Nuovi farmaci ipoglicemizzanti ed outcomes cardiovascolari Dr M










- Slides: 46

Nuovi farmaci ipoglicemizzanti ed outcomes cardiovascolari Dr. M. A. Fulantelli Specialista Diabetologia ASP PA V Edizione: APPROCCIO MULTIDISCIPLINARE ALLE PATOLOGIE CARDIOVASCOLARI: DALLA PRESCRIZIONE AL TRATTAMENTO Splendid Hotel La Torre Mondello - Palermo 28 -29 ott. 2016



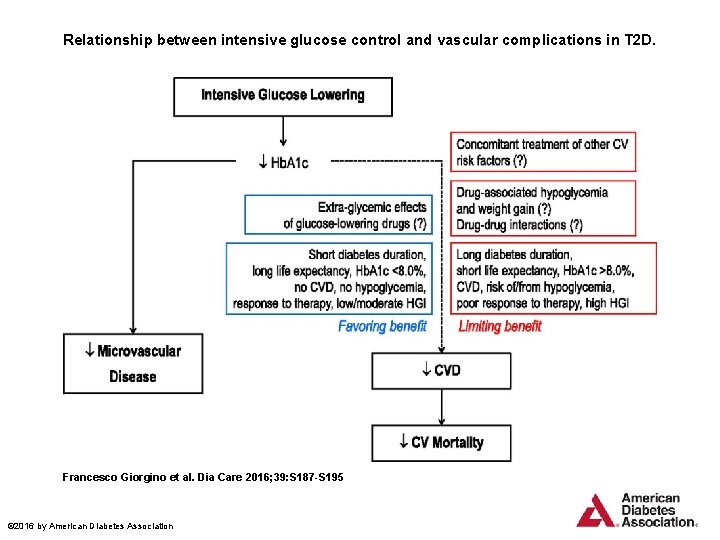
Relationship between intensive glucose control and vascular complications in T 2 D. Francesco Giorgino et al. Dia Care 2016; 39: S 187 -S 195 © 2016 by American Diabetes Association

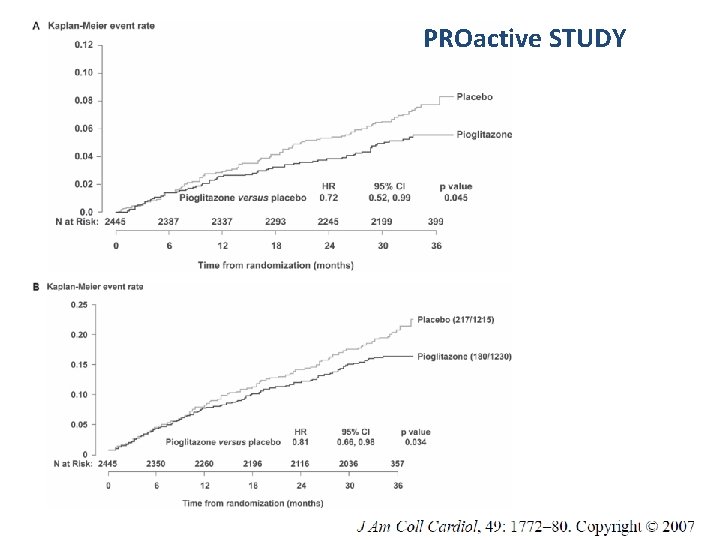
PROactive STUDY


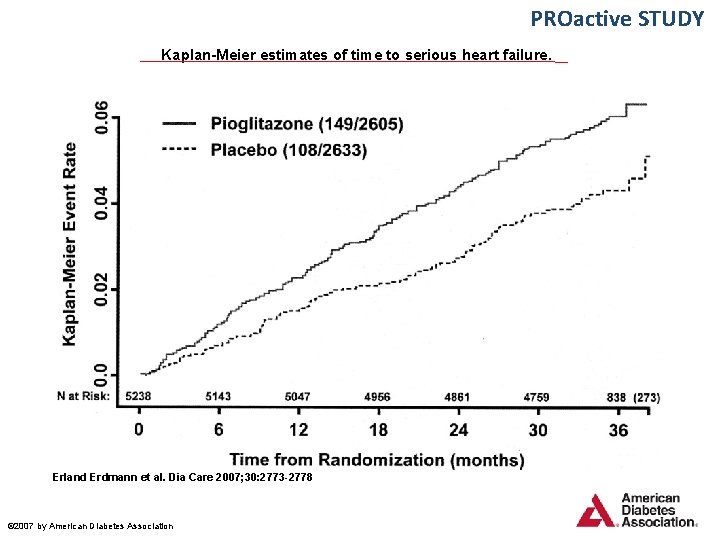
PROactive STUDY Kaplan-Meier estimates of time to serious heart failure. Erland Erdmann et al. Dia Care 2007; 30: 2773 -2778 © 2007 by American Diabetes Association

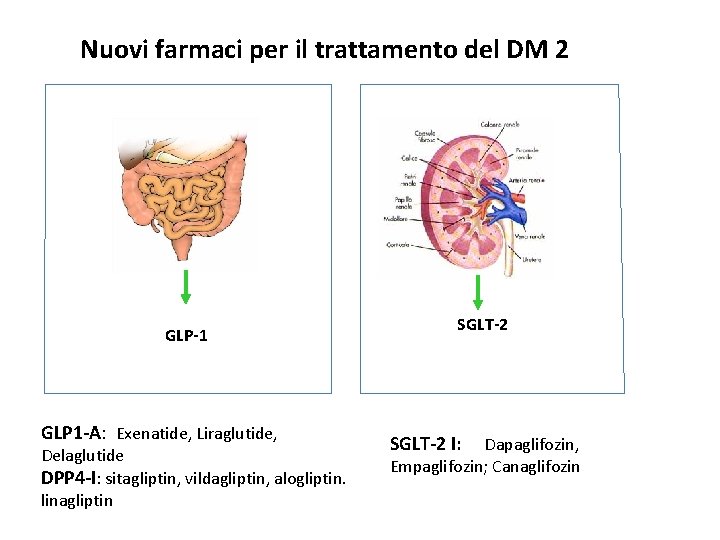
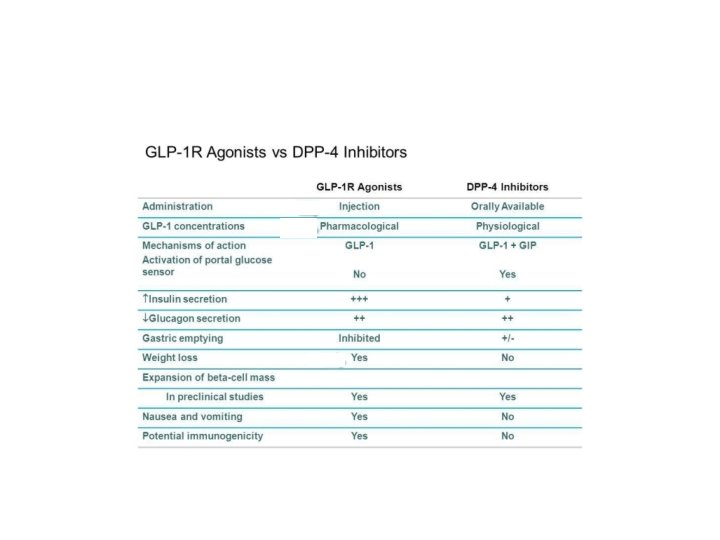
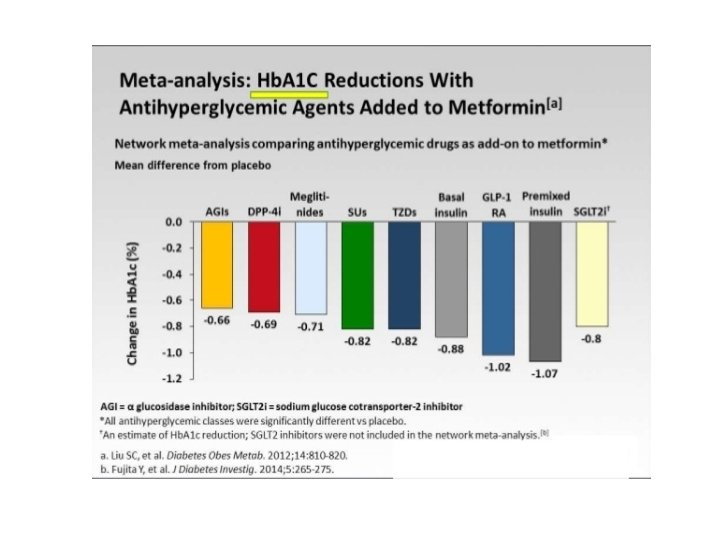
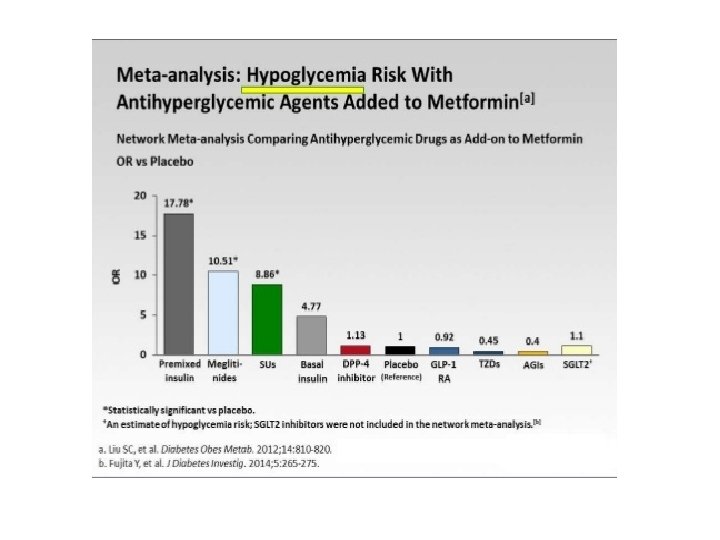
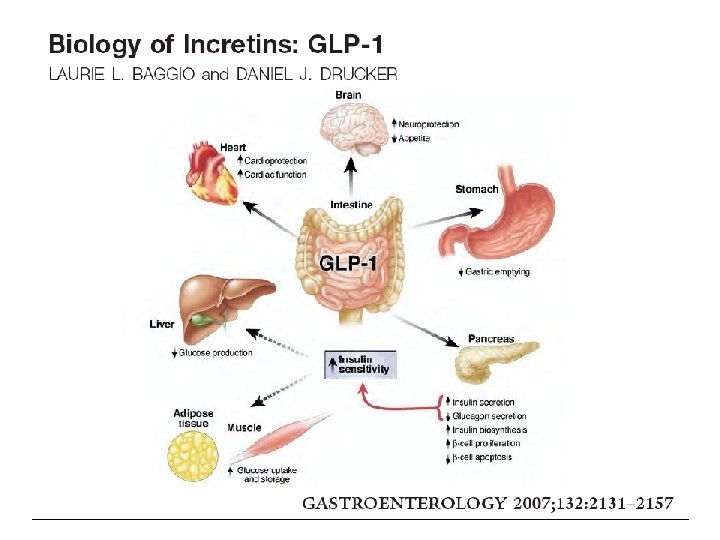
Nuovi farmaci per il trattamento del DM 2 GLP-1 GLP 1 -A: Exenatide, Liraglutide, Delaglutide DPP 4 -I: sitagliptin, vildagliptin, alogliptin. linagliptin SGLT-2 I: Dapaglifozin, Empaglifozin; Canaglifozin





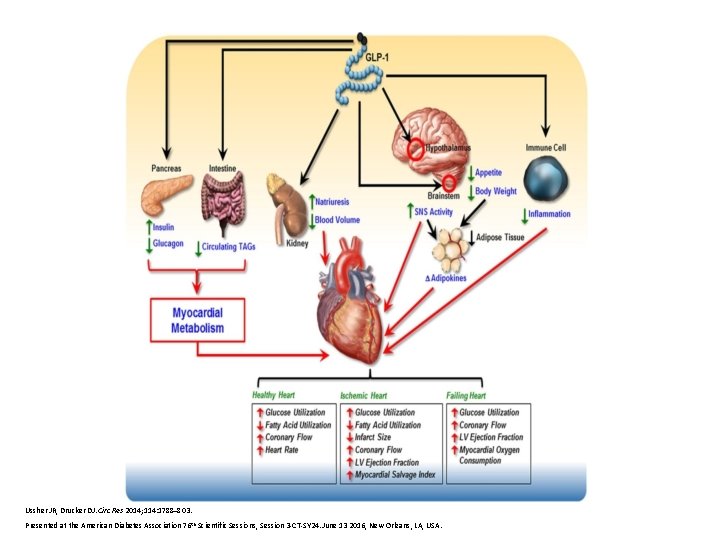
Ussher JR, Drucker DJ. Circ Res 2014; 114: 1788– 803. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.



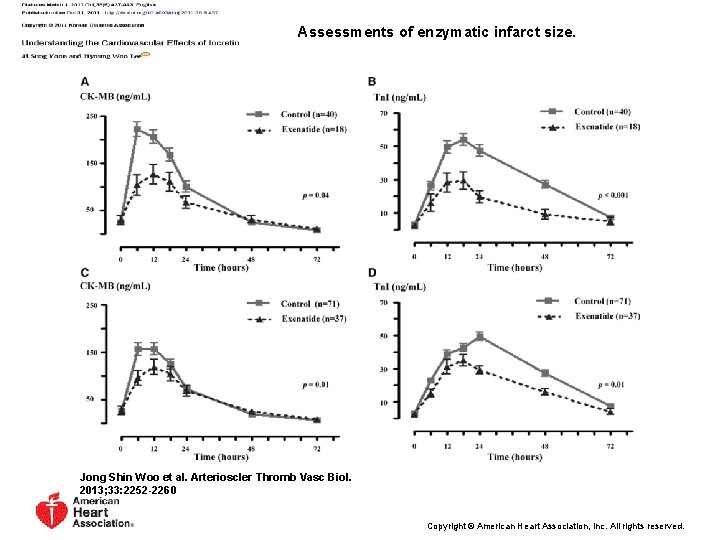
Assessments of enzymatic infarct size. Jong Shin Woo et al. Arterioscler Thromb Vasc Biol. 2013; 33: 2252 -2260 Copyright © American Heart Association, Inc. All rights reserved.

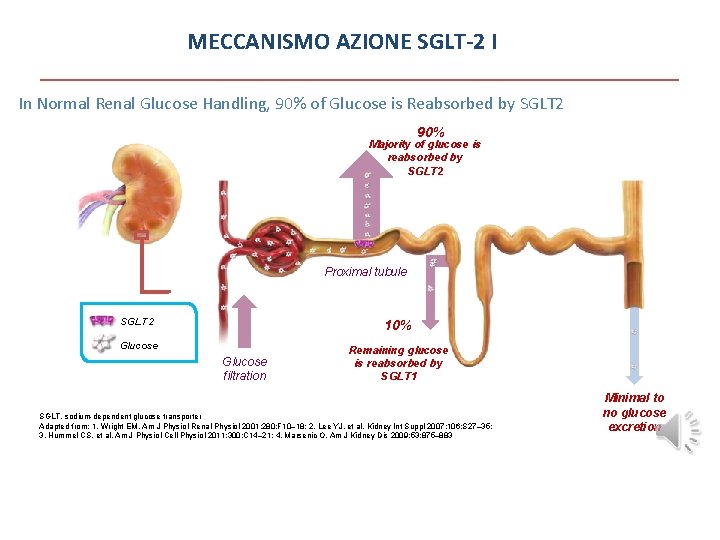
MECCANISMO AZIONE SGLT-2 I In Normal Renal Glucose Handling, 90% of Glucose is Reabsorbed by SGLT 2 90% Majority of glucose is reabsorbed by SGLT 2 Proximal tubule SGLT 2 10% Glucose filtration Remaining glucose is reabsorbed by SGLT 1 SGLT, sodium-dependent glucose transporter Adapted from: 1. Wright EM. Am J Physiol Renal Physiol 2001; 280: F 10– 18; 2. Lee YJ, et al. Kidney Int Suppl 2007; 106: S 27– 35; 3. Hummel CS, et al. Am J Physiol Cell Physiol 2011; 300: C 14– 21; 4. Marsenic O. Am J Kidney Dis 2009; 53: 875– 883 Minimal to no glucose excretion

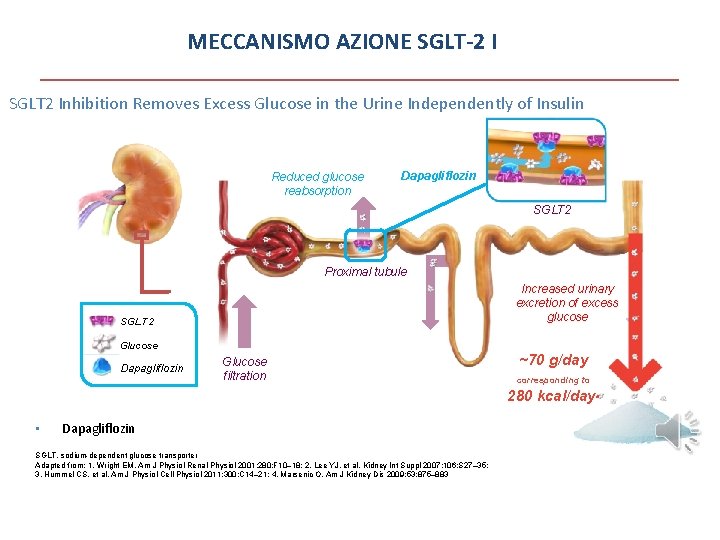
MECCANISMO AZIONE SGLT-2 I SGLT 2 Inhibition Removes Excess Glucose in the Urine Independently of Insulin Reduced glucose reabsorption Dapagliflozin SGLT 2 Proximal tubule Increased urinary excretion of excess glucose SGLT 2 Glucose Dapagliflozin Glucose filtration ~70 g/day corresponding to 280 kcal/daya • Dapagliflozin is >1400 -times more selective for SGLT 2 than SGLT 11 SGLT, sodium-dependent glucose transporter Adapted from: 1. Wright EM. Am J Physiol Renal Physiol 2001; 280: F 10– 18; 2. Lee YJ, et al. Kidney Int Suppl 2007; 106: S 27– 35; 3. Hummel CS, et al. Am J Physiol Cell Physiol 2011; 300: C 14– 21; 4. Marsenic O. Am J Kidney Dis 2009; 53: 875– 883

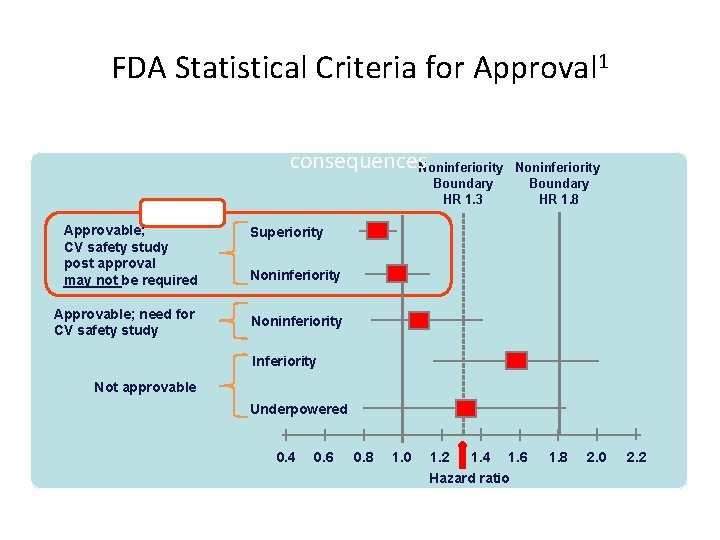
FDA Statistical Criteria for Approval 1 Five hypothetical examples of possible HRs, and regulatory consequences. Noninferiority Boundary HR 1. 3 BEST Approvable; CV safety study post approval may not be required Approvable; need for CV safety study Boundary HR 1. 8 Superiority Noninferiority Inferiority Not approvable Underpowered 0. 4 0. 6 0. 8 1. 0 1. 2 1. 4 1. 6 Hazard ratio FDA = Food and Drug Administration; HR = hazard ratio; CV = cardiovascular. 1. Reproduced with permission from Hirshberg B et al. Diabetes Care. 2011: 34 (Suppl 2); S 101–S 106. 1. 8 2. 0 2. 2

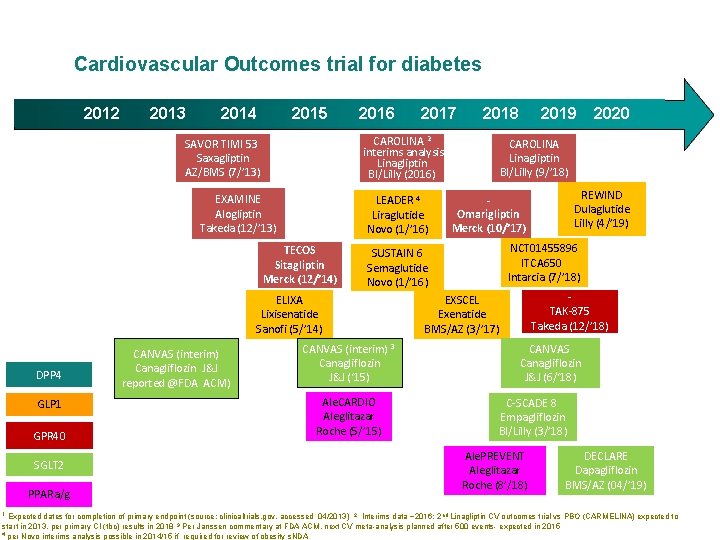
Cardiovascular Outcomes trial for diabetes 2012 2013 2014 2015 2016 EXAMINE Alogliptin Takeda (12/’ 13) LEADER 4 Liraglutide Novo (1/’ 16) TECOS Sitagliptin Merck (12/’ 14) GLP 1 GPR 40 SGLT 2 PPARa/g 1 CANVAS (interim) 3 Canagliflozin J&J (‘ 15) Ale. CARDIO Aleglitazar Roche (5/’ 15) 2019 2020 CAROLINA Linagliptin BI/Lilly (9/’ 18) REWIND Dulaglutide Lilly (4/’ 19) Omarigliptin Merck (10/’ 17) NCT 01455896 ITCA 650 Intarcia (7/’ 18) SUSTAIN 6 Semaglutide Novo (1/’ 16) ELIXA Lixisenatide Sanofi (5/’ 14) DPP 4 2018 CAROLINA 2 interims analysis Linagliptin BI/Lilly (2016) SAVOR TIMI 53 Saxagliptin AZ/BMS (7/’ 13) CANVAS (interim) Canagliflozin J&J reported @FDA ACM) 2017 TAK-875 Takeda (12/’ 18) EXSCEL Exenatide BMS/AZ (3/’ 17) CANVAS Canagliflozin J&J (6/‘ 18) C-SCADE 8 Empagliflozin BI/Lilly (3/’ 18) Ale. PREVENT Aleglitazar Roche (8’/18) DECLARE Dapagliflozin BMS/AZ (04/’ 19) Expected dates for completion of primary endpoint (source: clinicaltrials. gov, accessed 04/2013) 2 Interims data ~2016; 2 nd Linagliptin CV outcomes trial vs PBO (CARMELINA) expected to start in 2013, per primary CI (tbc) results in 2018 3 Per Janssen commentary at FDA ACM, next CV meta-analysis planned after 500 events- expected in 2015 4 per Novo interims analysis possible in 2014/15 if required for review of obesity s. NDA

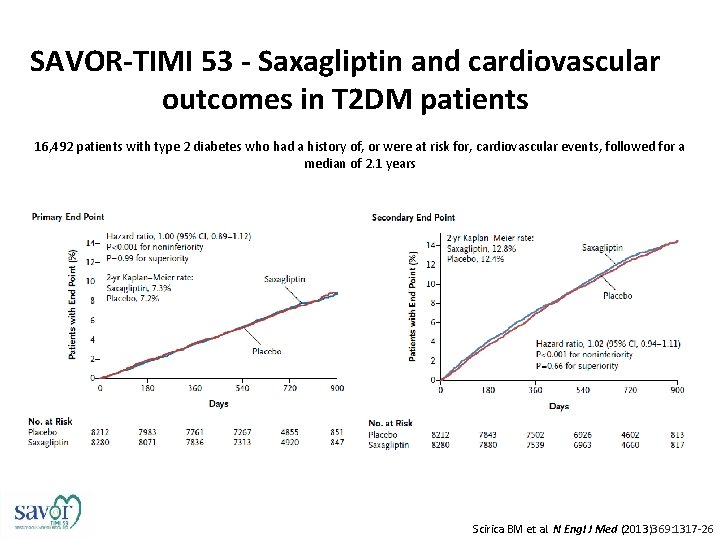
SAVOR-TIMI 53 - Saxagliptin and cardiovascular outcomes in T 2 DM patients 16, 492 patients with type 2 diabetes who had a history of, or were at risk for, cardiovascular events, followed for a median of 2. 1 years Scirica BM et al. N Engl J Med (2013)369: 1317 -26

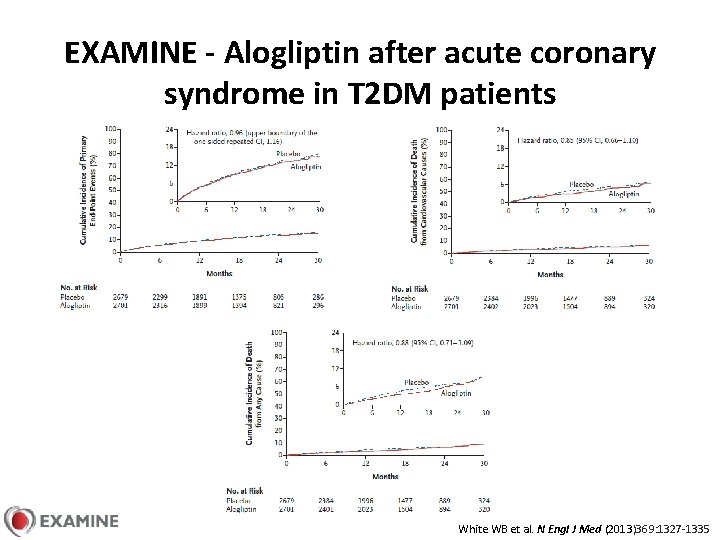
EXAMINE - Alogliptin after acute coronary syndrome in T 2 DM patients White WB et al. N Engl J Med (2013)369: 1327 -1335

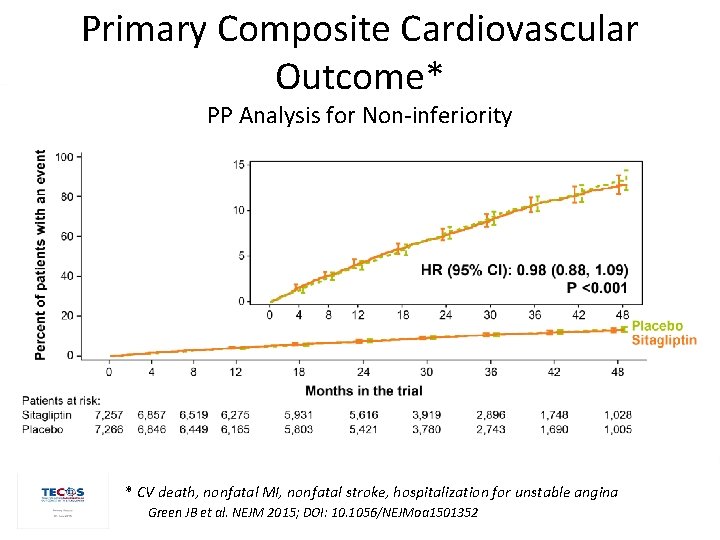
Primary Composite Cardiovascular Outcome* PP Analysis for Non-inferiority * CV death, nonfatal MI, nonfatal stroke, hospitalization for unstable angina Green JB et al. NEJM 2015; DOI: 10. 1056/NEJMoa 1501352

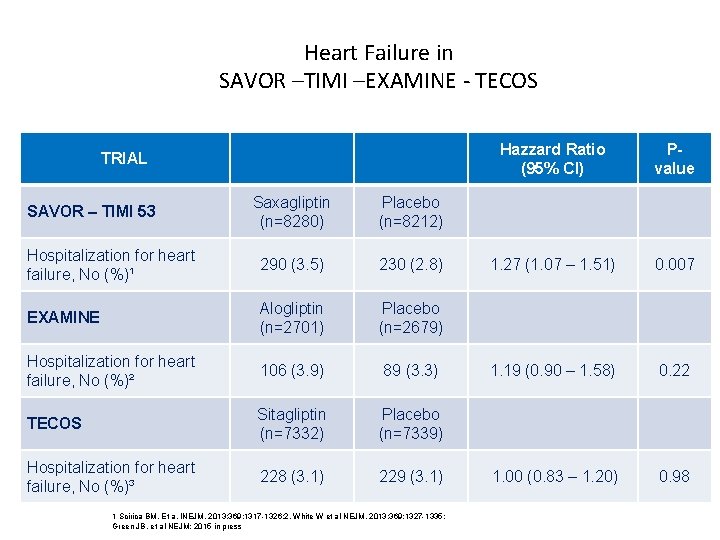
Heart Failure in SAVOR –TIMI –EXAMINE - TECOS TRIAL Saxagliptin (n=8280) Placebo (n=8212) Hospitalization for heart failure, No (%)¹ 290 (3. 5) 230 (2. 8) EXAMINE Alogliptin (n=2701) Placebo (n=2679) Hospitalization for heart failure, No (%)² 106 (3. 9) 89 (3. 3) TECOS Sitagliptin (n=7332) Placebo (n=7339) Hospitalization for heart failure, No (%)³ 228 (3. 1) 229 (3. 1) SAVOR – TIMI 53 1 Scirica BM. Et a. INEJM. 2013; 369; 1317 -1326; 2, White W et al NEJM. 2013; 369; 1327 -1335; Green JB, et al NEJM; 2015 in press Hazzard Ratio (95% CI) Pvalue 1. 27 (1. 07 – 1. 51) 0. 007 1. 19 (0. 90 – 1. 58) 0. 22 1. 00 (0. 83 – 1. 20) 0. 98

Studio in doppio cieco randomizzato, placebo controllato Obiettivo: valutare gli effetti a lungo temine di empaglifozin vs placebo, in aggiunta alla cura standard, su morbidità e mortalità cdv in pz diabetici ad alto rischio di eventi

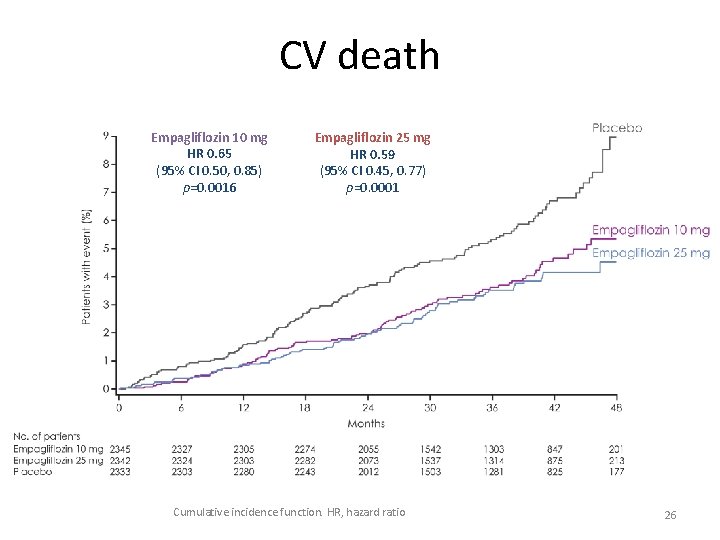
CV death Empagliflozin 10 mg HR 0. 65 (95% CI 0. 50, 0. 85) p=0. 0016 Empagliflozin 25 mg HR 0. 59 (95% CI 0. 45, 0. 77) p=0. 0001 Cumulative incidence function. HR, hazard ratio 26

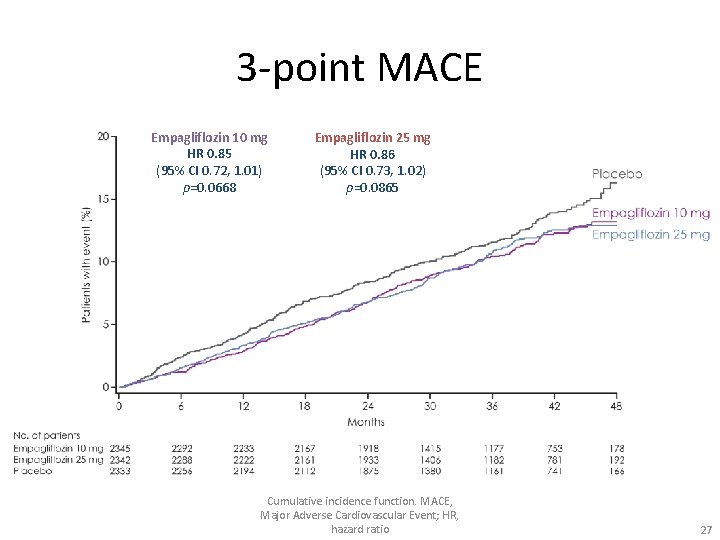
3 -point MACE Empagliflozin 10 mg HR 0. 85 (95% CI 0. 72, 1. 01) p=0. 0668 Empagliflozin 25 mg HR 0. 86 (95% CI 0. 73, 1. 02) p=0. 0865 Cumulative incidence function. MACE, Major Adverse Cardiovascular Event; HR, hazard ratio 27

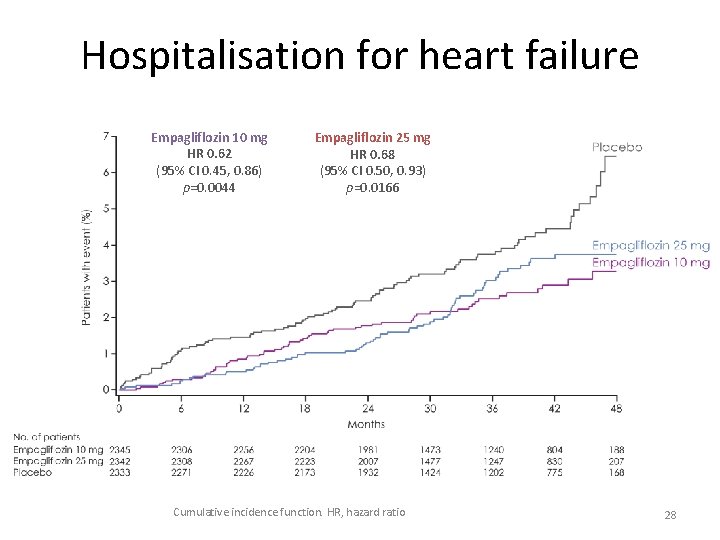
Hospitalisation for heart failure Empagliflozin 10 mg HR 0. 62 (95% CI 0. 45, 0. 86) p=0. 0044 Empagliflozin 25 mg HR 0. 68 (95% CI 0. 50, 0. 93) p=0. 0166 Cumulative incidence function. HR, hazard ratio 28

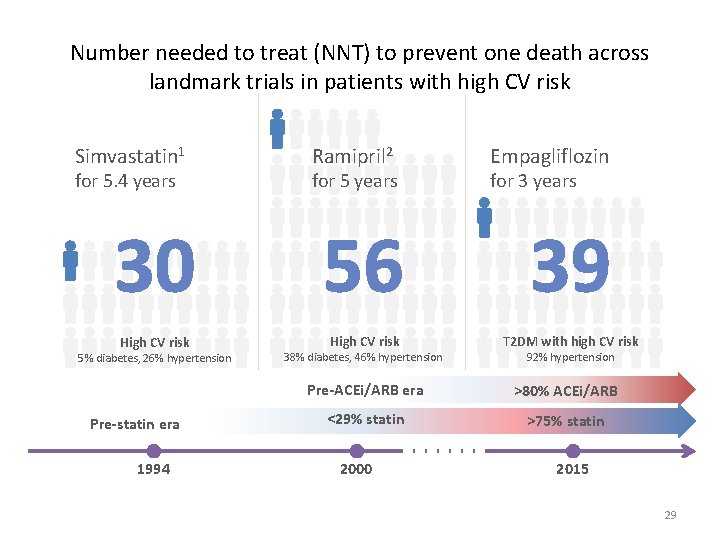
Number needed to treat (NNT) to prevent one death across landmark trials in patients with high CV risk Simvastatin 1 for 5. 4 years Ramipril 2 for 5 years Empagliflozin for 3 years 30 56 39 High CV risk T 2 DM with high CV risk 5% diabetes, 26% hypertension Pre-statin era 1994 38% diabetes, 46% hypertension 92% hypertension Pre-ACEi/ARB era >80% ACEi/ARB <29% statin >75% statin 2000 2015 29

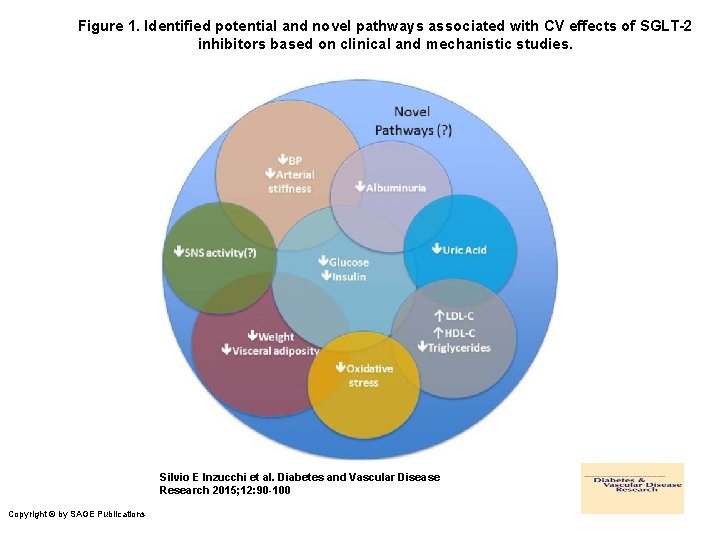
Figure 1. Identified potential and novel pathways associated with CV effects of SGLT-2 inhibitors based on clinical and mechanistic studies. Silvio E Inzucchi et al. Diabetes and Vascular Disease Research 2015; 12: 90 -100 Copyright © by SAGE Publications



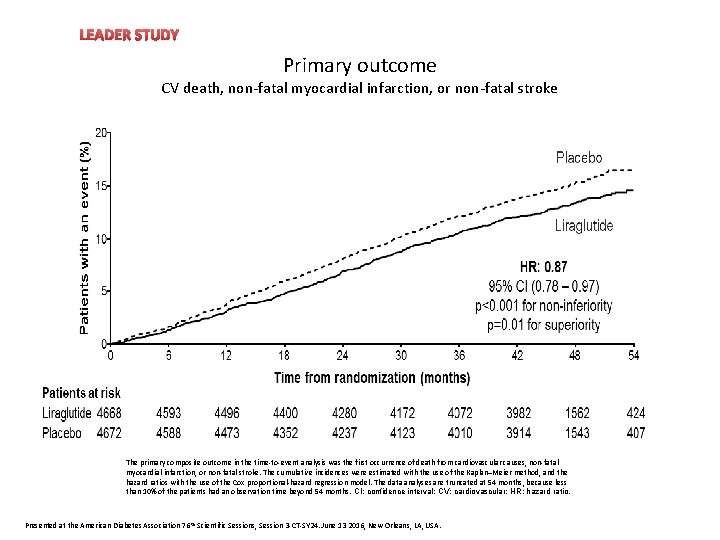
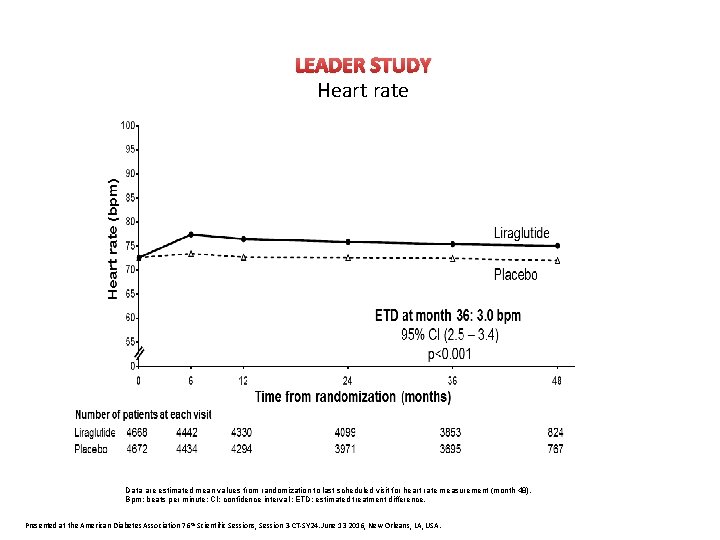
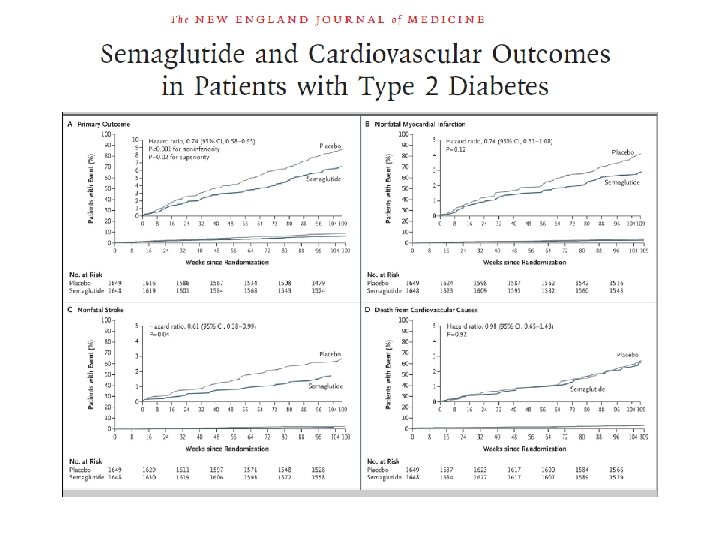
LEADER STUDY Heart rate Primary outcome CV death, non-fatal myocardial infarction, or non-fatal stroke The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke. The cumulative incidences were estimated with the use of the Kaplan–Meier method, and the hazard ratios with the use of the Cox proportional-hazard regression model. The data analyses are truncated at 54 months, because less than 10% of the patients had an observation time beyond 54 months. CI: confidence interval; CV: cardiovascular; HR: hazard ratio. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

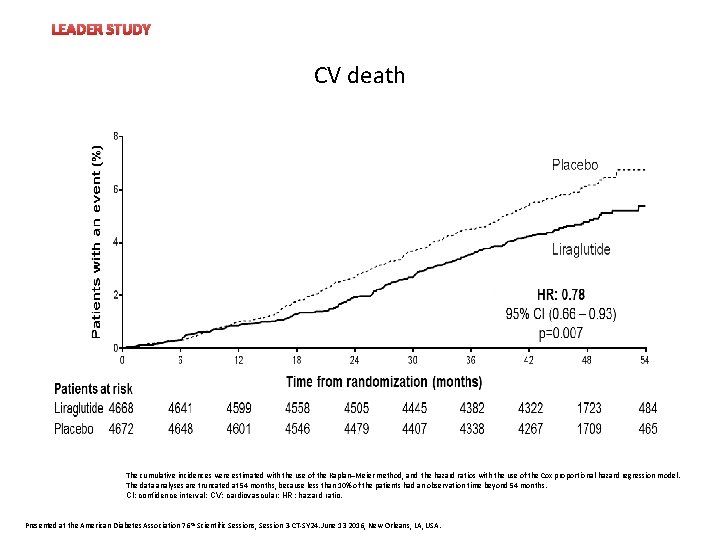
LEADER STUDY Heart rate CV death The cumulative incidences were estimated with the use of the Kaplan–Meier method, and the hazard ratios with the use of the Cox proportional-hazard regression model. The data analyses are truncated at 54 months, because less than 10% of the patients had an observation time beyond 54 months. CI: confidence interval; CV: cardiovascular; HR: hazard ratio. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

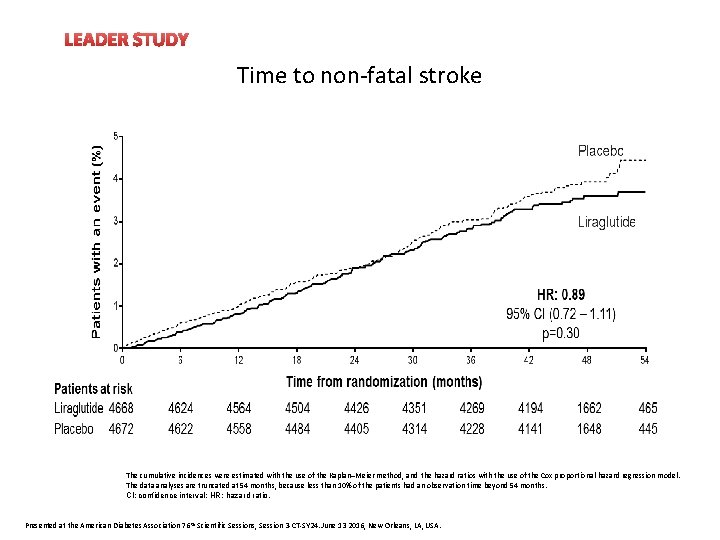
LEADER STUDY Time to non-fatal stroke The cumulative incidences were estimated with the use of the Kaplan–Meier method, and the hazard ratios with the use of the Cox proportional-hazard regression model. The data analyses are truncated at 54 months, because less than 10% of the patients had an observation time beyond 54 months. CI: confidence interval; HR: hazard ratio. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

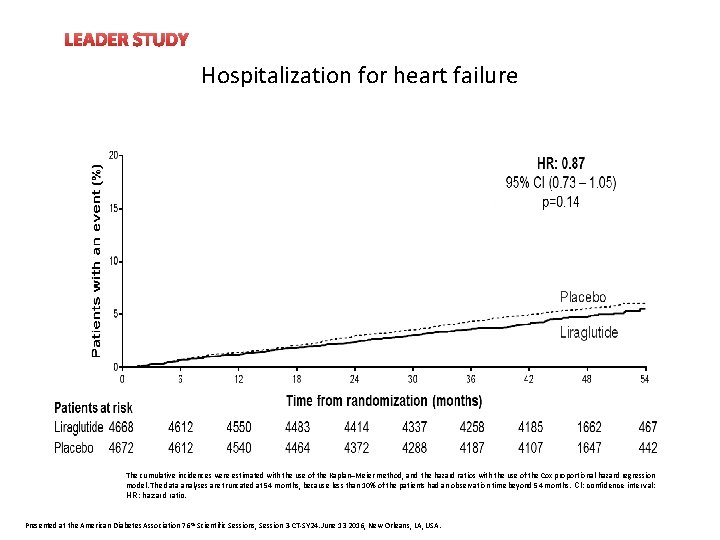
LEADER STUDY Hospitalization for heart failure The cumulative incidences were estimated with the use of the Kaplan–Meier method, and the hazard ratios with the use of the Cox proportional-hazard regression model. The data analyses are truncated at 54 months, because less than 10% of the patients had an observation time beyond 54 months. CI: confidence interval; HR: hazard ratio. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

LEADER STUDY Heart rate Data are estimated mean values from randomization to last scheduled visit for heart rate measurement (month 48). Bpm: beats per minute; CI: confidence interval ; ETD: estimated treatment difference. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

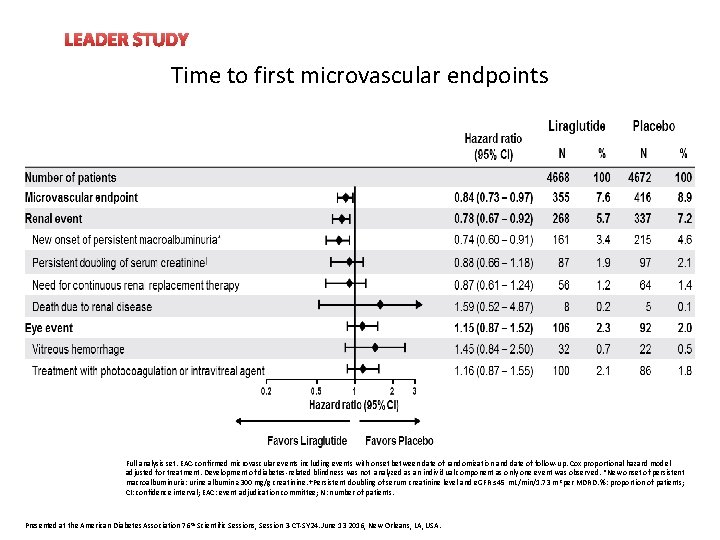
LEADER STUDY Time to first microvascular endpoints Full analysis set. EAC-confirmed microvascular events including events with onset between date of randomization and date of follow-up. Cox proportional hazard model adjusted for treatment. Development of diabetes-related blindness was not analyzed as an individual component as only one event was observed. *New onset of persistent macroalbuminuria: urine albumin ≥ 300 mg/g creatinine. †Persistent doubling of serum creatinine level and e. GFR ≤ 45 m. L/min/1. 73 m 2 per MDRD. %: proportion of patients; CI: confidence interval; EAC: event adjudication committee; N: number of patients. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

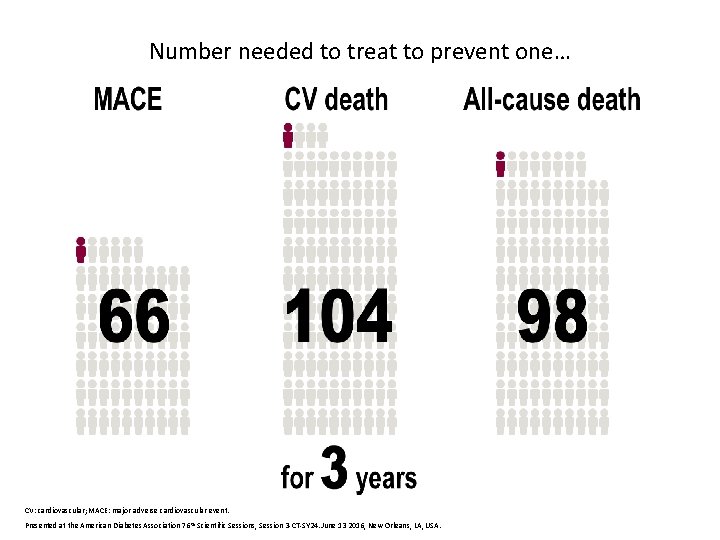
Number needed to treat to prevent one… CV: cardiovascular; MACE: major adverse cardiovascular event. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

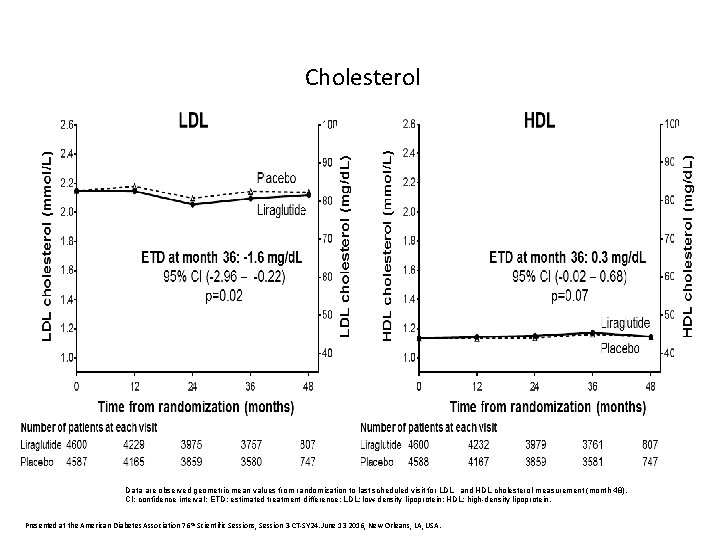
Cholesterol Data are observed geometric mean values from randomization to last scheduled visit for LDL and HDL cholesterol measurement (month 48). CI: confidence interval; ETD: estimated treatment difference; LDL: low-density lipoprotein; HDL: high-density lipoprotein. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.

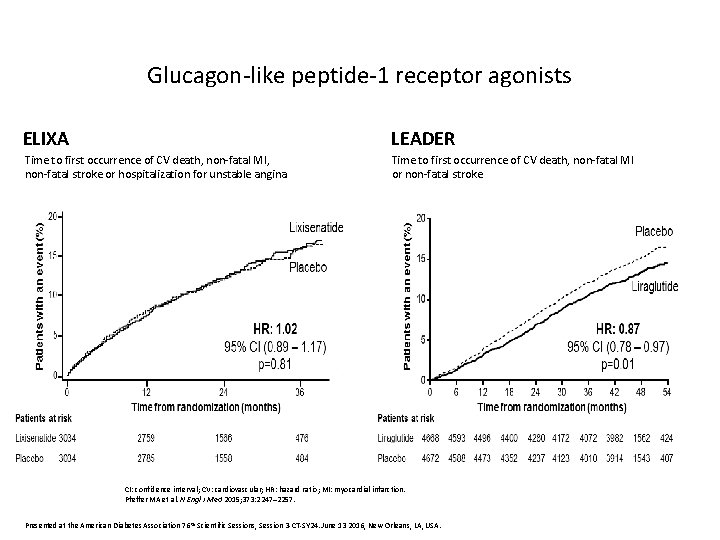
Glucagon-like peptide-1 receptor agonists ELIXA LEADER Time to first occurrence of CV death, non-fatal MI, non-fatal stroke or hospitalization for unstable angina Time to first occurrence of CV death, non-fatal MI or non-fatal stroke CI: confidence interval; CV: cardiovascular; HR: hazard ratio; MI: myocardial infarction. Pfeffer MA et al. N Engl J Med 2015; 373: 2247– 2257. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.


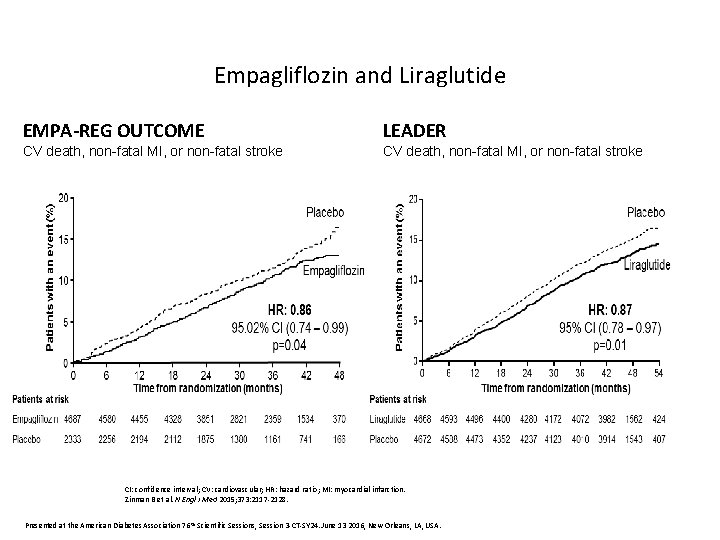
Empagliflozin and Liraglutide EMPA-REG OUTCOME LEADER CV death, non-fatal MI, or non-fatal stroke CI: confidence interval; CV: cardiovascular; HR: hazard ratio; MI: myocardial infarction. Zinman B et al. N Engl J Med 2015; 373: 2117 -2128. Presented at the American Diabetes Association 76 th Scientific Sessions, Session 3 -CT-SY 24. June 13 2016, New Orleans, LA, USA.



 Bisprololo
Bisprololo Indirizzi tecnici
Indirizzi tecnici Nuovi antidiabetici orali
Nuovi antidiabetici orali La rivoluzione francese
La rivoluzione francese Nuovi prodotti alimentari
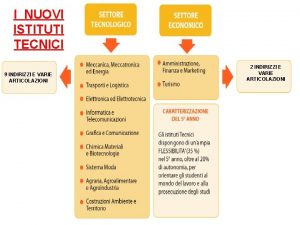
Nuovi prodotti alimentari Nuovi istituti tecnici
Nuovi istituti tecnici Nuovi prodotti alimentari
Nuovi prodotti alimentari Nuovi percorsi professionali
Nuovi percorsi professionali Per vino nuovo otri nuovi
Per vino nuovo otri nuovi Nuovi antidiabetici orali
Nuovi antidiabetici orali Ssri farmaci
Ssri farmaci Vie parenterali
Vie parenterali Come scalare dalmadorm
Come scalare dalmadorm Challenge test farmaci
Challenge test farmaci Ssri farmaci
Ssri farmaci Farmaci antibatterici
Farmaci antibatterici Tabella equivalenza statine
Tabella equivalenza statine Algoritmo als farmaci
Algoritmo als farmaci Farmaci antimuscarinici
Farmaci antimuscarinici Neuroplasticità
Neuroplasticità Farmaci ipolipidemizzanti cosa sono
Farmaci ipolipidemizzanti cosa sono Circolo enteroepatico farmaci
Circolo enteroepatico farmaci Halichondrin b
Halichondrin b Glicosidi cardiaci
Glicosidi cardiaci Escrezione dei farmaci
Escrezione dei farmaci Astenia nervosa
Astenia nervosa Farmaci venotropi
Farmaci venotropi Positive practice positive outcomes
Positive practice positive outcomes Examples of ifsp outcomes and strategies
Examples of ifsp outcomes and strategies Ancient rome outcomes geography and early republic
Ancient rome outcomes geography and early republic Arithmetic sequence objectives
Arithmetic sequence objectives Learning objectives of digestive system
Learning objectives of digestive system Learning outcomes examples english
Learning outcomes examples english Outcomes focused regulation
Outcomes focused regulation Syllabus nsw
Syllabus nsw Missouri quality outcomes
Missouri quality outcomes Leov math
Leov math Expected outcomes in research examples
Expected outcomes in research examples Relationship between aims and objectives
Relationship between aims and objectives The collection of all possible outcomes
The collection of all possible outcomes Ppst curriculum and planning
Ppst curriculum and planning Youth work outcomes
Youth work outcomes Learning outcomes of ascending and descending order
Learning outcomes of ascending and descending order Fundamental counting principle examples
Fundamental counting principle examples Angina pectoris pathophysiology
Angina pectoris pathophysiology Measurable outcomes examples
Measurable outcomes examples Course objectives of database management systems
Course objectives of database management systems