LECTURE 01 Cast of Characters AOSC 434 Air




































- Slides: 58

LECTURE 01 Cast of Characters AOSC 434 Air Pollution Russell R. Dickerson www. atmos. umd. edu/~russ/syllabus 434. html


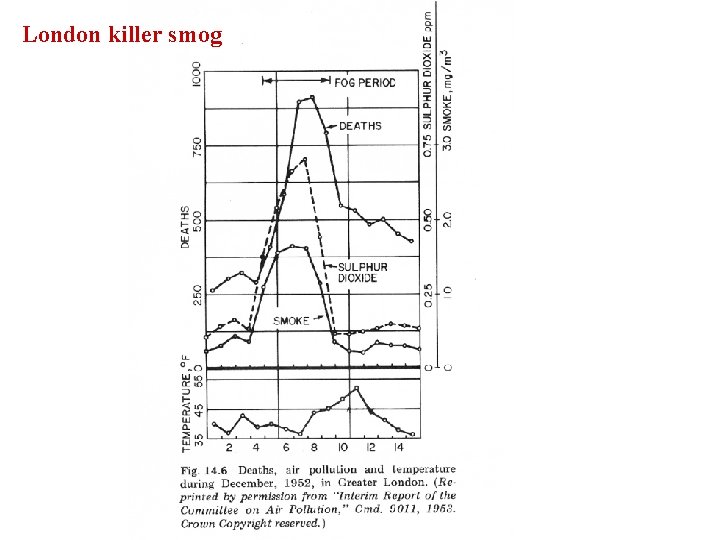
London killer smog

Donora, PA October 29, 1948; 2: 00 pm LST


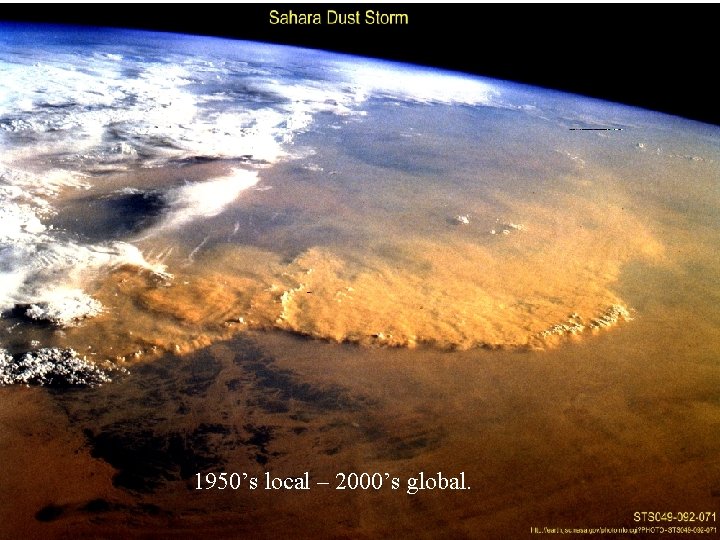
1950’s local – 2000’s global.


Washington Post Jan. 25, 2014 “China’s air pollution prompts creative, sometimes wacky, solutions”


Air pollution, seen here over Mexico City, has many effects that are detrimental to the economy. Joshua Graff Zivin, and Matthew Neidell Science 2018; 359: 39 -40 Published by AAAS

Pollution and Smog Seinfeld & Pandis Ch. 2 Finlayson-Pitts & Pitts Ch. 1 Wark & Warner Ch. 1 Jacob Chapters 12 & 13.

Definitions • Los Angeles Smog (photochemical smog) is the mixture of ozone, hydrocarbons, partially oxidized hydrocarbons, oxides of nitrogen and other trace gases that results from the action of sunlight on automobile exhaust and other pollutants. It is characterized by high temperatures stagnant winds (high barometric pressure), and sunny conditions. • London Smog (particulate, or sulfurous smog) is a mixture of sulfur dioxide and sulfate and sulfite aerosol resulting primarily from the combustion of high sulfur coal followed by conversion of SO 2 to H 2 SO 4. It is characterized by low temperatures, high humidity and stagnant winds.

Air Pollutants Photochemical and London Smog Species Involved Including Criteria Pollutants Limit here refers to the National Ambient Air Quality Standard (NAAQS) established by the US-EPA.

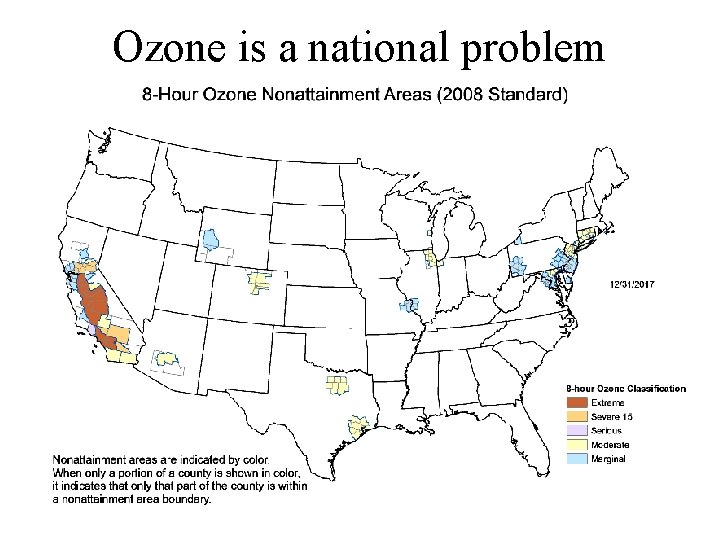
1. Ozone, O 3 (Photochemical Oxidant) criteria pollutant Secondary • Effects: 1. Respiration - premature aging of lungs (Bascom et al. , 1996); mortality (e. g. , Jerrett et al. , 2009). 4%/10 ppb. 300, 000 deaths/yr (Landrigan et al. 2017) 2. Phytotoxin, i. e. Vegetation damage (Heck et al. , JAPCA. , 1982; Schmalwieser et al. 2003; Mac. Kinzie and El-Ashry, 1988) 3. Materials damage - rubber 4. Greenhouse effect (9. 6 m) • Limit: (National Ambient Air Quality Standard) 80 ppb for 1 hr. 1971 120 ppb for 1 hr. 1979 84 ppb for 8 hr 1997 75 ppb for 8 hr 2010 70 ppb for 8 hr 2015 • Ozone is an indicator of smog. • Ozone regulates many other oxidants

Ozone damaged plants.



What does history tell us? • Denora, Pitt, and London were sulfurous smogs. • Early work in Los Angeles focused on SO 2 from refineries – smog got worse. • VOC’s targeted next – smog got worse. • Denora, London, etc. were worse in winter – LA was worse in summer. • Burning eyes in LA. 18

What does history tell us? P. L. Mc. Gill, Stanford Research Institute*, “The Los Angeles Smog Problem” Industrial and Engineering Chemistry, 2476 -86, 1949. “Unquestionably the most disagreeable aspect of smog is eye irritation. ” They blamed elemental sulfur. Mechanism of the Smog: “Weather conditions control the time of occurrence of eye-irritating smog in Los Angeles. ” Meteorology and topography. Identified temperature inversions and stagnant winds as contributors. No mention of combustion, ozone, photochemistry, or automobiles other than as a source of H 2 CO that did not cause eye irritation. *supported by The Western Oil and Gas Association. 19

Haagen-Smit (1952) “Photochemical action of nitrogen oxides oxidized the hydrocarbons and thereby forms ozone…. ” Almost right. 20

Ozone is a national problem

2. Nitrogen Dioxide, NO 2 criteria pollutant Primary Effects: 1. Lungs (acute chemical pneumonia) EPA Criteria Pollutant 2. Phytotoxin 3. Catalyst for ozone formation. 4. Atmospheric acidity (about 1/3 of problem and growing) Limit: 100 g m-3 (53 ppb) annual mean 200 g m-3 (100 ppb) hourly mean (2010)



3. Carbon Monoxide, CO criteria pollutant Primary Effects: 1. Respiration (acute); EPA Criteria Pollutant 2. Cardiovascular system (chronic) 3. Contributor to photochemical smog 4. Changes global HOx cycle (oxidizing capacity of atmosphere). Limits: 9. 0 ppm for 8 hr 35 ppm for 1 hr 50 ppm for 8 hr is the "level of significant harm"

3. Carbon Monoxide, CO (cont…) • • Affinity for hemoglobin 200 times that of O 2. Displaces O 2 at [CO] = 0. 2 x 106/ 200 = 103 ppm. Concentrations above 750 ppm are fatal. Concentrations > 100 ppm cause dizziness, headache, loss of visual & mental acuity. • Cigarette smoke contains ca. 400 ppm CO (also HCN, H 2 CO, Ni(CO)4, NO 2).


4. Peroxyacetyl Nitrate, "PAN" (CH 3 C(O)-O-O- NO 2) not a criteria pollutant Secondary Effects: 1. Eye irritation 2. Respiratory tract (carcinogen? ) 3. Phytotoxin Limits: None (too hard to measure) • Compound "X" in LA smog • NOx reservoir.

An interesting history Smogtown, by Jacobs and Kelly, Overlook Press, 2008. 29

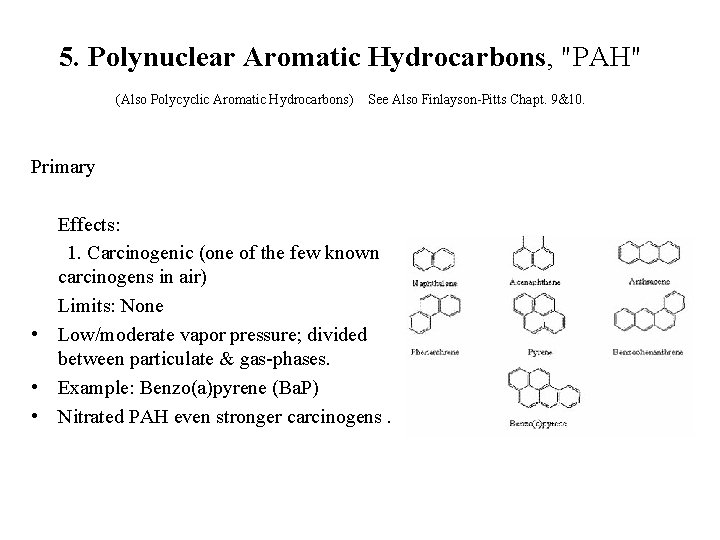
5. Polynuclear Aromatic Hydrocarbons, "PAH" (Also Polycyclic Aromatic Hydrocarbons) See Also Finlayson-Pitts Chapt. 9&10. Primary Effects: 1. Carcinogenic (one of the few known carcinogens in air) Limits: None • Low/moderate vapor pressure; divided between particulate & gas-phases. • Example: Benzo(a)pyrene (Ba. P) • Nitrated PAH even stronger carcinogens.

6. Ethylene, H 2 C=CH 2 Primary Effects: 1. Ozone formation 2. Plant hormone (e. g. oranges) Limits: None • Other biogenic hydrocarbons, isoprene, pinenes. • Some plants when stressed release more ethylene

7. Formaldehyde, H 2 CO Primary and secondary Effects: 1. Ozone formation 2. Eye irritant 3. Mutagen, suspected carcinogen Limits: None • Indoor air pollutant too, (ureaformaldehyde insulation) • Produced by HC oxidation • Represents class of partially oxidized HC

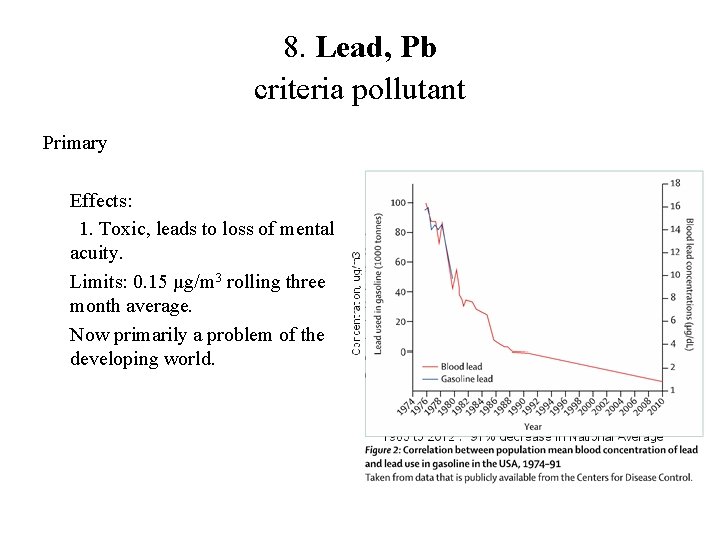
8. Lead, Pb criteria pollutant Primary Effects: 1. Toxic, leads to loss of mental acuity. Limits: 0. 15 µg/m 3 rolling three month average. Now primarily a problem of the developing world.

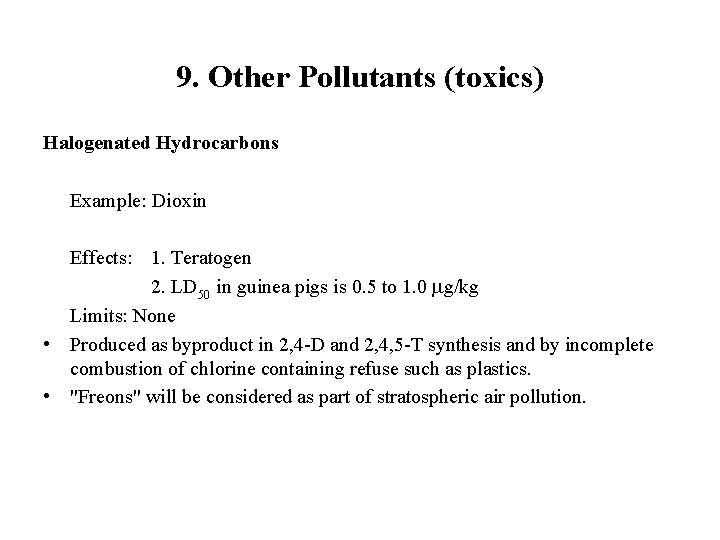
9. Other Pollutants (toxics) Halogenated Hydrocarbons Example: Dioxin Effects: 1. Teratogen 2. LD 50 in guinea pigs is 0. 5 to 1. 0 g/kg Limits: None • Produced as byproduct in 2, 4 -D and 2, 4, 5 -T synthesis and by incomplete combustion of chlorine containing refuse such as plastics. • "Freons" will be considered as part of stratospheric air pollution.



LONDON-TYPE SMOG 10. Sulfur Dioxide, SO 2 Primary Effects 1. Produces H 2 SO 4 found on particles and in precipitation - Acid Deposition 2. Cloud Condensation Nuclei (climate) 3. Materials degradation 4. Respiratory tract (esp. bisulfites, HSO 3 -) 5. Phytotoxin

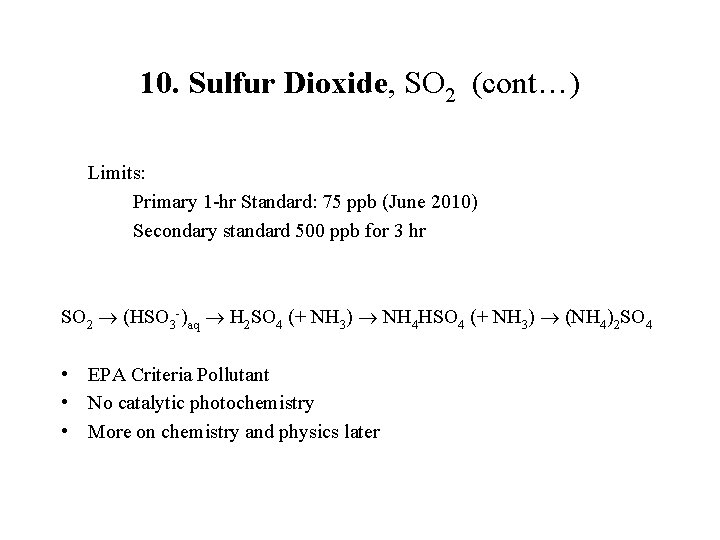
10. Sulfur Dioxide, SO 2 (cont…) Limits: Primary 1 -hr Standard: 75 ppb (June 2010) Secondary standard 500 ppb for 3 hr SO 2 (HSO 3 -)aq H 2 SO 4 (+ NH 3) NH 4 HSO 4 (+ NH 3) (NH 4)2 SO 4 • EPA Criteria Pollutant • No catalytic photochemistry • More on chemistry and physics later


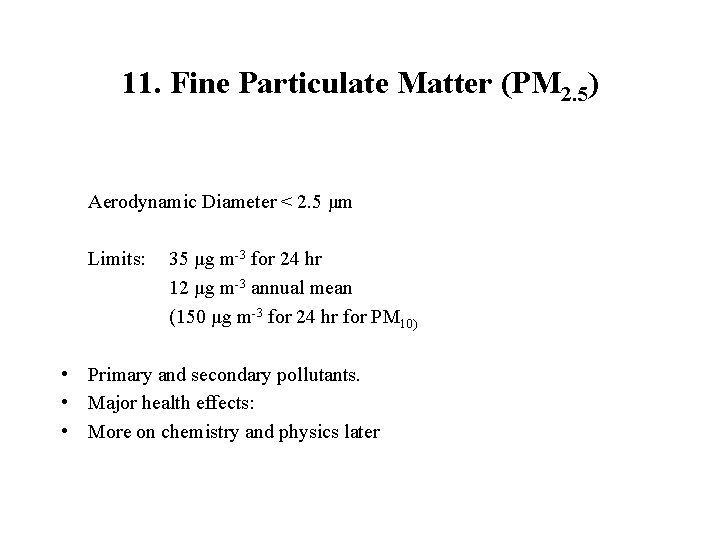
11. Fine Particulate Matter (PM 2. 5) Aerodynamic Diameter < 2. 5 μm Limits: 35 μg m-3 for 24 hr 12 μg m-3 annual mean (150 μg m-3 for 24 hr for PM 10) • Primary and secondary pollutants. • Major health effects: • More on chemistry and physics later



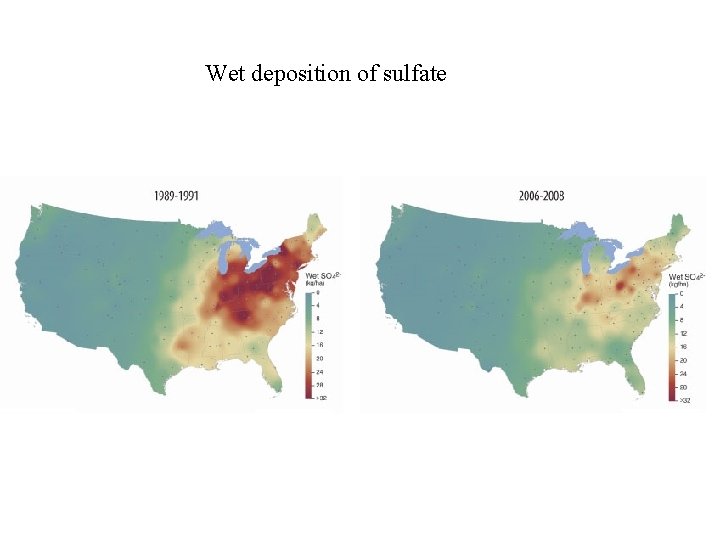
Wet deposition of sulfate

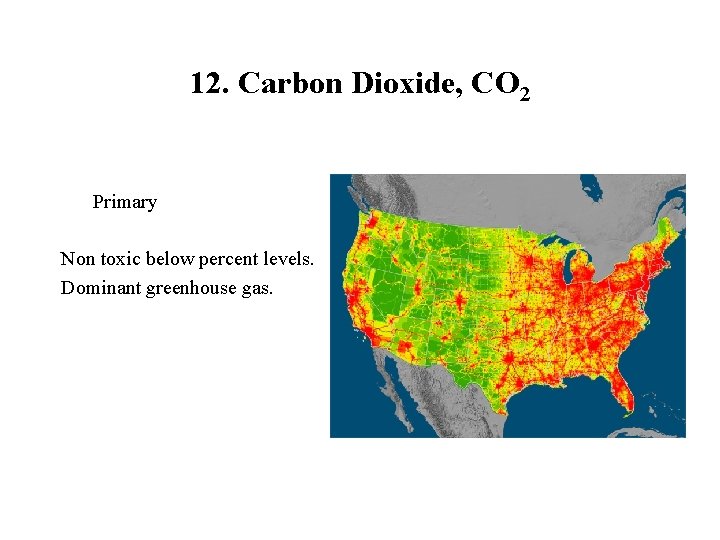
12. Carbon Dioxide, CO 2 Primary Non toxic below percent levels. Dominant greenhouse gas.

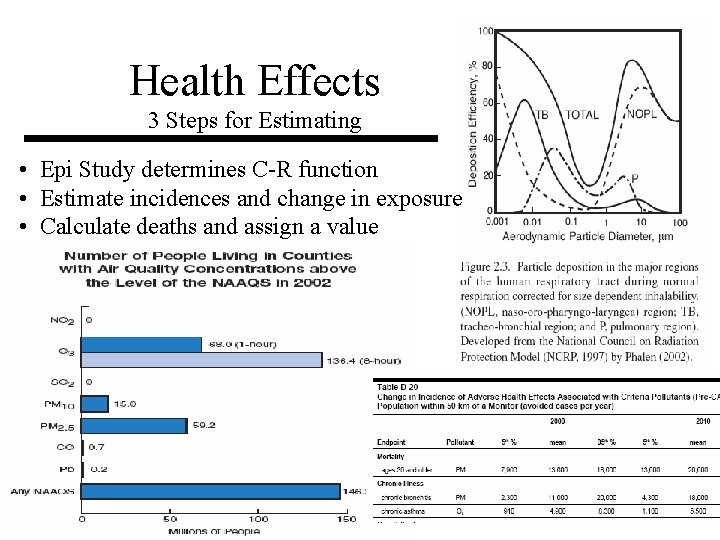
Health Effects 3 Steps for Estimating • Epi Study determines C-R function • Estimate incidences and change in exposure • Calculate deaths and assign a value



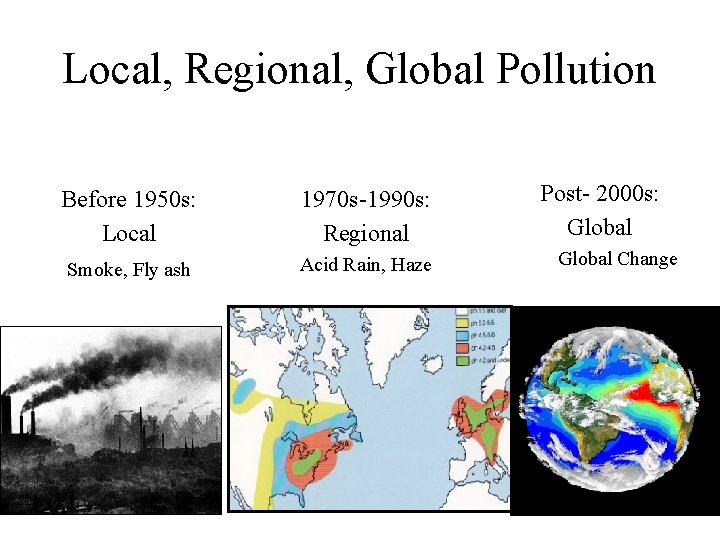
Local, Regional, Global Pollution Before 1950 s: Local Smoke, Fly ash 1970 s-1990 s: Regional Acid Rain, Haze Post- 2000 s: Global Change

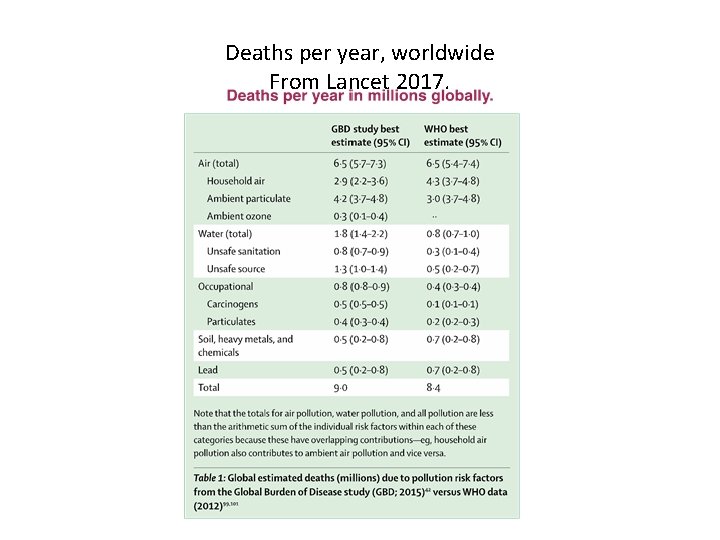
Deaths per year, worldwide From Lancet 2017.

Lecture Summary There a vast variety of pollutants, and the problem has changed from local to global. They have health, environmental, climate, and/or welfare effects. You will be expected to know the name and basic facts of each pollutant or pollutant family. This course will provide you with the tools to understand the impact, sources, chemistry, transport, meteorology, trends, and sinks for all of these pollutants – and some that have not been discovered yet.

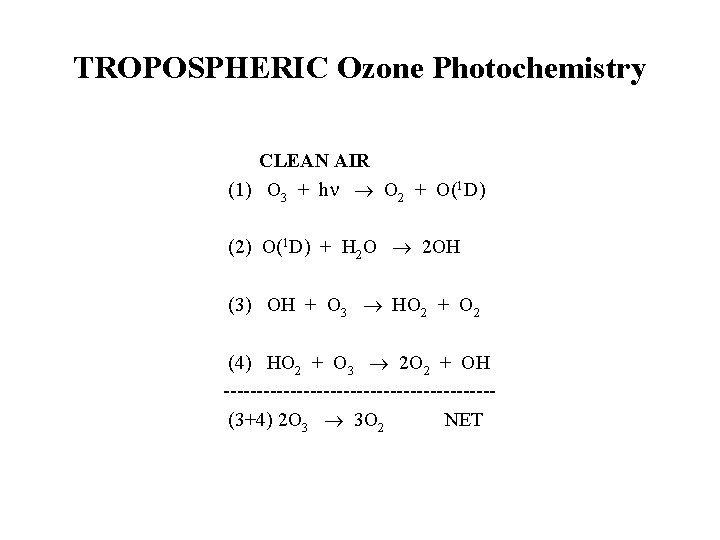
TROPOSPHERIC Ozone Photochemistry CLEAN AIR (1) O 3 + h O 2 + O(1 D) (2) O(1 D) + H 2 O 2 OH (3) OH + O 3 HO 2 + O 2 (4) HO 2 + O 3 2 O 2 + OH -------------------- (3+4) 2 O 3 3 O 2 NET

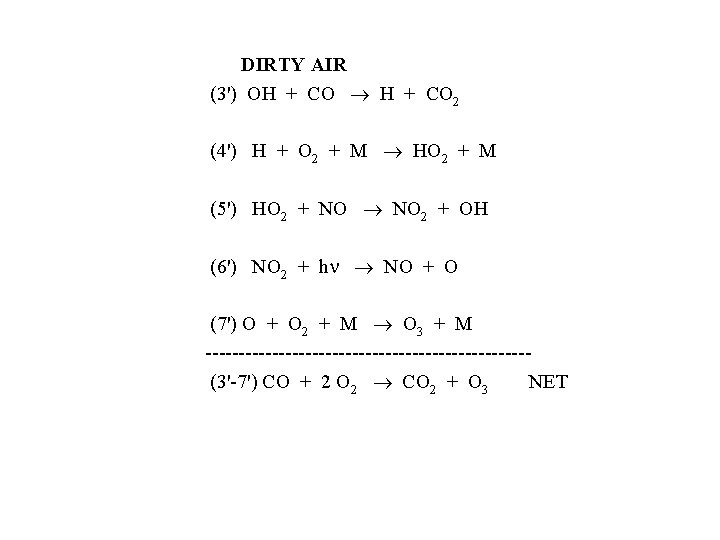
DIRTY AIR (3') OH + CO 2 (4') H + O 2 + M HO 2 + M (5') HO 2 + NO NO 2 + OH (6') NO 2 + h NO + O (7') O + O 2 + M O 3 + M ------------------------ (3'-7') CO + 2 O 2 CO 2 + O 3 NET

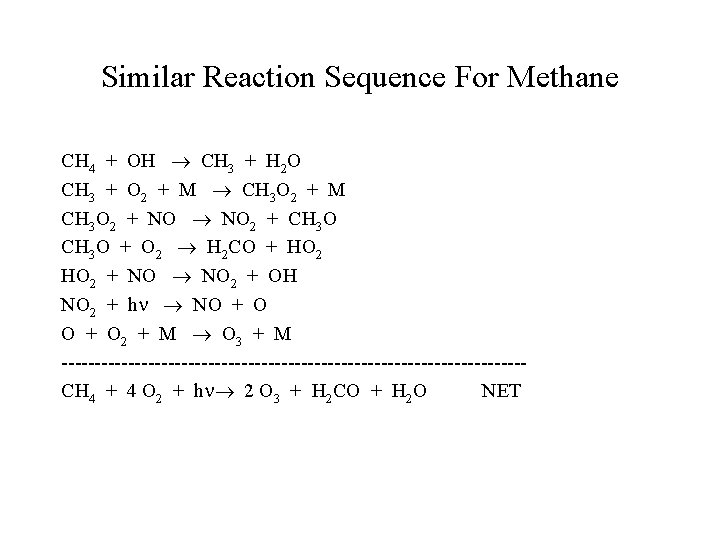
Similar Reaction Sequence For Methane CH 4 + OH CH 3 + H 2 O CH 3 + O 2 + M CH 3 O 2 + NO NO 2 + CH 3 O + O 2 H 2 CO + HO 2 + NO NO 2 + OH NO 2 + h NO + O 2 + M O 3 + M -----------------------------------CH 4 + 4 O 2 + h 2 O 3 + H 2 CO + H 2 O NET

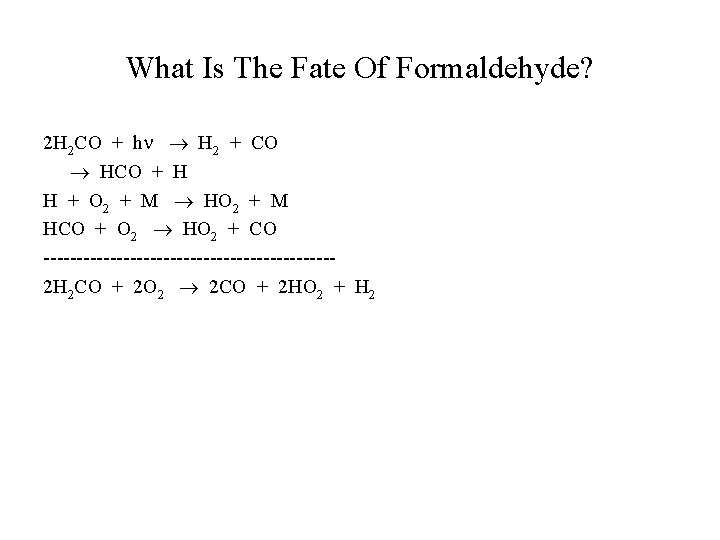
What Is The Fate Of Formaldehyde? 2 H 2 CO + h H 2 + CO HCO + H H + O 2 + M HCO + O 2 HO 2 + CO ----------------------2 H 2 CO + 2 O 2 2 CO + 2 HO 2 + H 2

This means two ozone molecules are produced per formaldehyde. The grand total for methane is four O 3 produced! Methane is a good model for all alkanes, but by itself reacts too slowly to form much ozone locally, it is, however, important on a global scale. The net production of ozone requires converting of NO to NO 2 without consuming O 2.

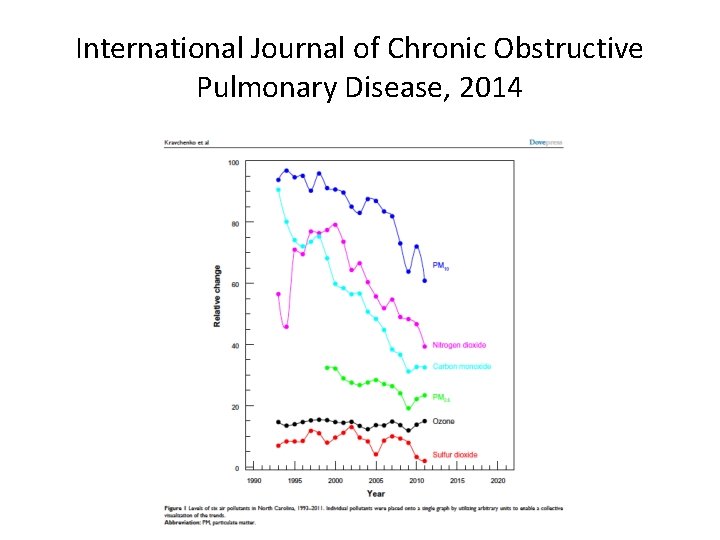
International Journal of Chronic Obstructive Pulmonary Disease, 2014


@AOSC 434
 Relief in rpd
Relief in rpd Pt tanah air sentosa
Pt tanah air sentosa Allah adalah kasih dan sumber kasih
Allah adalah kasih dan sumber kasih Anatomy team 434
Anatomy team 434 Astm d 2261
Astm d 2261 Anatomy team 434
Anatomy team 434 Ece 434
Ece 434 Ece 434
Ece 434 Ece 434
Ece 434 434 x 2
434 x 2 Ece 434
Ece 434 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Martha corey the crucible character analysis
Martha corey the crucible character analysis Romeo and juliet plot diagram
Romeo and juliet plot diagram Static chacter
Static chacter Andy harris mountain guide
Andy harris mountain guide Air hujan sanggup menjadi air tanah lantaran proses … *
Air hujan sanggup menjadi air tanah lantaran proses … * Contoh pelarut non air
Contoh pelarut non air Supply air throttling
Supply air throttling Chapter 12 section 3 acid precipitation
Chapter 12 section 3 acid precipitation Can you feel it in the air in the air
Can you feel it in the air in the air An air mass is created when a large body of air
An air mass is created when a large body of air Two cold air masses converge on a warm air mass
Two cold air masses converge on a warm air mass Put your left hand in
Put your left hand in Awan cumulus nimbus
Awan cumulus nimbus Is methane lighter than air
Is methane lighter than air Right hand in the air left hand in the air
Right hand in the air left hand in the air Pengertian perairan payau ialah
Pengertian perairan payau ialah A counterflow concentric tube heat exchanger
A counterflow concentric tube heat exchanger Maritime polar
Maritime polar Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Refrigeration cycle cop
Refrigeration cycle cop Flow control valve direction
Flow control valve direction 3/2 dcv symbol
3/2 dcv symbol Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Barometer
Barometer Mapel pipas adalah
Mapel pipas adalah A swirling center of low air pressure is called
A swirling center of low air pressure is called Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Cold air mass overtakes warm air mass
Cold air mass overtakes warm air mass Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Stationary front
Stationary front Okrasná část zahrady má tvar obdelníku
Okrasná část zahrady má tvar obdelníku Velik si tekst
Velik si tekst Casts in urine
Casts in urine Indirect tooling
Indirect tooling Cast trig diagram
Cast trig diagram The caste system
The caste system Barber shop cast
Barber shop cast Bae yong-kyun
Bae yong-kyun Turisticky nejpřitažlivější část usa
Turisticky nejpřitažlivější část usa 002
002 Half sectional view
Half sectional view He was treated like a ____ and cast out from his community
He was treated like a ____ and cast out from his community Bidai traksi
Bidai traksi Mehta casting
Mehta casting Tripoding in prosthodontics
Tripoding in prosthodontics Slab in orthopedics
Slab in orthopedics