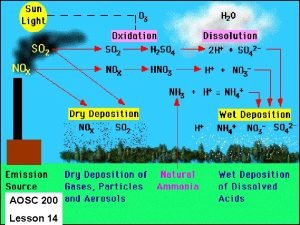
Chemistry of the Troposphere Introduction Photochemical Smog Nitrogen





- Slides: 34

Chemistry of the Troposphere

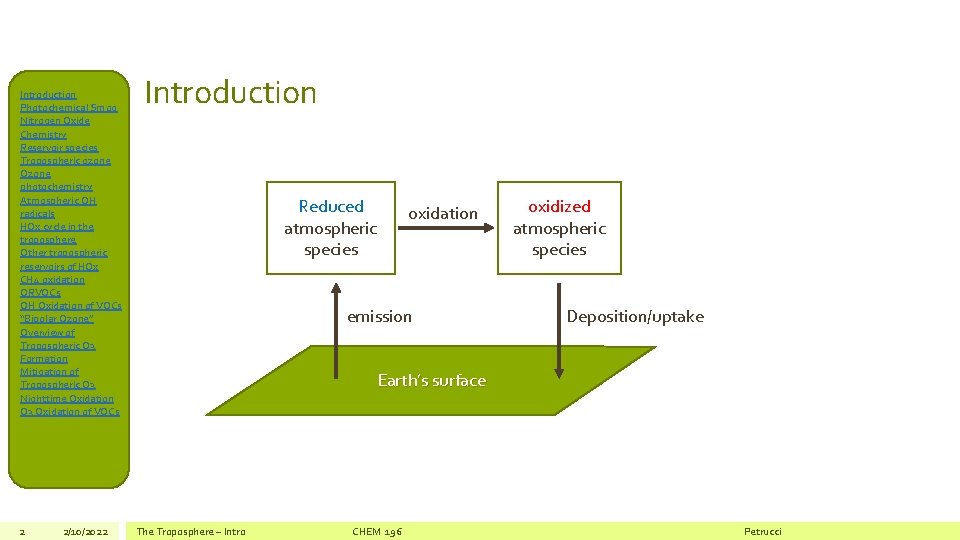
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 2 2/10/2022 Introduction Reduced atmospheric species oxidation emission oxidized atmospheric species Deposition/uptake Earth’s surface The Troposphere – Intro CHEM 196 Petrucci

Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 3 2/10/2022 • John Evelyn, English author and founding member of the Royal Society, published Fumigufium in 1661, describing London smog and offering suggestions for its mitigation “…a cloud of sea-coal, as if there be a semblance of hell upon Earth…” • Haagen-Smit, Ind. Eng. Chem. 44: 1342 (1952): Smog from photochemical oxidation of hydrocarbons in the presence of NOx; description of ozone, aerosol pollution: “Photochemical and other reactions change normally harmless compounds into objectionable ones. On the other hand, substances irritating when released may soon be converted into harmless ones. A proper evaluation of the contribution of air pollutants to the smog nuisance must include not only the time and place of their emission, but also their fate in the air. ” • Haagen-Smit, Ind. Eng. Chem. 45: 2086 (1953): Ozone from HCs and NOx “The release of large quantities of hydrocarbons to the air and the simultaneous presence of nitrogen oxides from combustion processes explains the relatively high ozone content. ” The Troposphere – Intro CHEM 196 Petrucci

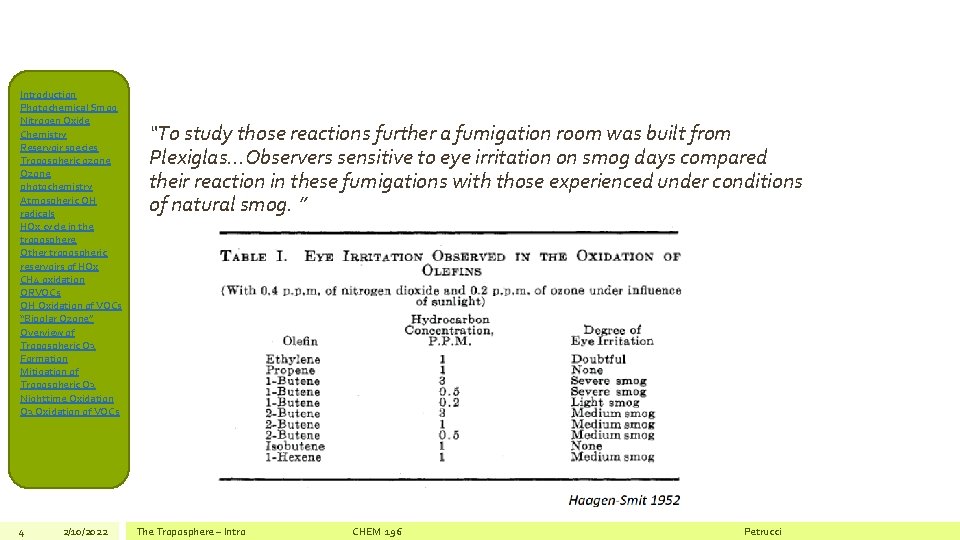
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 4 2/10/2022 “To study those reactions further a fumigation room was built from Plexiglas…Observers sensitive to eye irritation on smog days compared their reaction in these fumigations with those experienced under conditions of natural smog. ” The Troposphere – Intro CHEM 196 Petrucci

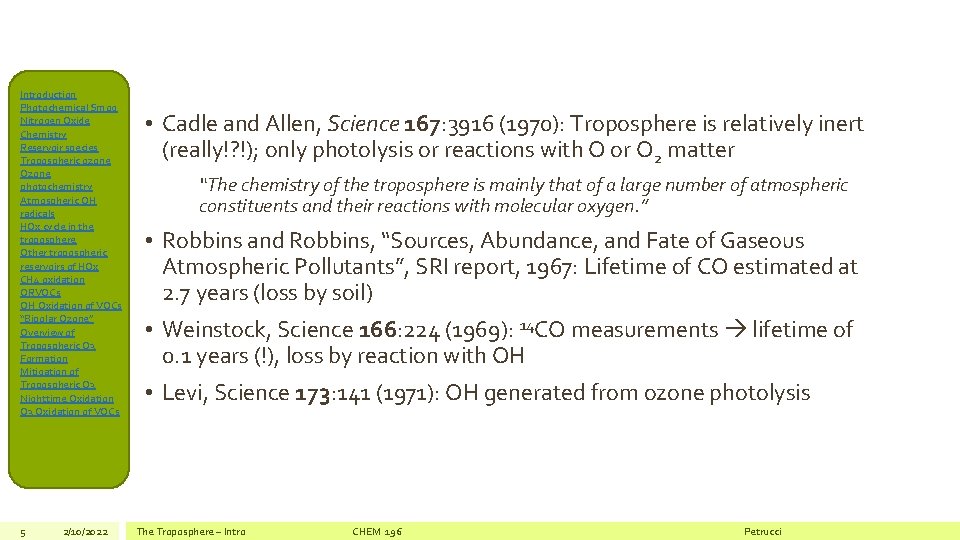
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 5 2/10/2022 • Cadle and Allen, Science 167: 3916 (1970): Troposphere is relatively inert (really!? !); only photolysis or reactions with O or O 2 matter “The chemistry of the troposphere is mainly that of a large number of atmospheric constituents and their reactions with molecular oxygen. ” • Robbins and Robbins, “Sources, Abundance, and Fate of Gaseous Atmospheric Pollutants”, SRI report, 1967: Lifetime of CO estimated at 2. 7 years (loss by soil) • Weinstock, Science 166: 224 (1969): 14 CO measurements lifetime of 0. 1 years (!), loss by reaction with OH • Levi, Science 173: 141 (1971): OH generated from ozone photolysis The Troposphere – Intro CHEM 196 Petrucci

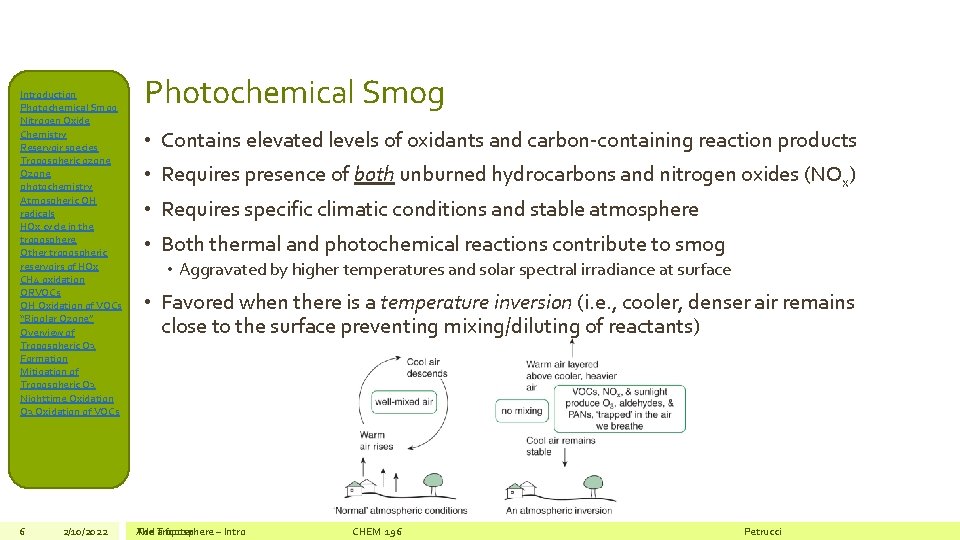
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 6 2/10/2022 Photochemical Smog • Contains elevated levels of oxidants and carbon-containing reaction products • Requires presence of both unburned hydrocarbons and nitrogen oxides (NOx) • Requires specific climatic conditions and stable atmosphere • Both thermal and photochemical reactions contribute to smog • Aggravated by higher temperatures and solar spectral irradiance at surface • Favored when there is a temperature inversion (i. e. , cooler, denser air remains close to the surface preventing mixing/diluting of reactants) The Add Troposphere a footer – Intro CHEM 196 Petrucci

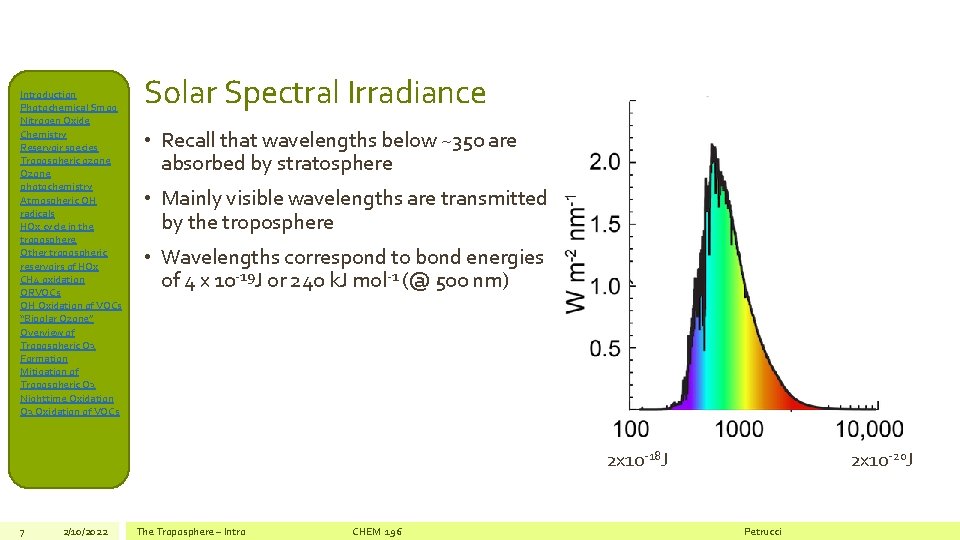
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs Solar Spectral Irradiance • Recall that wavelengths below ~350 are absorbed by stratosphere • Mainly visible wavelengths are transmitted by the troposphere • Wavelengths correspond to bond energies of 4 x 10 -19 J or 240 k. J mol-1 (@ 500 nm) 2 x 10 -20 J 2 x 10 -18 J 7 2/10/2022 The Troposphere – Intro CHEM 196 Petrucci

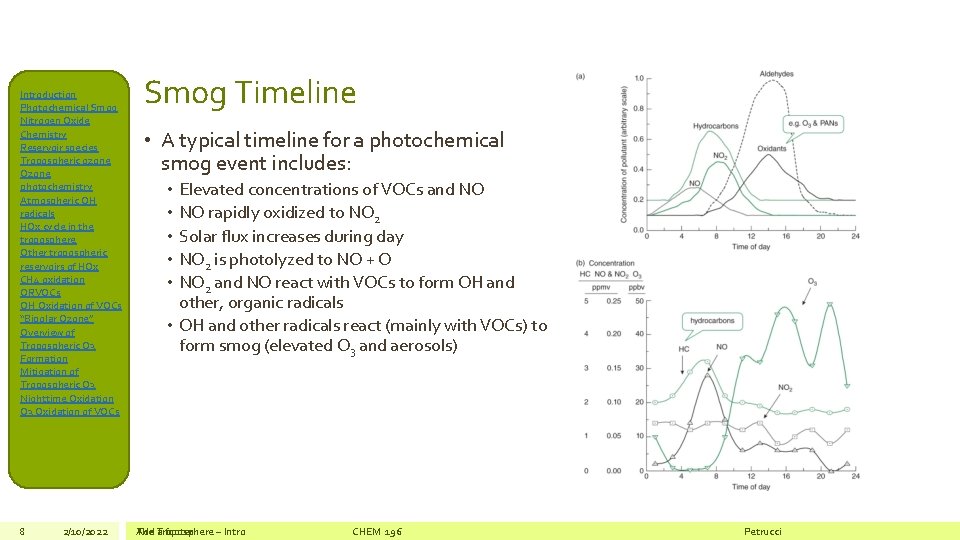
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 8 2/10/2022 Smog Timeline • A typical timeline for a photochemical smog event includes: Elevated concentrations of VOCs and NO NO rapidly oxidized to NO 2 Solar flux increases during day NO 2 is photolyzed to NO + O NO 2 and NO react with VOCs to form OH and other, organic radicals • OH and other radicals react (mainly with VOCs) to form smog (elevated O 3 and aerosols) • • • The Add Troposphere a footer – Intro CHEM 196 Petrucci

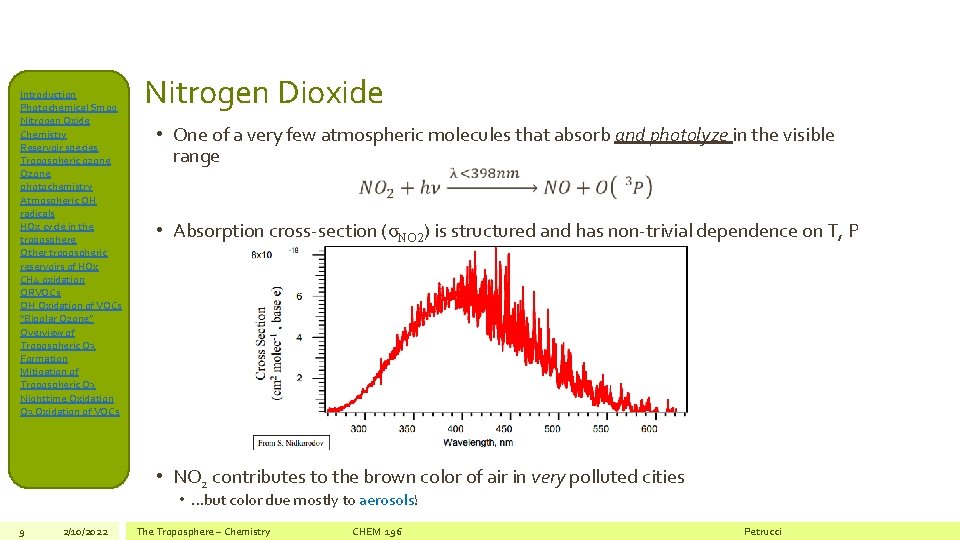
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs Nitrogen Dioxide • One of a very few atmospheric molecules that absorb and photolyze in the visible range • Absorption cross-section (σNO 2) is structured and has non-trivial dependence on T, P • NO 2 contributes to the brown color of air in very polluted cities • …but color due mostly to aerosols! 9 2/10/2022 The Troposphere – Chemistry CHEM 196 Petrucci

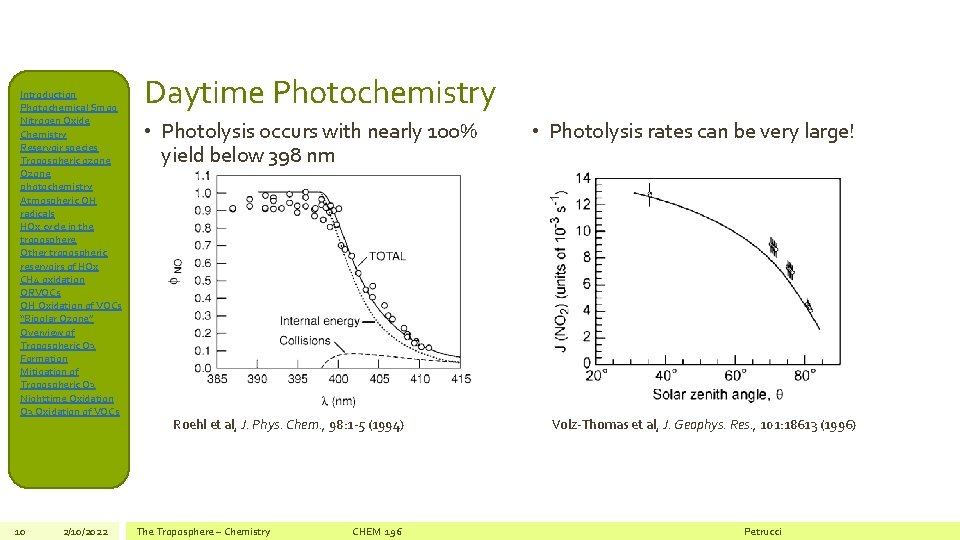
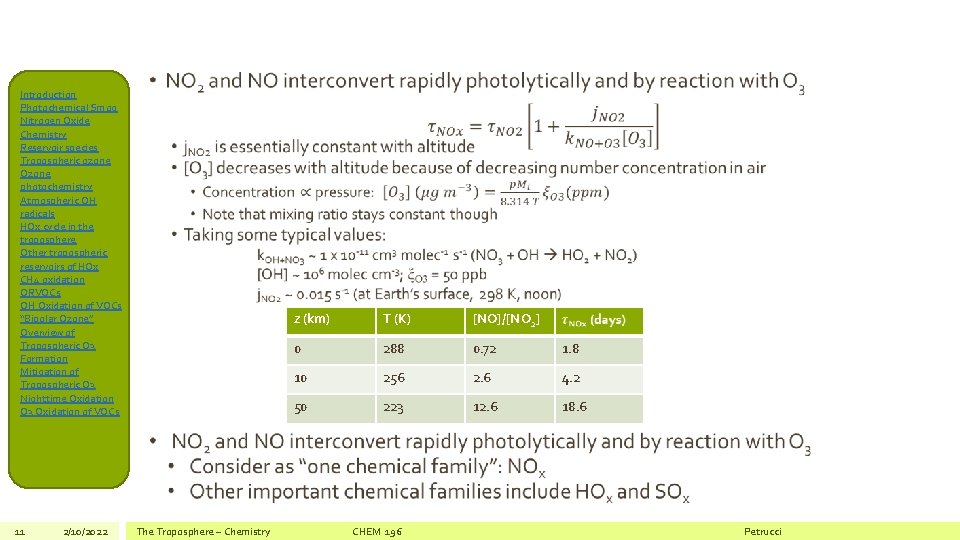
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 10 2/10/2022 Daytime Photochemistry • Photolysis occurs with nearly 100% yield below 398 nm Roehl et al, J. Phys. Chem. , 98: 1 -5 (1994) The Troposphere – Chemistry CHEM 196 • Photolysis rates can be very large! Volz-Thomas et al, J. Geophys. Res. , 101: 18613 (1996) Petrucci

Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 11 2/10/2022 • The Troposphere – Chemistry z (km) T (K) [NO]/[NO 2] 0 288 0. 72 1. 8 10 256 2. 6 4. 2 50 223 12. 6 18. 6 CHEM 196 Petrucci

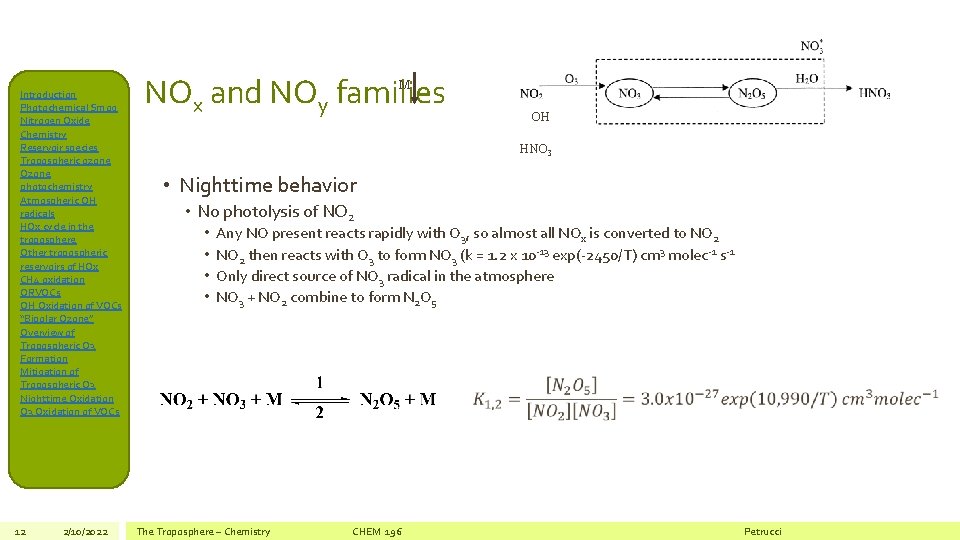
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 12 2/10/2022 NOx and NOy families M OH HNO 3 • Nighttime behavior • No photolysis of NO 2 • • Any NO present reacts rapidly with O 3, so almost all NOx is converted to NO 2 then reacts with O 3 to form NO 3 (k = 1. 2 x 10 -13 exp(-2450/T) cm 3 molec-1 s-1 Only direct source of NO 3 radical in the atmosphere NO 3 + NO 2 combine to form N 2 O 5 The Troposphere – Chemistry CHEM 196 Petrucci

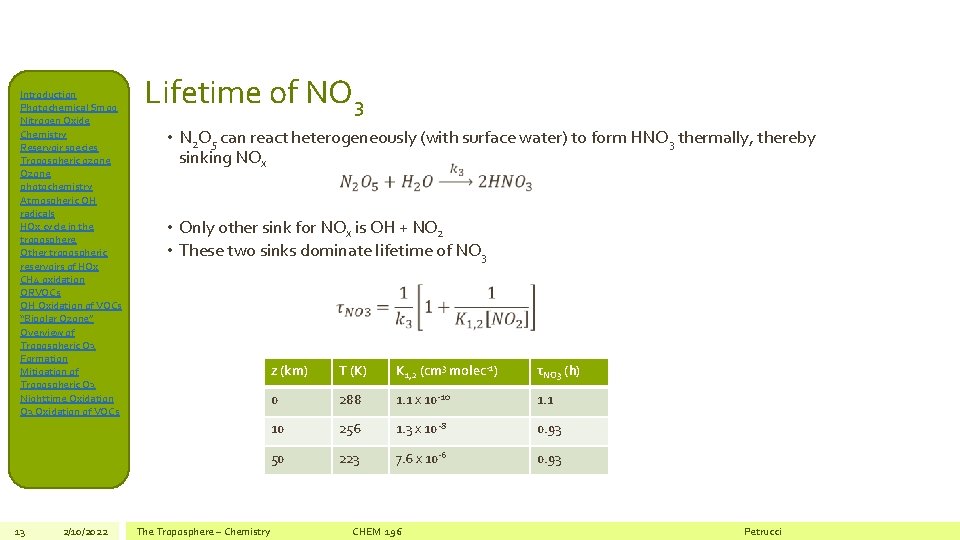
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 13 2/10/2022 Lifetime of NO 3 • N 2 O 5 can react heterogeneously (with surface water) to form HNO 3 thermally, thereby sinking NOx • Only other sink for NOx is OH + NO 2 • These two sinks dominate lifetime of NO 3 The Troposphere – Chemistry z (km) T (K) K 1, 2 (cm 3 molec-1) τNO 3 (h) 0 288 1. 1 x 10 -10 1. 1 10 256 1. 3 x 10 -8 0. 93 50 223 7. 6 x 10 -6 0. 93 CHEM 196 Petrucci

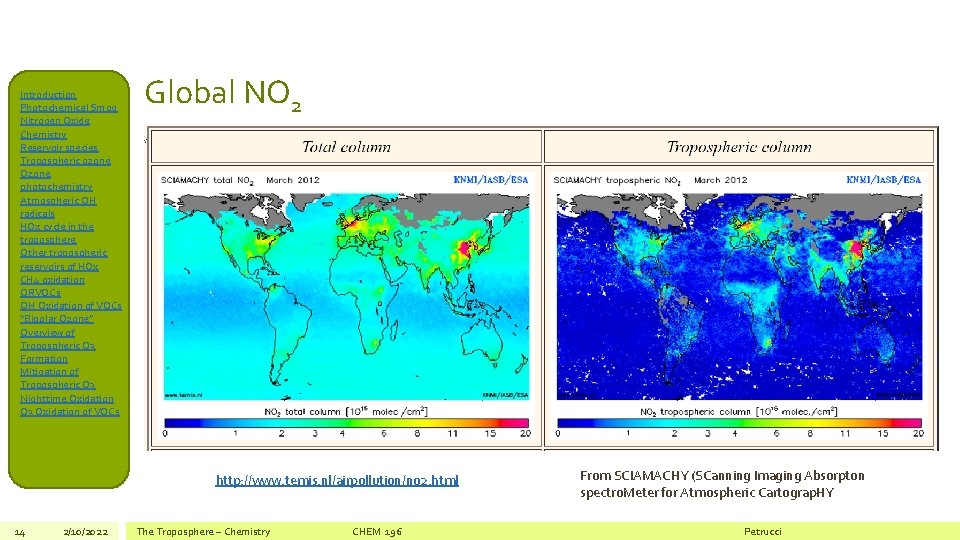
Introduction Photochemical Smog Nitrogen Oxide Chemistry Reservoir species Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs Global NO 2 http: //www. temis. nl/airpollution/no 2. html 14 2/10/2022 The Troposphere – Chemistry CHEM 196 From SCIAMACHY (SCanning Imaging Absorpton spectro. Meter for Atmospheric Cartograp. HY Petrucci

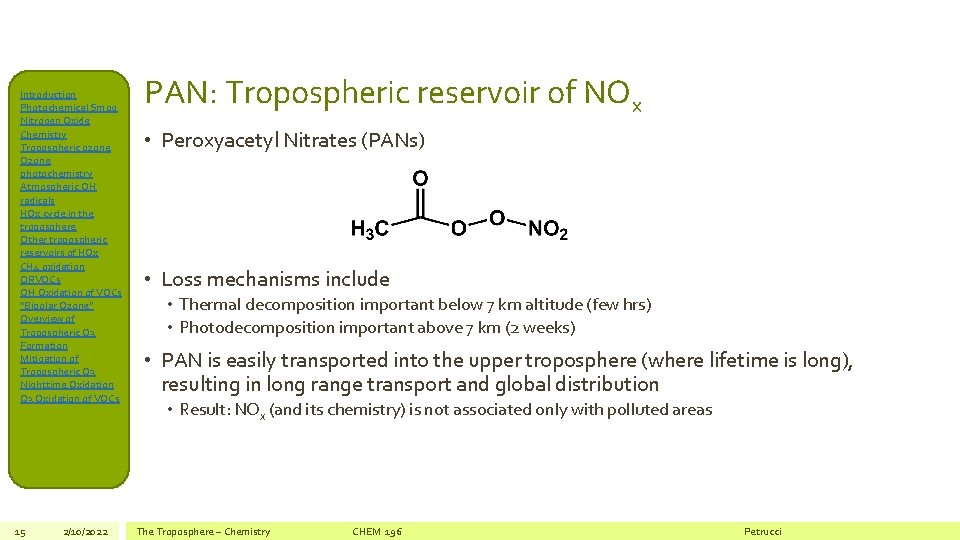
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 15 2/10/2022 PAN: Tropospheric reservoir of NOx • Peroxyacetyl Nitrates (PANs) • Loss mechanisms include • Thermal decomposition important below 7 km altitude (few hrs) • Photodecomposition important above 7 km (2 weeks) • PAN is easily transported into the upper troposphere (where lifetime is long), resulting in long range transport and global distribution • Result: NOx (and its chemistry) is not associated only with polluted areas The Troposphere – Chemistry CHEM 196 Petrucci

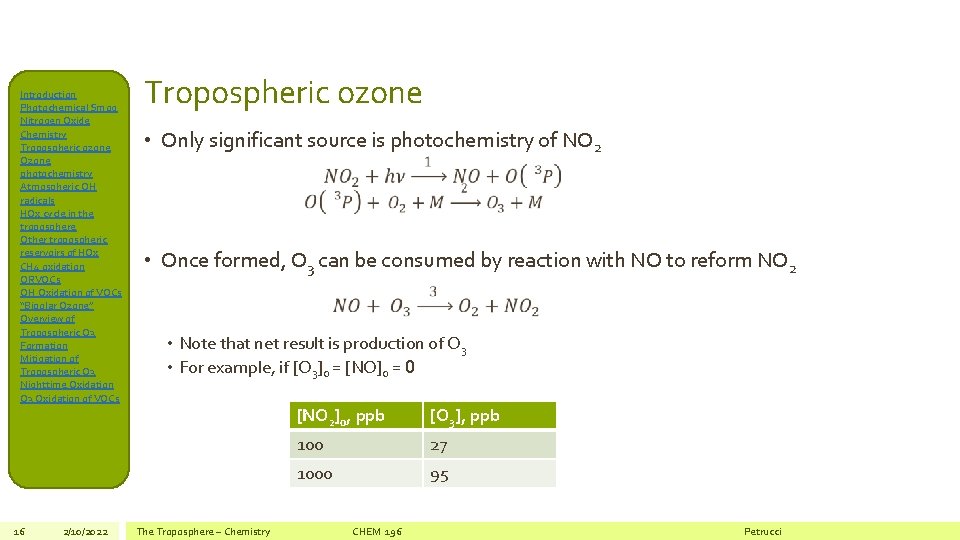
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Ozone photochemistry Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 16 2/10/2022 Tropospheric ozone • Only significant source is photochemistry of NO 2 • Once formed, O 3 can be consumed by reaction with NO to reform NO 2 • Note that net result is production of O 3 • For example, if [O 3]0 = [NO]0 = 0 The Troposphere – Chemistry [NO 2]0, ppb [O 3], ppb 100 27 1000 95 CHEM 196 Petrucci

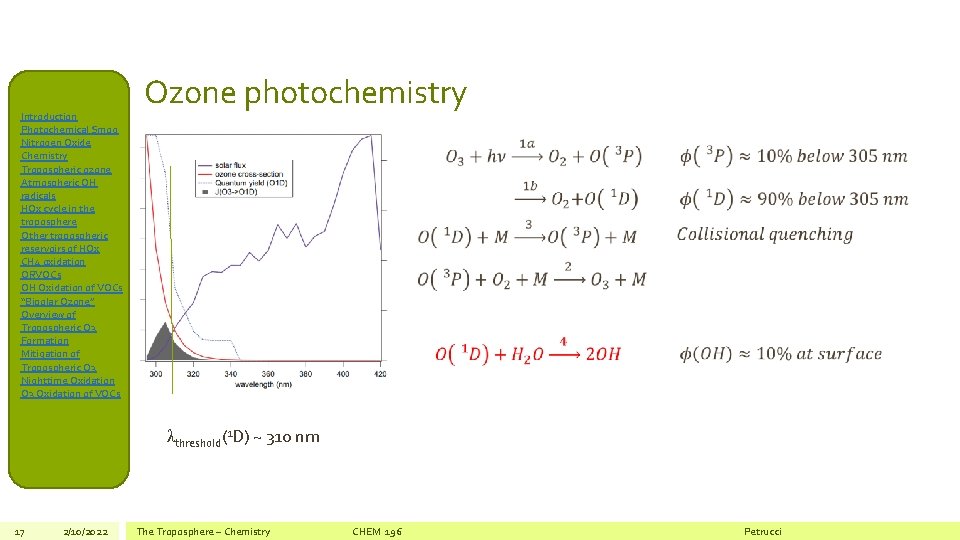
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs Ozone photochemistry λthreshold(1 D) ~ 310 nm 17 2/10/2022 The Troposphere – Chemistry CHEM 196 Petrucci

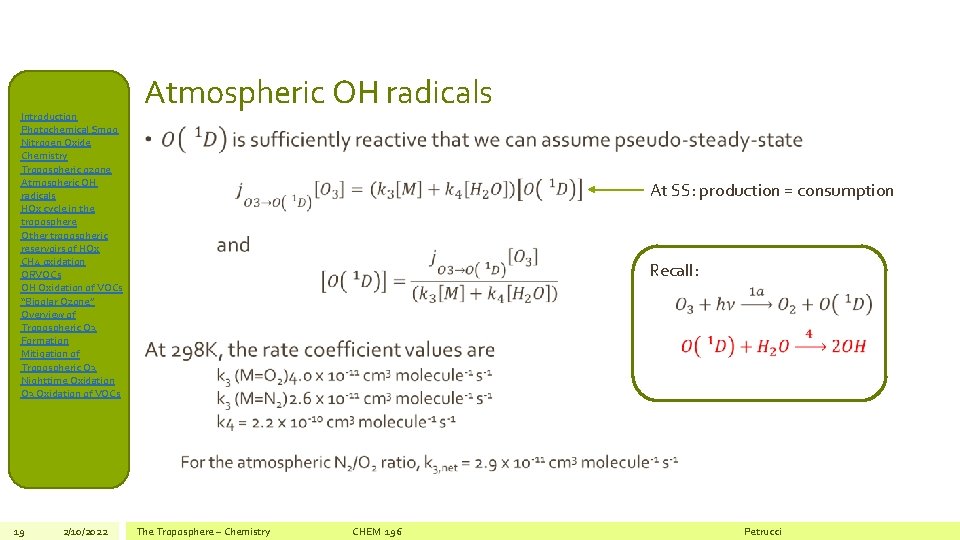
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 19 2/10/2022 Atmospheric OH radicals • At SS: production = consumption Recall: The Troposphere – Chemistry CHEM 196 Petrucci

Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 20 2/10/2022 • The Troposphere – Chemistry CHEM 196 Petrucci

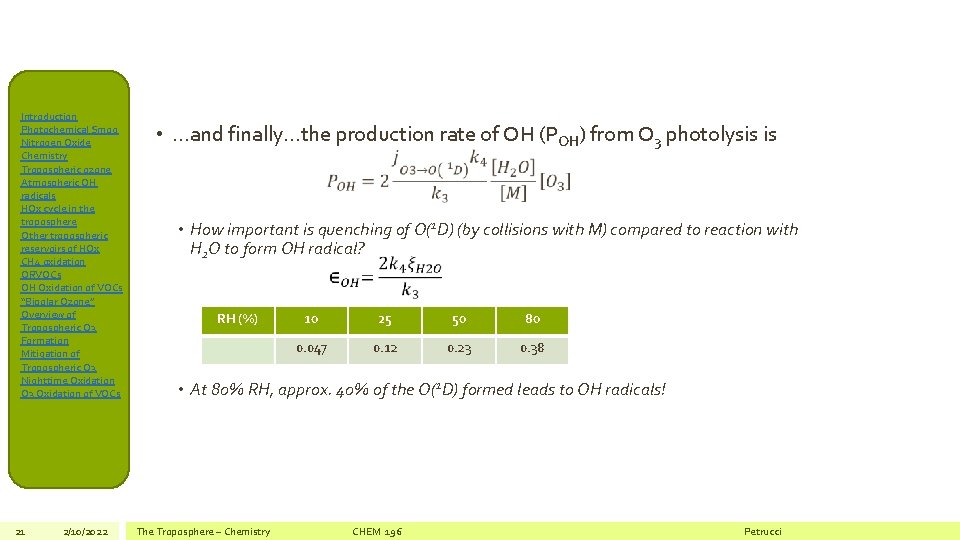
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 21 2/10/2022 • …and finally…the production rate of OH (POH) from O 3 photolysis is • How important is quenching of O(1 D) (by collisions with M) compared to reaction with H 2 O to form OH radical? RH (%) 10 25 50 80 0. 047 0. 12 0. 23 0. 38 • At 80% RH, approx. 40% of the O(1 D) formed leads to OH radicals! The Troposphere – Chemistry CHEM 196 Petrucci

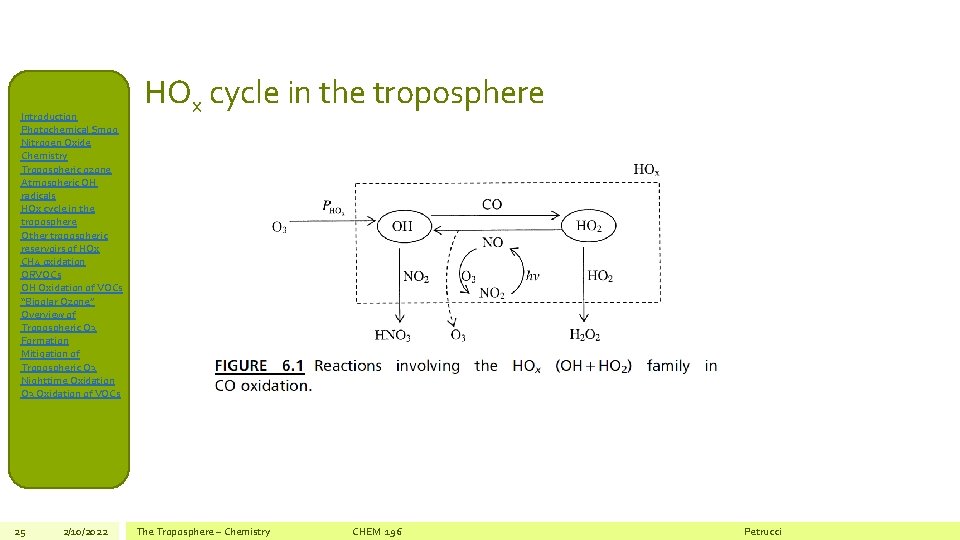
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere Other tropospheric reservoirs of HOx CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 25 2/10/2022 HOx cycle in the troposphere The Troposphere – Chemistry CHEM 196 Petrucci

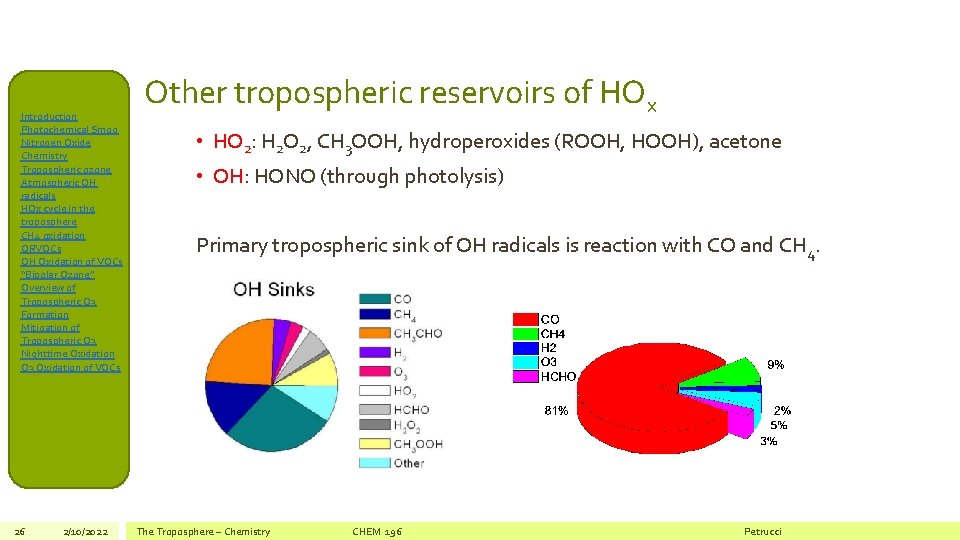
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 26 2/10/2022 Other tropospheric reservoirs of HOx • HO 2: H 2 O 2, CH 3 OOH, hydroperoxides (ROOH, HOOH), acetone • OH: HONO (through photolysis) Primary tropospheric sink of OH radicals is reaction with CO and CH 4. The Troposphere – Chemistry CHEM 196 Petrucci

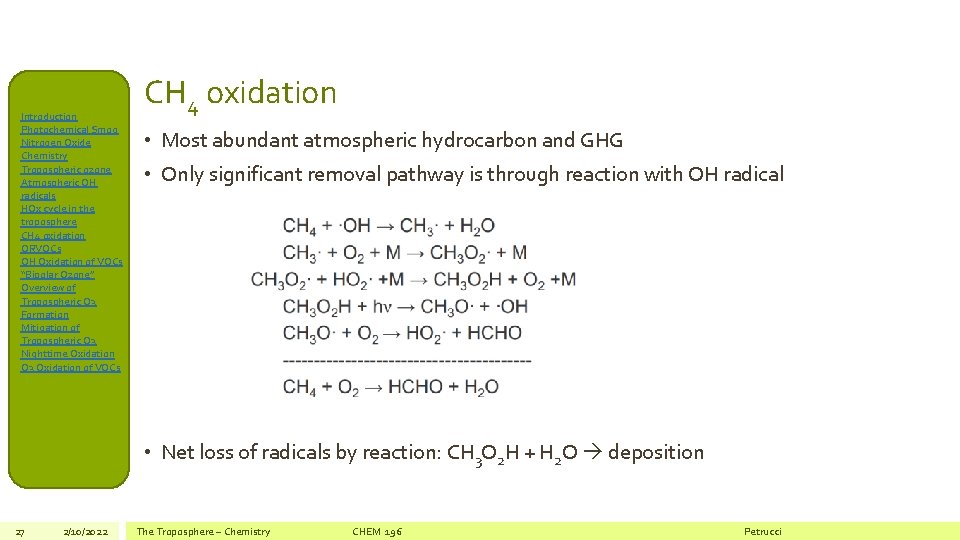
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs CH 4 oxidation • Most abundant atmospheric hydrocarbon and GHG • Only significant removal pathway is through reaction with OH radical • Net loss of radicals by reaction: CH 3 O 2 H + H 2 O deposition 27 2/10/2022 The Troposphere – Chemistry CHEM 196 Petrucci

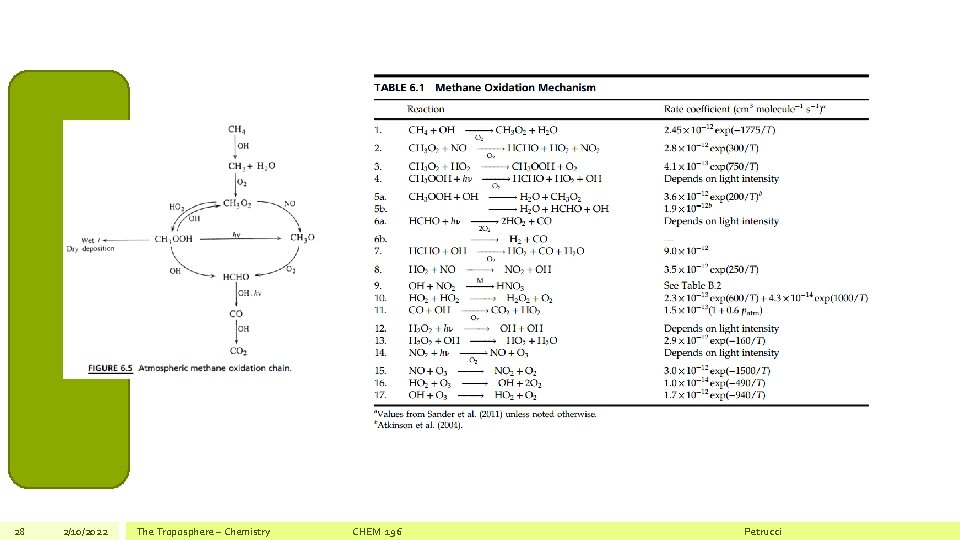
28 2/10/2022 The Troposphere – Chemistry CHEM 196 Petrucci

Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 29 2/10/2022 ORVOCs • Other Reactive Volatile Organic Compounds in the atmosphere • Although minor components, they play outsized role in aerosol production • Biogenic and anthropogenic sources • Unlike CH 4, also highly reactive with O 3 and other atmospheric oxidants • OH and O 3 oxidation occurs by very different pathways. The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 30 2/10/2022 OH Oxidation of VOCs • ALL VOCs are subject to OH oxidation • General reaction steps include: • Initiation involves a hydrogen abstraction, leaving a carbon-centered radical • Molecular oxygen adds to the radical, creating an alkylperoxy radical • Alkylperoxy radical transfers O-atom to NO molecule • Hydroperoxyl radical and aldehyde products result • Aldehydes react with NO 2 to form PANs • PAN decomposition products propagate chain reaction The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

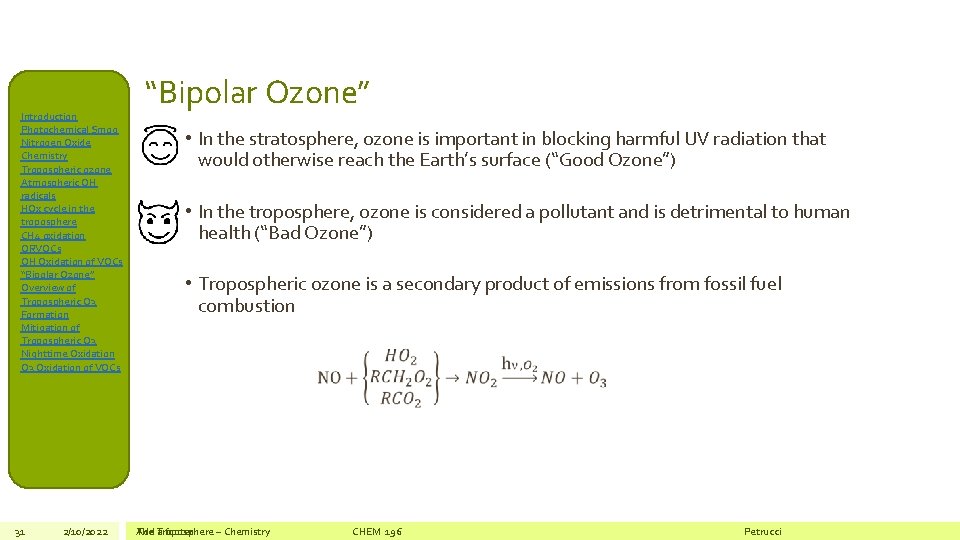
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 31 2/10/2022 “Bipolar Ozone” • In the stratosphere, ozone is important in blocking harmful UV radiation that would otherwise reach the Earth’s surface (“Good Ozone”) • In the troposphere, ozone is considered a pollutant and is detrimental to human health (“Bad Ozone”) • Tropospheric ozone is a secondary product of emissions from fossil fuel combustion The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

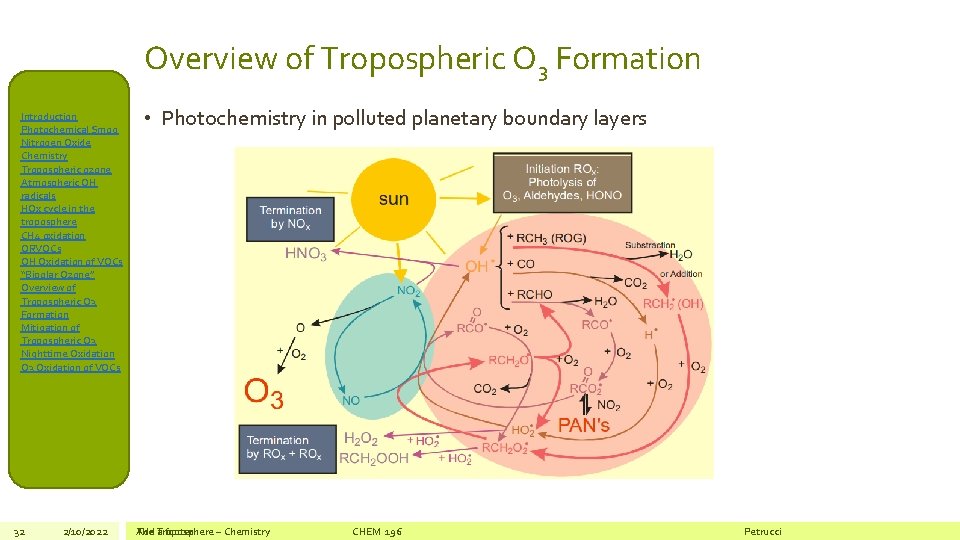
Overview of Tropospheric O 3 Formation Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Overview of Tropospheric O 3 Formation Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 32 2/10/2022 • Photochemistry in polluted planetary boundary layers The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

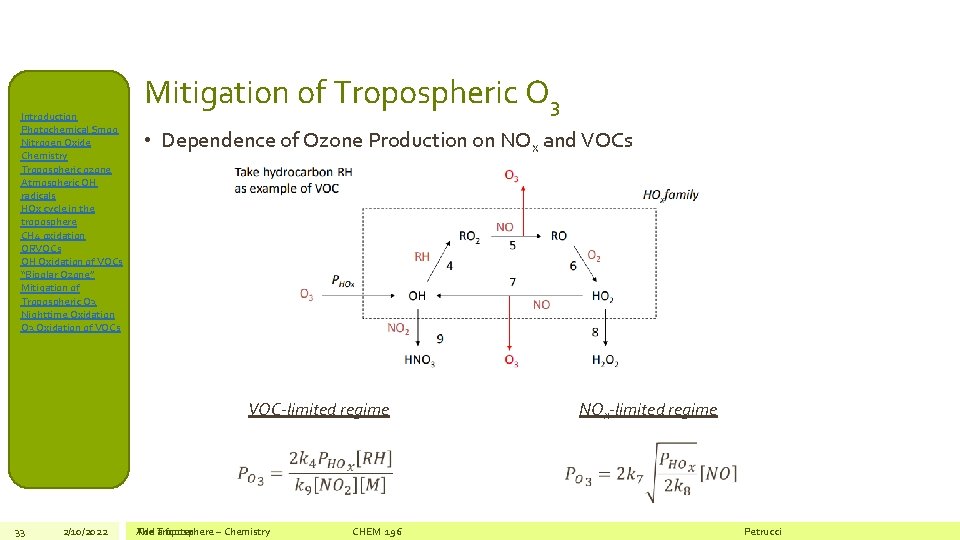
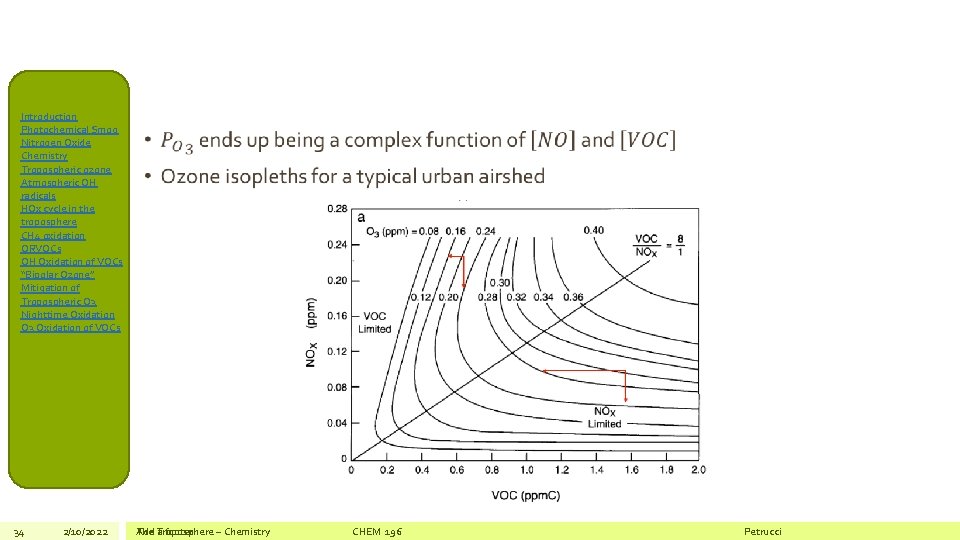
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs Mitigation of Tropospheric O 3 • Dependence of Ozone Production on NOx and VOCs VOC-limited regime 33 2/10/2022 The Add Troposphere a footer – Chemistry CHEM 196 NOx-limited regime Petrucci

Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 34 2/10/2022 • The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

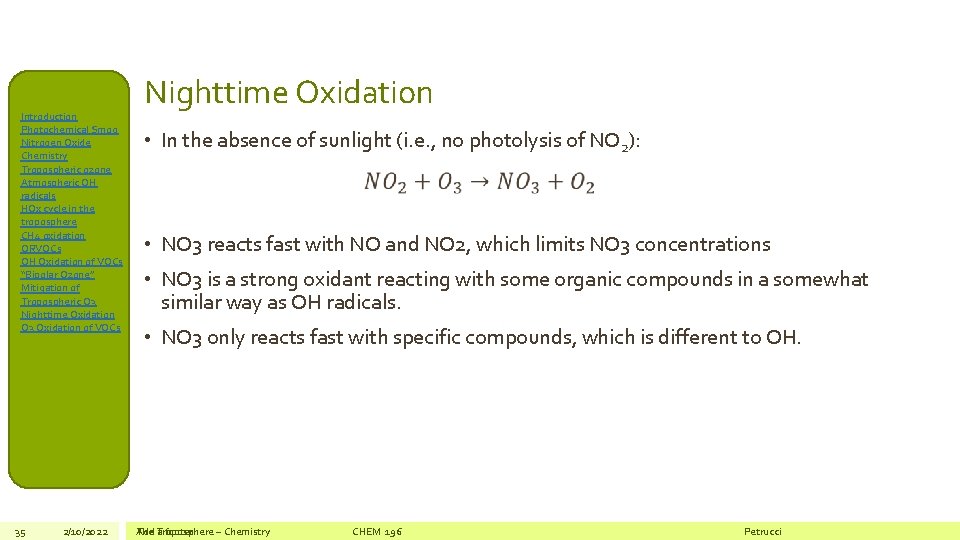
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 35 2/10/2022 Nighttime Oxidation • In the absence of sunlight (i. e. , no photolysis of NO 2): • NO 3 reacts fast with NO and NO 2, which limits NO 3 concentrations • NO 3 is a strong oxidant reacting with some organic compounds in a somewhat similar way as OH radicals. • NO 3 only reacts fast with specific compounds, which is different to OH. The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

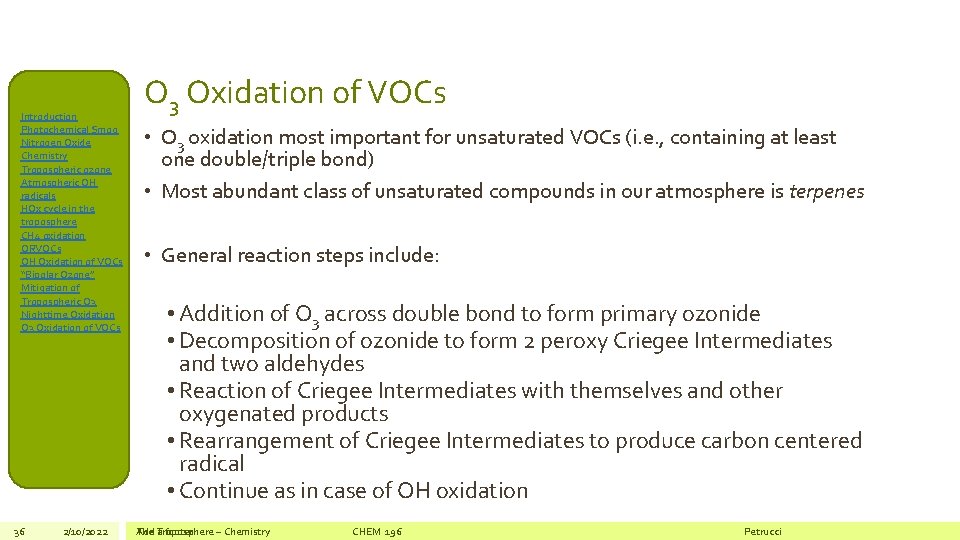
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 36 2/10/2022 O 3 Oxidation of VOCs • O 3 oxidation most important for unsaturated VOCs (i. e. , containing at least one double/triple bond) • Most abundant class of unsaturated compounds in our atmosphere is terpenes • General reaction steps include: • Addition of O 3 across double bond to form primary ozonide • Decomposition of ozonide to form 2 peroxy Criegee Intermediates and two aldehydes • Reaction of Criegee Intermediates with themselves and other oxygenated products • Rearrangement of Criegee Intermediates to produce carbon centered radical • Continue as in case of OH oxidation The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

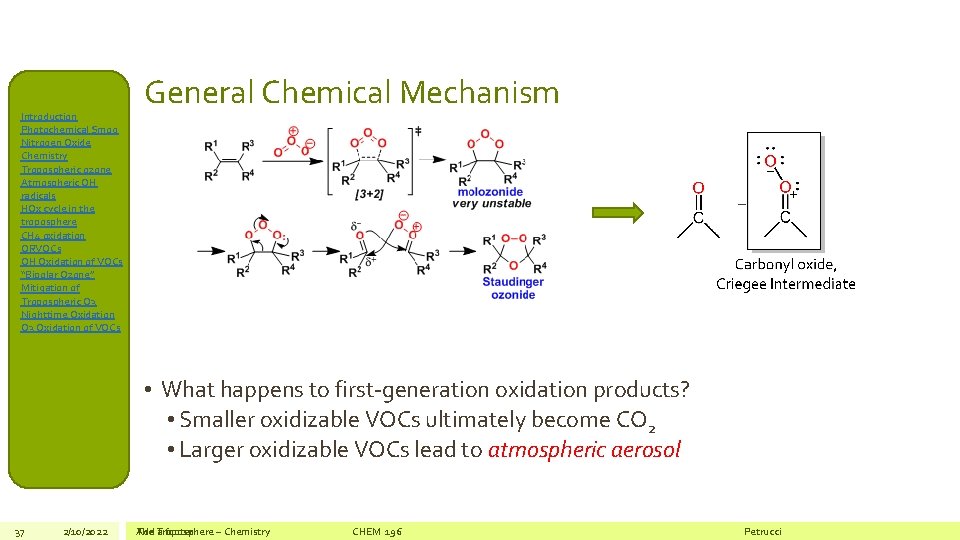
Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs General Chemical Mechanism • What happens to first-generation oxidation products? • Smaller oxidizable VOCs ultimately become CO 2 • Larger oxidizable VOCs lead to atmospheric aerosol 37 2/10/2022 The Add Troposphere a footer – Chemistry CHEM 196 Petrucci

Introduction Photochemical Smog Nitrogen Oxide Chemistry Tropospheric ozone Atmospheric OH radicals HOx cycle in the troposphere CH 4 oxidation ORVOCs OH Oxidation of VOCs “Bipolar Ozone” Mitigation of Tropospheric O 3 Nighttime Oxidation O 3 Oxidation of VOCs 38 2/10/2022 The Add Troposphere a footer – Chemistry CHEM 196 Petrucci
 Photochemical smog
Photochemical smog Effects of photochemical smog
Effects of photochemical smog Grothus draper law of photochemistry
Grothus draper law of photochemistry Define quantum yield of a photochemical reaction
Define quantum yield of a photochemical reaction Troposphere characteristics
Troposphere characteristics Troposphere stratosphere mesosphere thermosphere exosphere
Troposphere stratosphere mesosphere thermosphere exosphere Stratopause
Stratopause Troposphere characteristics
Troposphere characteristics Troposphere facts
Troposphere facts Tropopause folding
Tropopause folding Why is the mesosphere important
Why is the mesosphere important How many layers of atmosphere
How many layers of atmosphere Klb chemistry book 3 nitrogen and its compounds
Klb chemistry book 3 nitrogen and its compounds Smog formation
Smog formation Smog
Smog Sulfur dioxide smog
Sulfur dioxide smog Smog fotoquimico ecuacion quimica
Smog fotoquimico ecuacion quimica How is smog formed
How is smog formed Smog
Smog Smog hog electrostatic precipitators nyc
Smog hog electrostatic precipitators nyc Causes of smog
Causes of smog Smog
Smog Two sources of air pollution
Two sources of air pollution Sources of smog
Sources of smog Earth day quiz
Earth day quiz Kamilia smog
Kamilia smog Kamilia smog
Kamilia smog Smog basics
Smog basics El smog fotoquímico
El smog fotoquímico Types of smog
Types of smog Pengertian smog
Pengertian smog Denora smog
Denora smog Smog reductor
Smog reductor Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry