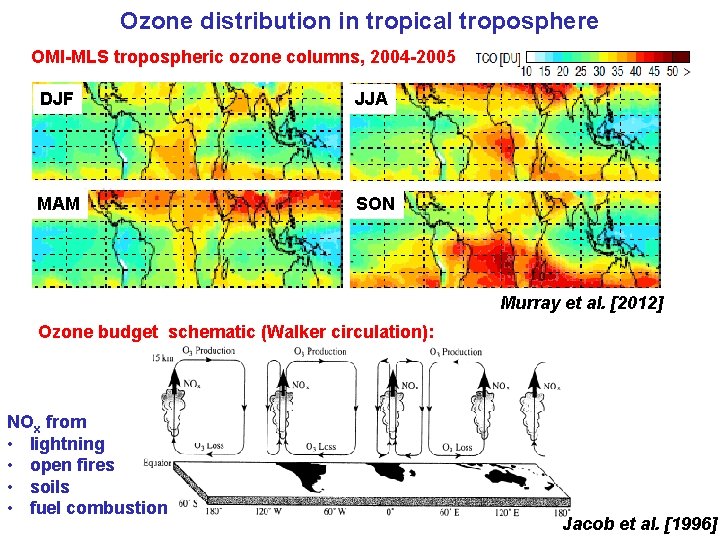
Ozone distribution in tropical troposphere OMI-MLS tropospheric ozone columns, 2004 -2005 DJF JJA MAM SON Murray et al. [2012] Ozone budget schematic (Walker circulation): NOx from • lightning • open fires • soils • fuel combustion Jacob et al. [1996]

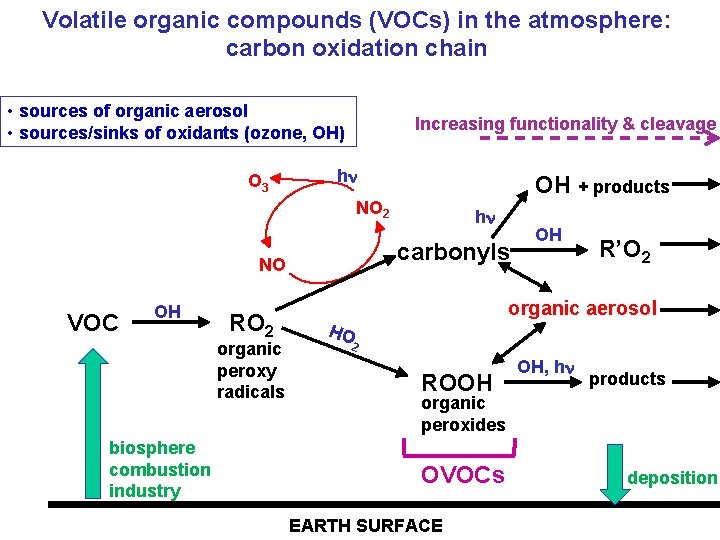
Volatile organic compounds (VOCs) in the atmosphere: carbon oxidation chain • sources of organic aerosol • sources/sinks of oxidants (ozone, OH) O 3 Increasing functionality & cleavage h OH + products NO 2 carbonyls NO VOC OH RO 2 organic peroxy radicals biosphere combustion industry h OH R’O 2 organic aerosol HO 2 ROOH OH, h products organic peroxides OVOCs EARTH SURFACE deposition

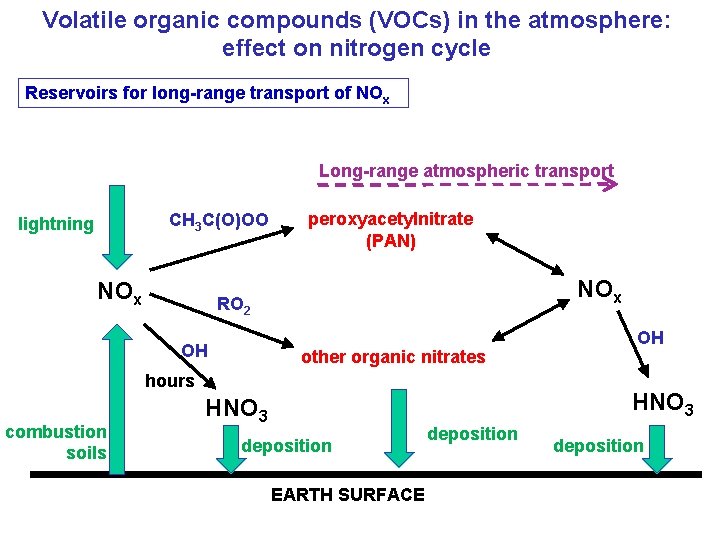
Volatile organic compounds (VOCs) in the atmosphere: effect on nitrogen cycle Reservoirs for long-range transport of NOx Long-range atmospheric transport CH 3 C(O)OO lightning NOx peroxyacetylnitrate (PAN) NOx RO 2 OH other organic nitrates hours combustion soils OH HNO 3 deposition EARTH SURFACE deposition

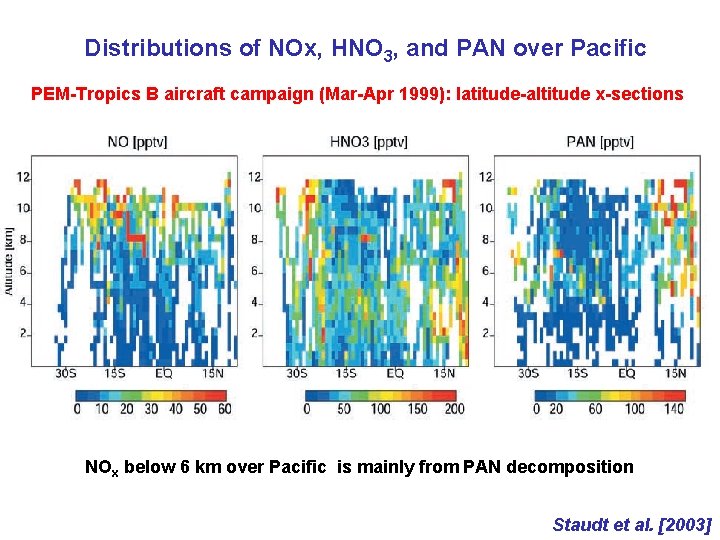
Distributions of NOx, HNO 3, and PAN over Pacific PEM-Tropics B aircraft campaign (Mar-Apr 1999): latitude-altitude x-sections NOx below 6 km over Pacific is mainly from PAN decomposition Staudt et al. [2003]

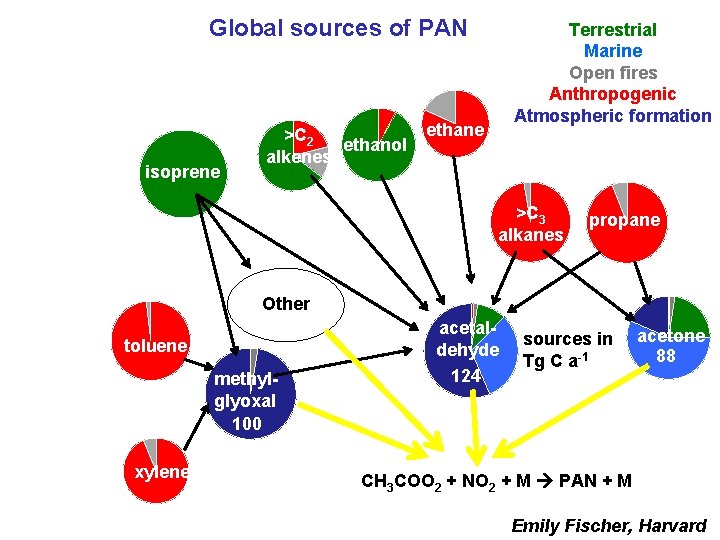
Global sources of PAN isoprene >C 2 ethanol alkenes Terrestrial Marine Open fires Anthropogenic Atmospheric formation ethane >C 3 alkanes propane Other toluene methylglyoxal 100 xylene acetaldehyde 124 sources in Tg C a-1 acetone 88 CH 3 COO 2 + NO 2 + M PAN + M Emily Fischer, Harvard

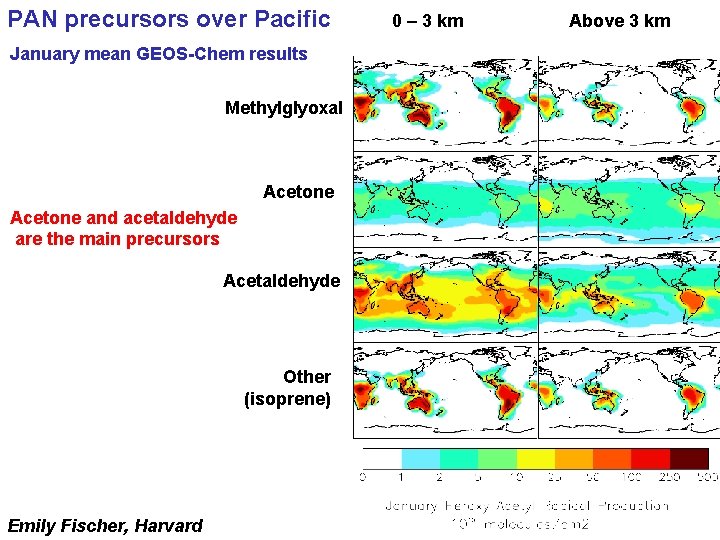
PAN precursors over Pacific January mean GEOS-Chem results Methylglyoxal Acetone and acetaldehyde are the main precursors Acetaldehyde Other (isoprene) Emily Fischer, Harvard 0 – 3 km Above 3 km

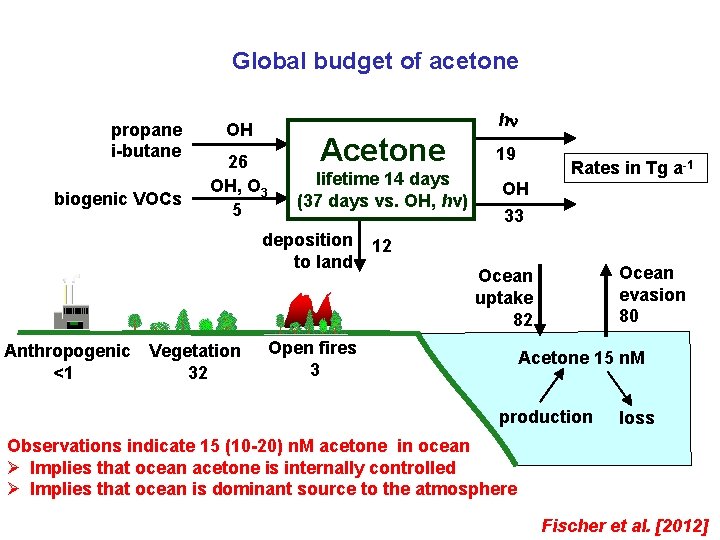
Global budget of acetone propane i-butane biogenic VOCs h OH 26 OH, O 3 5 Acetone lifetime 14 days (37 days vs. OH, hv) deposition to land Anthropogenic Vegetation <1 32 19 Rates in Tg a-1 OH 33 12 Ocean evasion 80 Ocean uptake 82 Open fires 3 Acetone 15 n. M production loss Observations indicate 15 (10 -20) n. M acetone in ocean Ø Implies that ocean acetone is internally controlled Ø Implies that ocean is dominant source to the atmosphere Fischer et al. [2012]

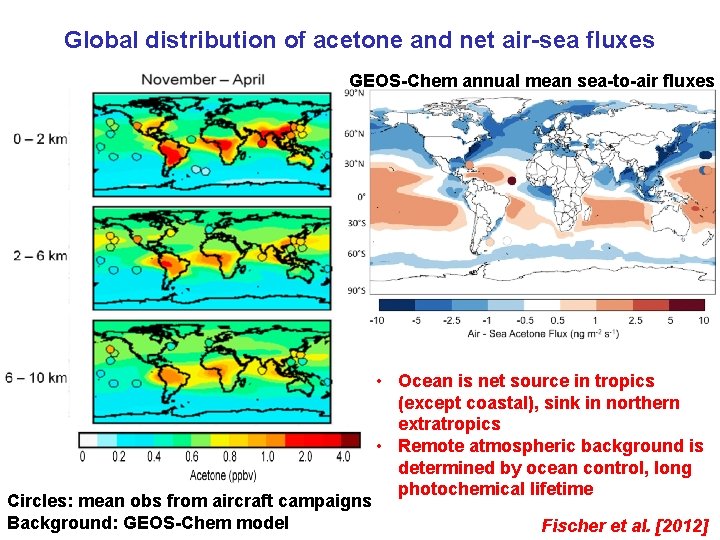
Global distribution of acetone and net air-sea fluxes GEOS-Chem annual mean sea-to-air fluxes Circles: mean obs from aircraft campaigns Background: GEOS-Chem model • Ocean is net source in tropics (except coastal), sink in northern extratropics • Remote atmospheric background is determined by ocean control, long photochemical lifetime Fischer et al. [2012]

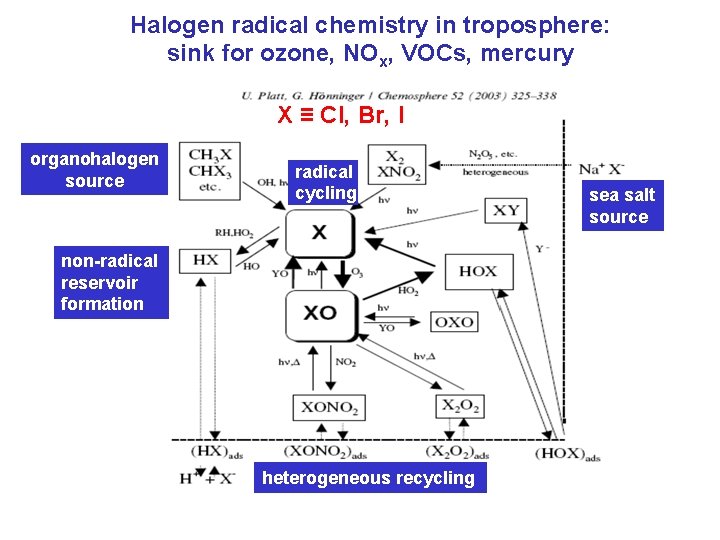
Halogen radical chemistry in troposphere: sink for ozone, NOx, VOCs, mercury X ≡ Cl, Br, I organohalogen source radical cycling non-radical reservoir formation heterogeneous recycling sea salt source

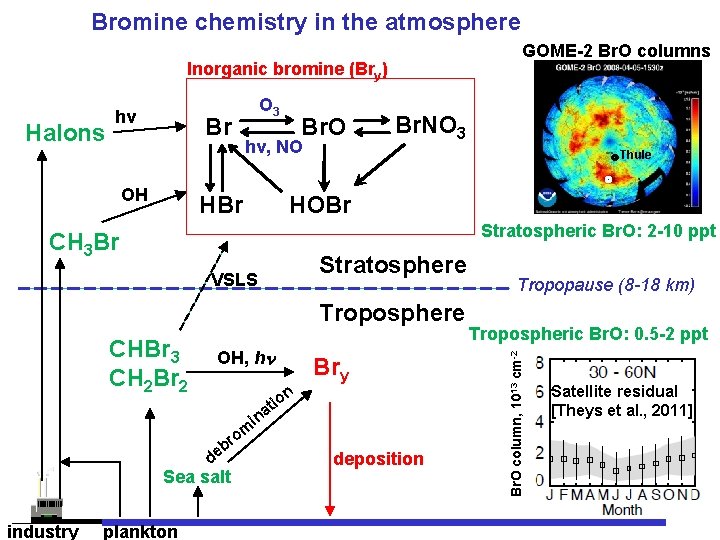
Bromine chemistry in the atmosphere GOME-2 Br. O columns Inorganic bromine (Bry) Br OH O 3 Br. O hv, NO HBr Br. NO 3 Thule HOBr Stratospheric Br. O: 2 -10 ppt CH 3 Br VSLS Stratosphere Troposphere CHBr 3 CH 2 Br 2 OH, h n io t a in om r b de Sea salt industry plankton Bry deposition Tropopause (8 -18 km) Tropospheric Br. O: 0. 5 -2 ppt Br. O column, 1013 cm-2 Halons hv Satellite residual [Theys et al. , 2011]

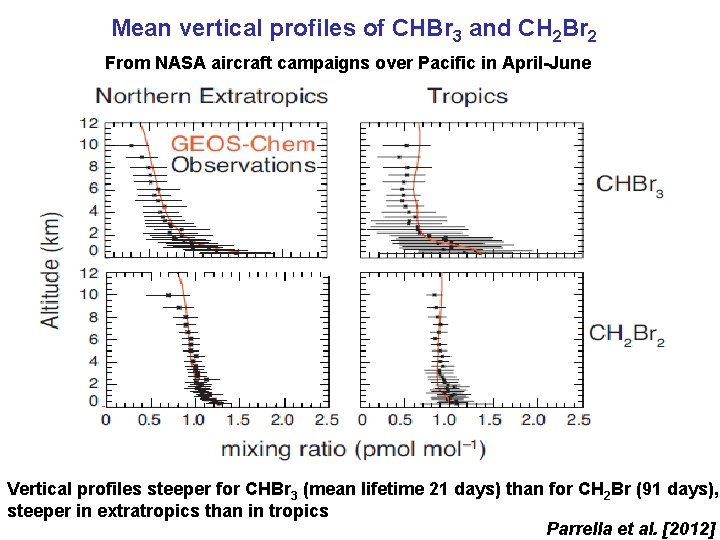
Mean vertical profiles of CHBr 3 and CH 2 Br 2 From NASA aircraft campaigns over Pacific in April-June Vertical profiles steeper for CHBr 3 (mean lifetime 21 days) than for CH 2 Br (91 days), steeper in extratropics than in tropics Parrella et al. [2012]
![Global tropospheric Bry budget in GEOSChem Gg Br a1 Liang et al 2010 stratospheric Global tropospheric Bry budget in GEOS-Chem (Gg Br a-1) Liang et al. [2010] stratospheric](https://slidetodoc.com/presentation_image_h/243c10838855251c7c1785c117f0f1d5/image-15.jpg)
Global tropospheric Bry budget in GEOS-Chem (Gg Br a-1) Liang et al. [2010] stratospheric Bry model (upper boundary conditions) STRATOSPHERE 36 56 CH 3 Br CH 2 Br 2 CHBr 3 57 407 Deposition lifetime 7 days 1420 (5 -15) Volcanoes 7 -9 ppt Bry 3. 2 ppt TROPOSPHERE Marine biosphere Sea-salt debromination (50% of 1 -10 µm particles) SURFACE Sea salt is the dominant global source but is released in marine boundary layer where lifetime against deposition is short; CHBr 3 is major source in the free troposphere Parrella et al. [2012]

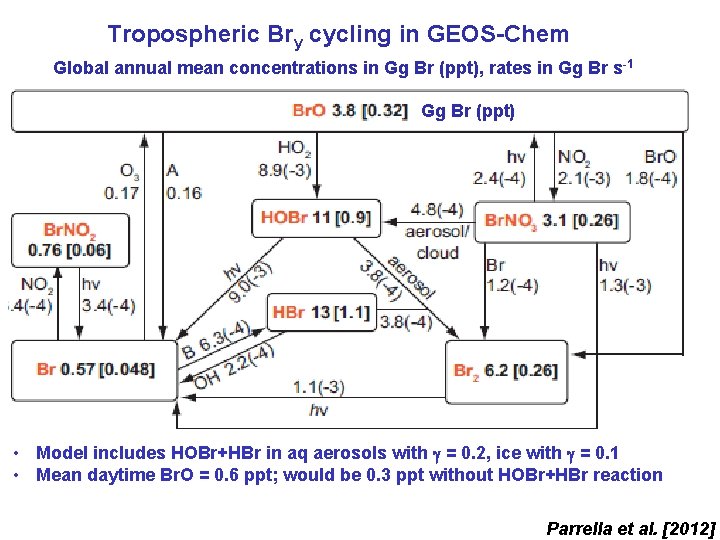
Tropospheric Bry cycling in GEOS-Chem Global annual mean concentrations in Gg Br (ppt), rates in Gg Br s -1 Gg Br (ppt) • Model includes HOBr+HBr in aq aerosols with = 0. 2, ice with = 0. 1 • Mean daytime Br. O = 0. 6 ppt; would be 0. 3 ppt without HOBr+HBr reaction Parrella et al. [2012]

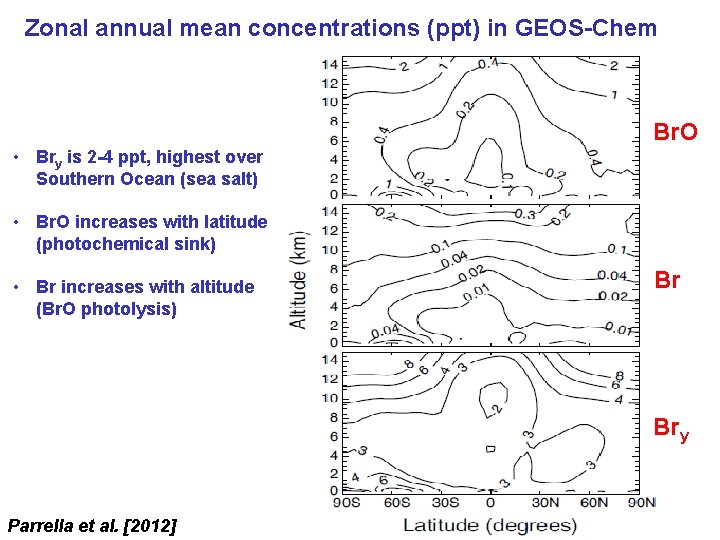
Zonal annual mean concentrations (ppt) in GEOS-Chem Br. O • Bry is 2 -4 ppt, highest over Southern Ocean (sea salt) • Br. O increases with latitude (photochemical sink) • Br increases with altitude (Br. O photolysis) Br Bry Parrella et al. [2012]
![Comparison to seasonal satellite data for tropospheric Br O Theys et al 2011 Comparison to seasonal satellite data for tropospheric Br. O [Theys et al. , 2011]](https://slidetodoc.com/presentation_image_h/243c10838855251c7c1785c117f0f1d5/image-18.jpg)
Comparison to seasonal satellite data for tropospheric Br. O [Theys et al. , 2011] (9: 30 am) model • TOMCAT has lower =0. 02 for HOBr+HBr than GEOS-Chem, large polar spring source from blowing snow • HOBr+HBr reaction critical for increasing Br. O with latitude, winter/spring NH max in GEOS -Chem Parrella et al. [2012]

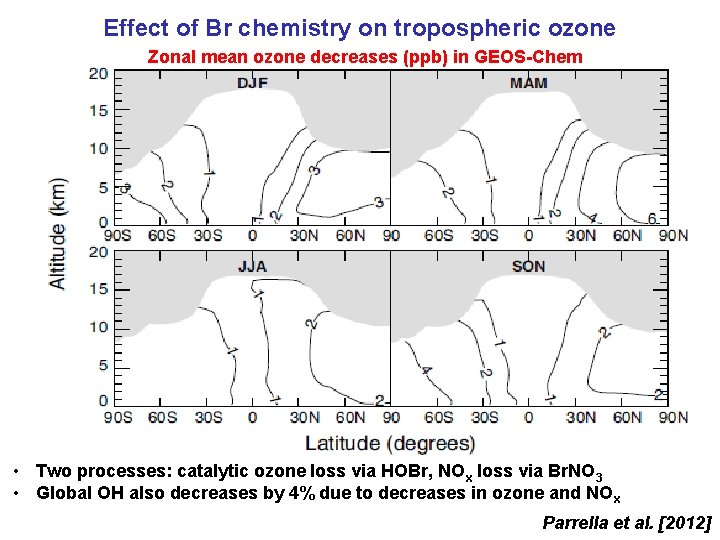
Effect of Br chemistry on tropospheric ozone Zonal mean ozone decreases (ppb) in GEOS-Chem • Two processes: catalytic ozone loss via HOBr, NOx loss via Br. NO 3 • Global OH also decreases by 4% due to decreases in ozone and NO x Parrella et al. [2012]

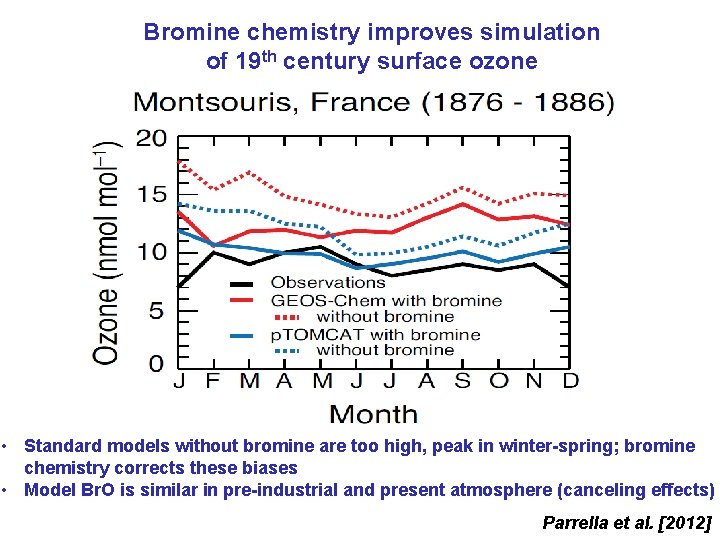
Bromine chemistry improves simulation of 19 th century surface ozone • Standard models without bromine are too high, peak in winter-spring; bromine chemistry corrects these biases • Model Br. O is similar in pre-industrial and present atmosphere (canceling effects) Parrella et al. [2012]

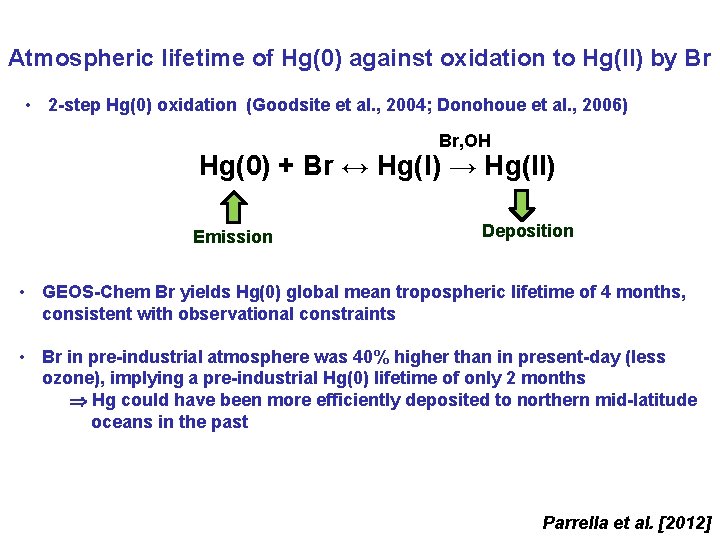
Atmospheric lifetime of Hg(0) against oxidation to Hg(II) by Br • 2 -step Hg(0) oxidation (Goodsite et al. , 2004; Donohoue et al. , 2006) Br, OH Hg(0) + Br ↔ Hg(I) → Hg(II) Emission Deposition • GEOS-Chem Br yields Hg(0) global mean tropospheric lifetime of 4 months, consistent with observational constraints • Br in pre-industrial atmosphere was 40% higher than in present-day (less ozone), implying a pre-industrial Hg(0) lifetime of only 2 months Hg could have been more efficiently deposited to northern mid-latitude oceans in the past Parrella et al. [2012]

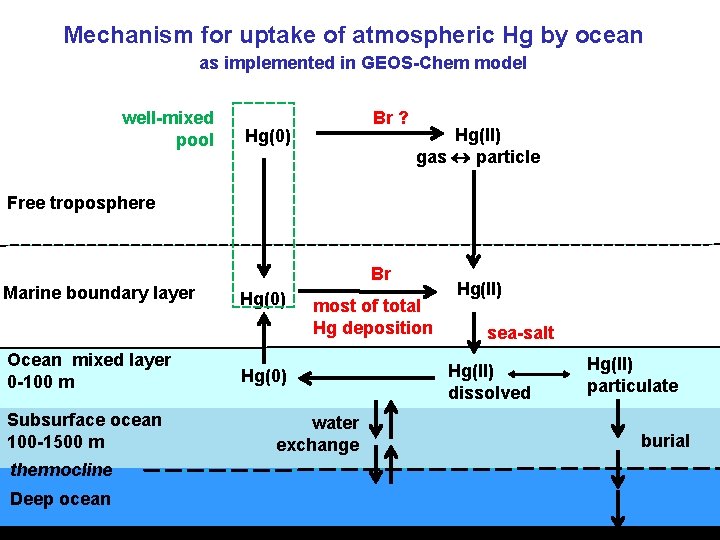
Mechanism for uptake of atmospheric Hg by ocean as implemented in GEOS-Chem model well-mixed pool Br ? Hg(0) Hg(II) gas particle Free troposphere Marine boundary layer Ocean mixed layer 0 -100 m Subsurface ocean 100 -1500 m thermocline Deep ocean Br Hg(0) most of total Hg deposition Hg(0) water exchange Hg(II) sea-salt Hg(II) dissolved Hg(II) particulate burial

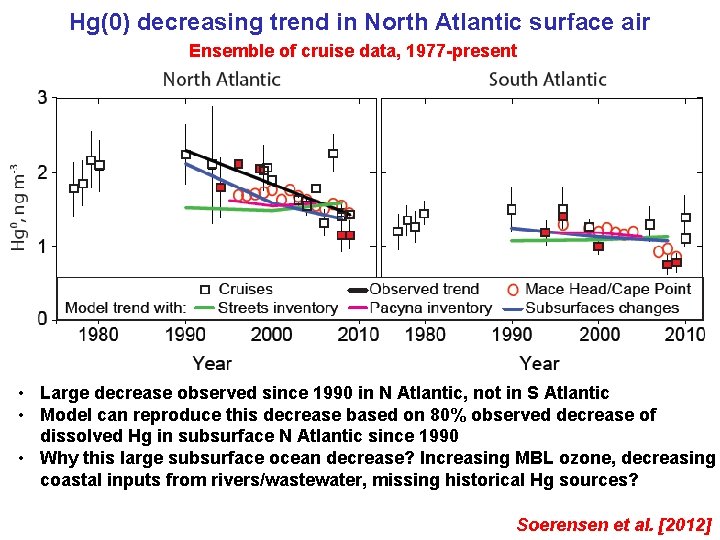
Hg(0) decreasing trend in North Atlantic surface air Ensemble of cruise data, 1977 -present • Large decrease observed since 1990 in N Atlantic, not in S Atlantic • Model can reproduce this decrease based on 80% observed decrease of dissolved Hg in subsurface N Atlantic since 1990 • Why this large subsurface ocean decrease? Increasing MBL ozone, decreasing coastal inputs from rivers/wastewater, missing historical Hg sources? Soerensen et al. [2012]

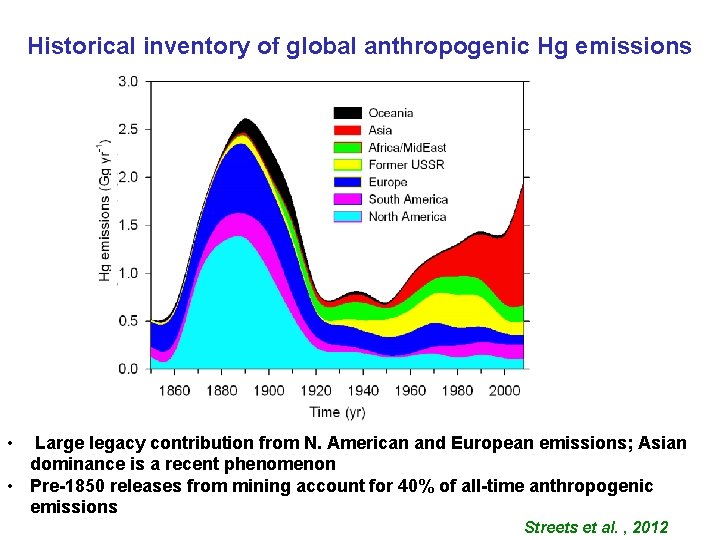
Historical inventory of global anthropogenic Hg emissions • Large legacy contribution from N. American and European emissions; Asian dominance is a recent phenomenon • Pre-1850 releases from mining account for 40% of all-time anthropogenic emissions Streets et al. , 2012

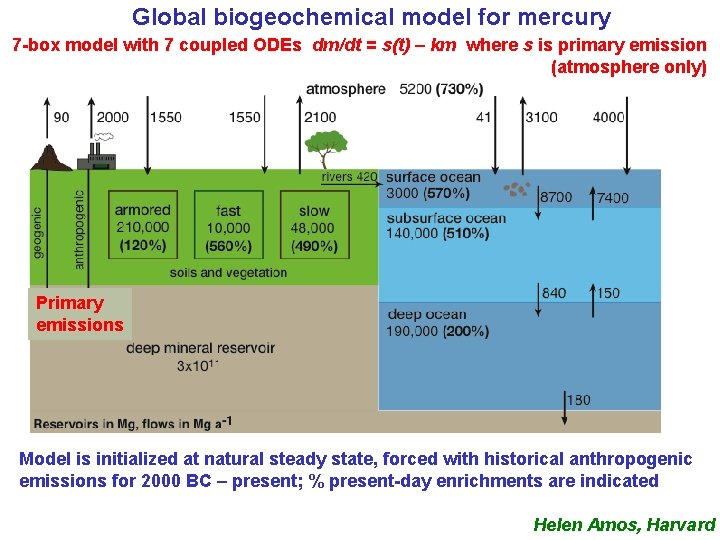
Global biogeochemical model for mercury 7 -box model with 7 coupled ODEs dm/dt = s(t) – km where s is primary emission (atmosphere only) Primary emissions Model is initialized at natural steady state, forced with historical anthropogenic emissions for 2000 BC – present; % present-day enrichments are indicated Helen Amos, Harvard

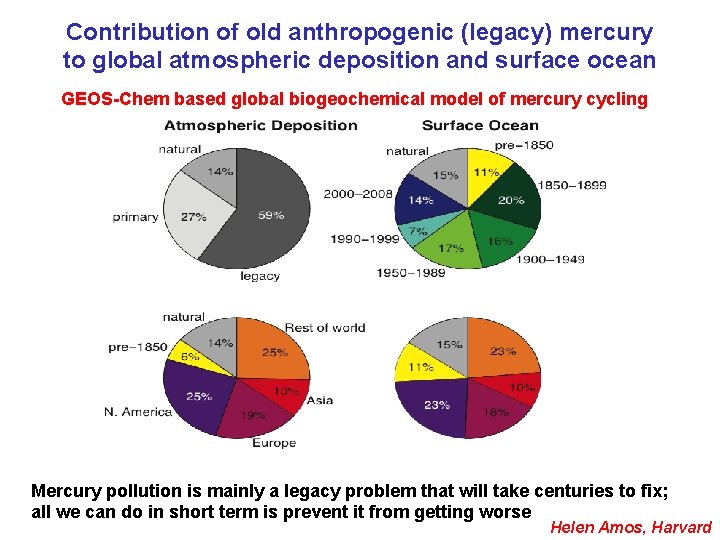
Contribution of old anthropogenic (legacy) mercury to global atmospheric deposition and surface ocean GEOS-Chem based global biogeochemical model of mercury cycling Mercury pollution is mainly a legacy problem that will take centuries to fix; all we can do in short term is prevent it from getting worse Helen Amos, Harvard
 Oxidant
Oxidant Troposphere characteristics
Troposphere characteristics How thick is the atmosphere
How thick is the atmosphere Characteristics of troposphere
Characteristics of troposphere Layers of the atmosphere
Layers of the atmosphere Troposphere characteristics
Troposphere characteristics Troposphere facts
Troposphere facts Troposphere stratosphere mesosphere thermosphere exosphere
Troposphere stratosphere mesosphere thermosphere exosphere Tropopause folding
Tropopause folding Role making role taking beispiele
Role making role taking beispiele Statuses and their related roles determine
Statuses and their related roles determine Azure web role worker role example
Azure web role worker role example Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết Số.nguyên tố
Số.nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Tư thế worms-breton
Tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin