Nuevos frmacos Pere Domingo Malalties Infeccioses Hospital de















- Slides: 60

Nuevos fármacos Pere Domingo Malalties Infeccioses Hospital de la Santa Creu i Sant Pau Barcelona pdomingo@santpau. cat

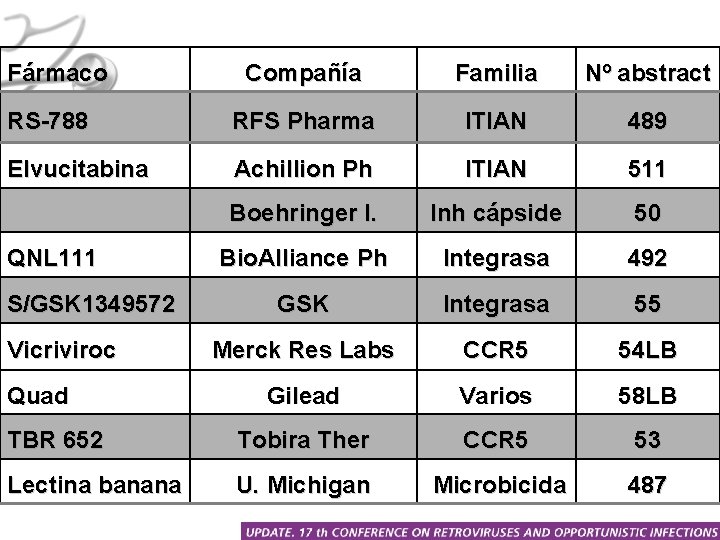
Fármaco Compañía Familia Nº abstract RS-788 RFS Pharma ITIAN 489 Elvucitabina Achillion Ph ITIAN 511 Boehringer I. Inh cápside 50 Bio. Alliance Ph Integrasa 492 GSK Integrasa 55 Merck Res Labs CCR 5 54 LB Gilead Varios 58 LB TBR 652 Tobira Ther CCR 5 53 Lectina banana U. Michigan Microbicida 487 QNL 111 S/GSK 1349572 Vicriviroc Quad

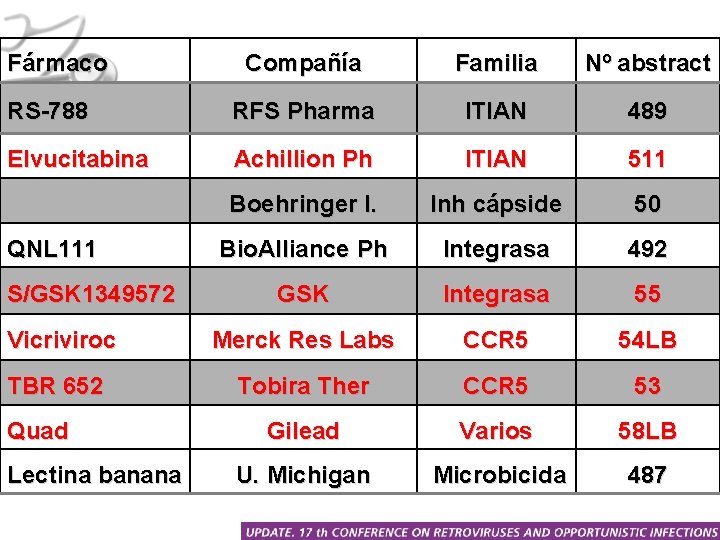
Fármaco Compañía Familia Nº abstract RS-788 RFS Pharma ITIAN 489 Elvucitabina Achillion Ph ITIAN 511 Boehringer I. Inh cápside 50 Bio. Alliance Ph Integrasa 492 GSK Integrasa 55 Vicriviroc Merck Res Labs CCR 5 54 LB TBR 652 Tobira Ther CCR 5 53 Gilead Varios 58 LB U. Michigan Microbicida 487 QNL 111 S/GSK 1349572 Quad Lectina banana

Análogos de nucleósidos

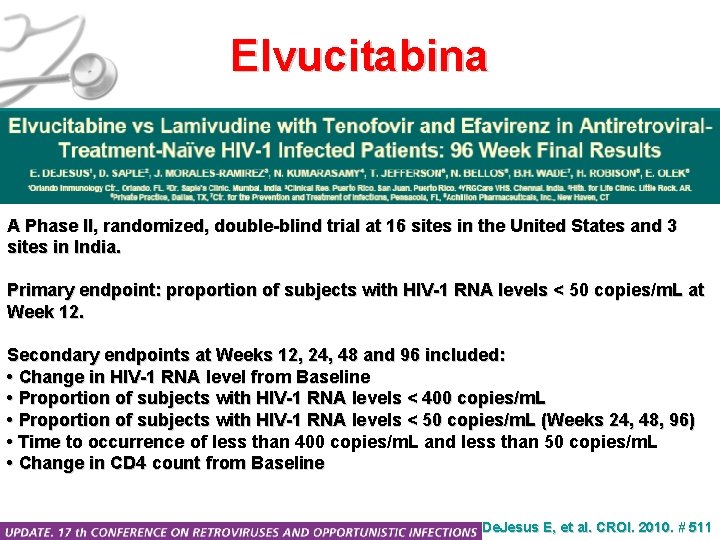
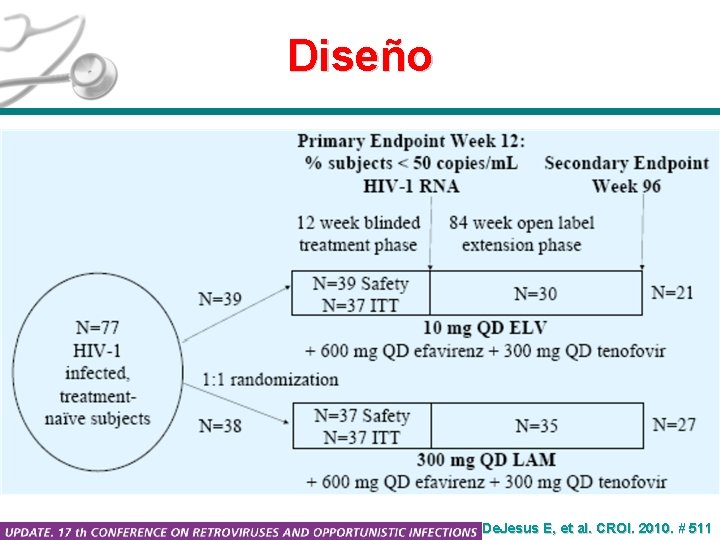
Elvucitabina A Phase II, randomized, double-blind trial at 16 sites in the United States and 3 sites in India. Primary endpoint: proportion of subjects with HIV-1 RNA levels < 50 copies/m. L at Week 12. Secondary endpoints at Weeks 12, 24, 48 and 96 included: • Change in HIV-1 RNA level from Baseline • Proportion of subjects with HIV-1 RNA levels < 400 copies/m. L • Proportion of subjects with HIV-1 RNA levels < 50 copies/m. L (Weeks 24, 48, 96) • Time to occurrence of less than 400 copies/m. L and less than 50 copies/m. L • Change in CD 4 count from Baseline De. Jesus E, et al. CROI. 2010. # 511

Diseño De. Jesus E, et al. CROI. 2010. # 511

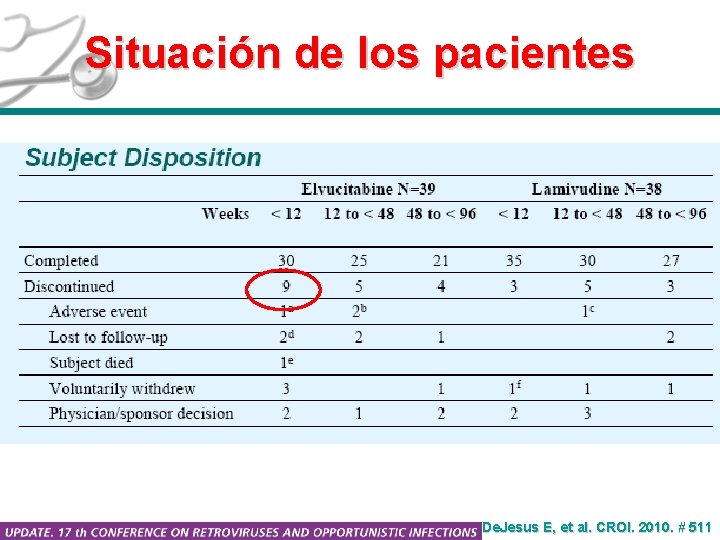
Situación de los pacientes De. Jesus E, et al. CROI. 2010. # 511

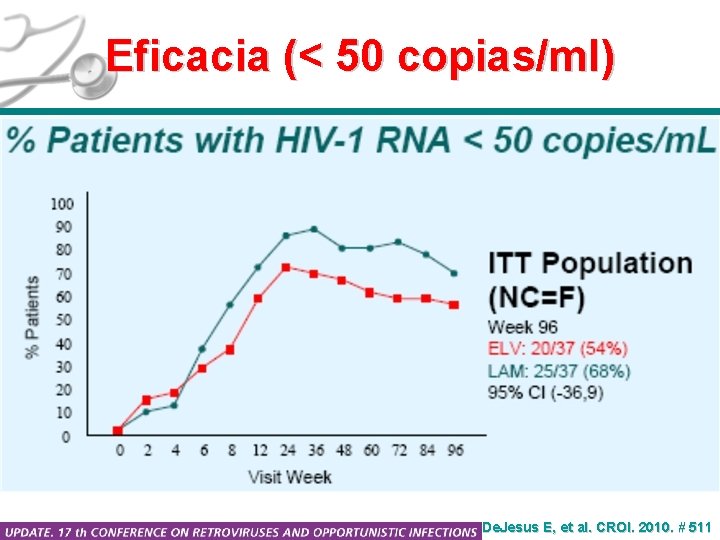
Eficacia (< 50 copias/ml) De. Jesus E, et al. CROI. 2010. # 511

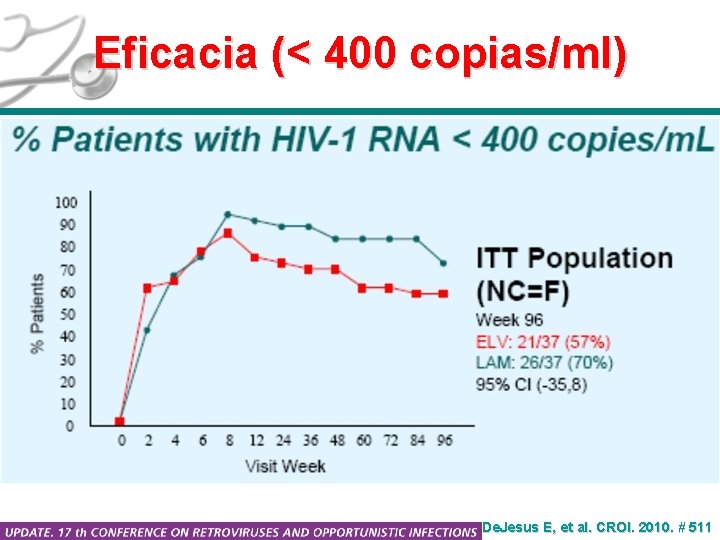
Eficacia (< 400 copias/ml) De. Jesus E, et al. CROI. 2010. # 511

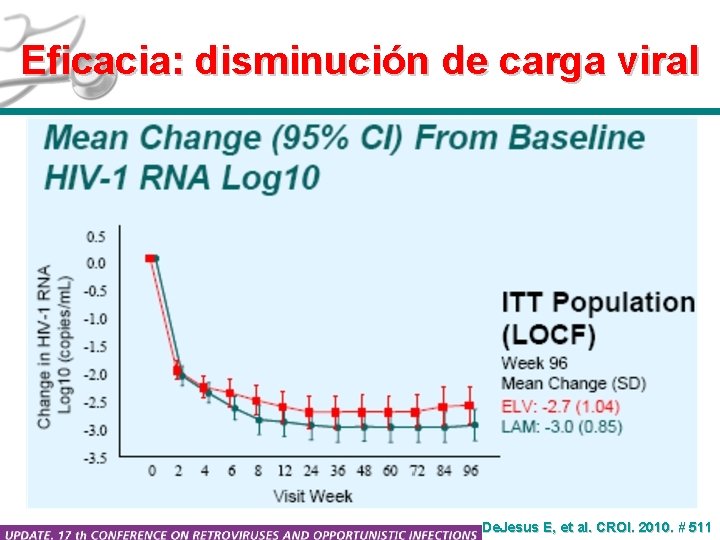
Eficacia: disminución de carga viral De. Jesus E, et al. CROI. 2010. # 511

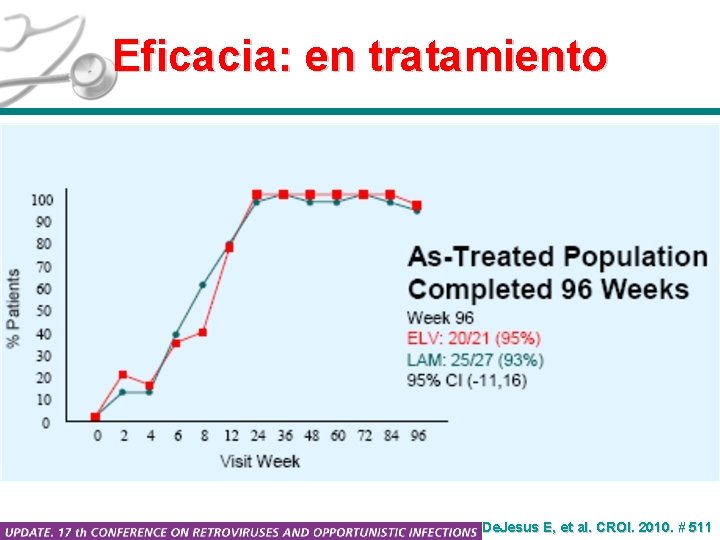
Eficacia: en tratamiento De. Jesus E, et al. CROI. 2010. # 511

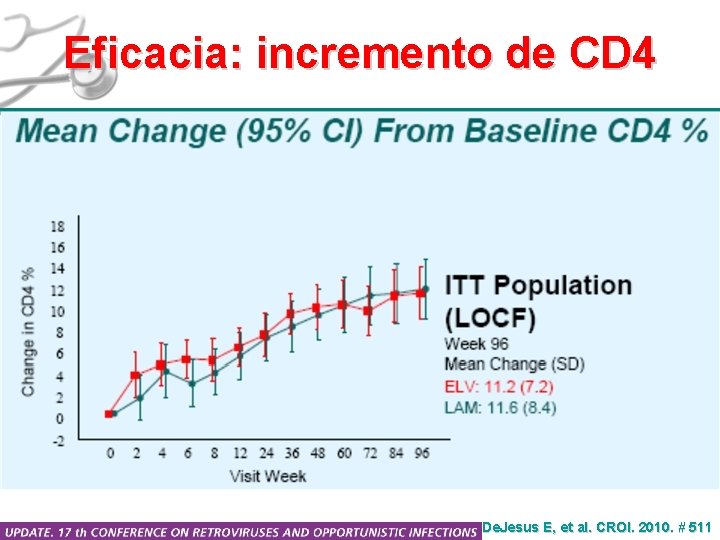
Eficacia: incremento de CD 4 De. Jesus E, et al. CROI. 2010. # 511

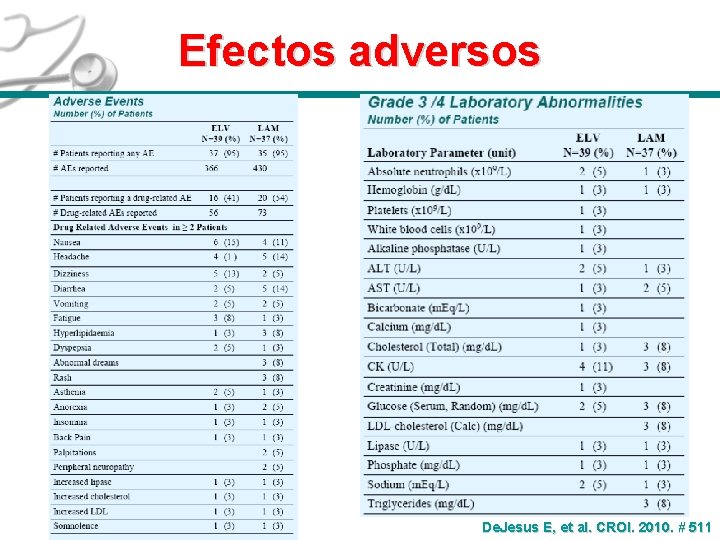
Efectos adversos De. Jesus E, et al. CROI. 2010. # 511

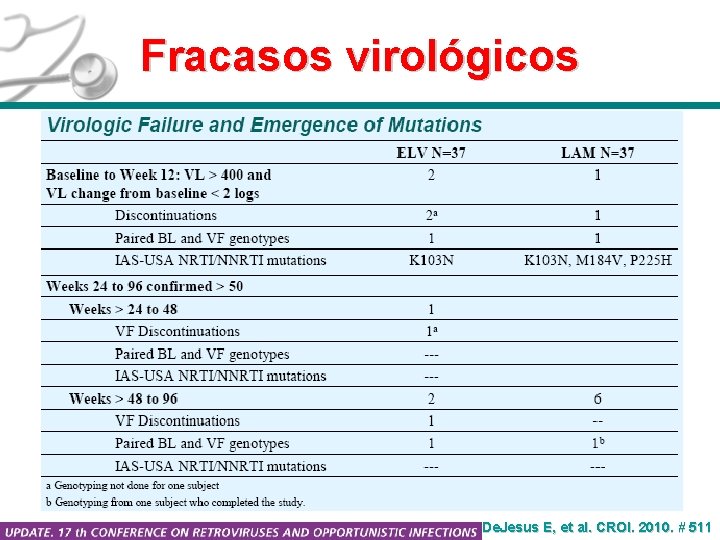
Fracasos virológicos De. Jesus E, et al. CROI. 2010. # 511

Conclusiones De. Jesus E, et al. CROI. 2010. # 511

Antagonistas de CCR 5

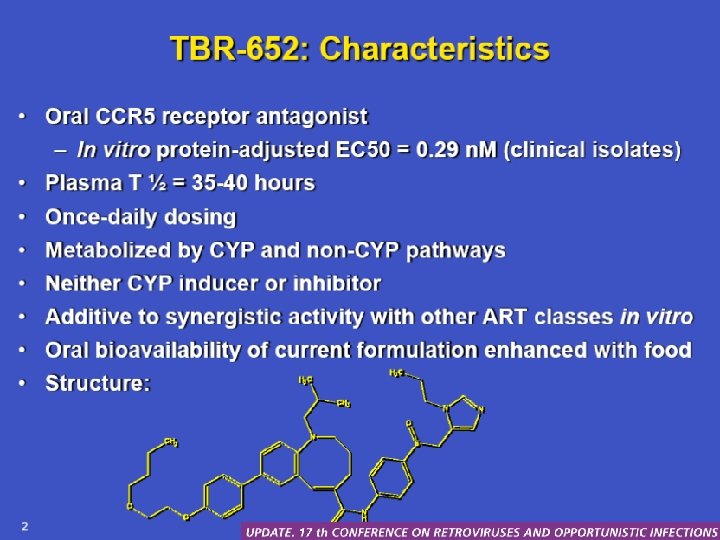
TBR-652 • Oral CCR 5 receptor antagonist – In vitro protein-adjusted EC 50 = 0, 29 n. M (clinical isolates) • Plasma t ½ = 35 -40 hours • Once-daily dosing • Metabolized by CYP and non-CYP pathways • Neither CYP inducer nor inhibitor • Additive or synergistic activity with other ART classes in vitro • Oral bioavailability of current formulation enhanced by food Palleja S, et al. CROI. 2010. Abstract 51

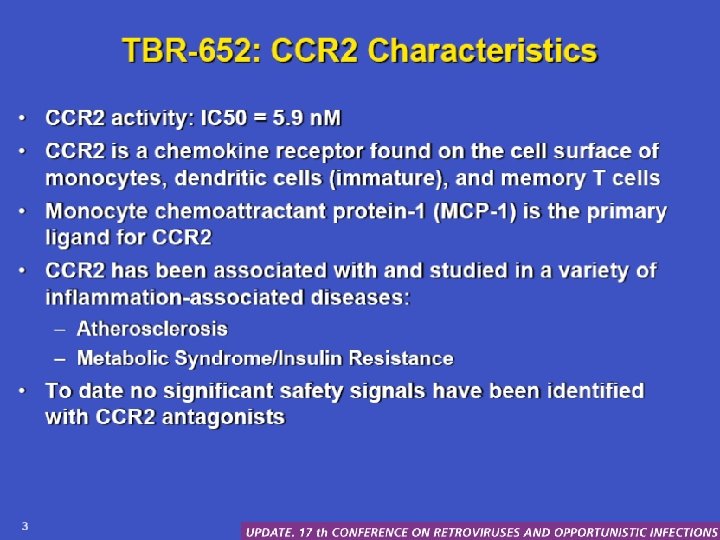
TBR-652: CCR 2 characteristics • CCR 2 activity: IC 50 = 5. 9 n. M • CCR 2 is a chemokine receptor found on the cell surface of monocytes, dendritic celles (immature) and memory T cells • Monocyte chemoatractant protein 1 (MCP-1) is the primary ligand for CCR 2 • CCR 2 has been associated with and studied in a variety of inflammation-associated diseases: – Atherosclerosis – Metabolic syndrome/insulin resistance • To date no significant safety signals have been identified with CCR 2 antagonists Palleja S, et al. CROI. 2010. Abstract 51

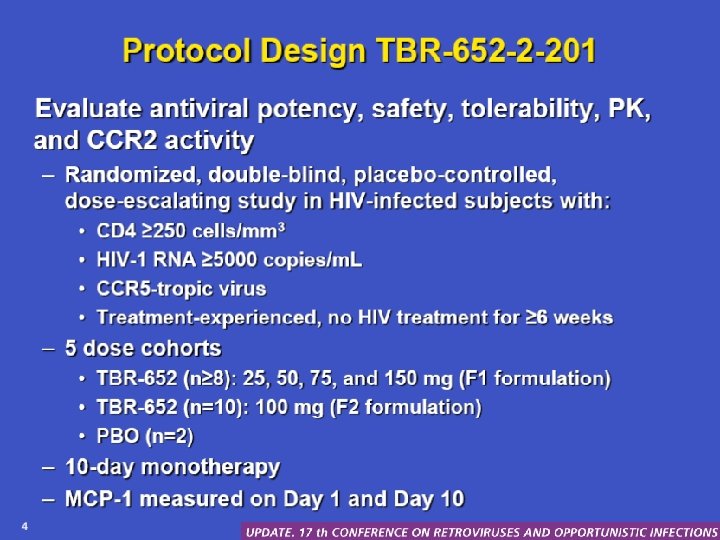
Protocol design. TBR-652 -201 • Evaluate antiviral potency, safety, tolerability, PK, and CCR 2 activity – Randomized, double-blind, placebo-controlled doseescalating study in HIV-infected subjects with: • • CD 4 ≥ 250 cells/mm 3 HIV-1 RNA > 5000 copies/ml CCR 5 -tropic virus Treatment experienced, no HIV treatment for ≥ 6 weeks – 5 dose cohorts • TBR-652 (≥ 8): 25, 50, 75, and 150 mg (F 1 formulation) • TBR-652 (n = 10): 100 mg (F 2 formulation) • PBO (n = 2) – 10 -day monotherapy – MCP-1 measured on day 1 and 10 Thompson M, et al. CROI. 2009. Abstract 571 a







Phase 3 Trials of Vicriviroc in Treatment. Experienced Subjects Demonstrate Safety but Not Significantly Superior Efficacy over Potent Background Regimens J. Gathe, 1 R. Diaz, 2 G. Fätkenheuer, 3 J. Zeinecker, 4 C. Mak, 5 R. A. Vilchez, 5 W. Greaves, 5 S. Kumar, 5 C. Onyebuchi, 5 L. M. Dunkle 5 1 Therapeutic Concepts PA, Houston, Texas; 2 Federal University of São Paulo, Brazil; 3 University of Cologne, Koln, Germany; 4 Institute of Infectious Diseases and Molecular Medicine, Cape Town, South Africa; 5 Merck, Kenilworth, New Jersey

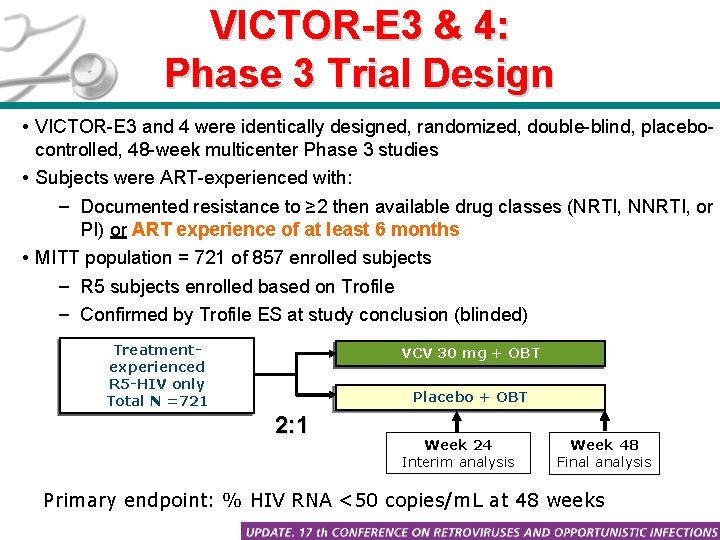
VICTOR-E 3 & 4: Phase 3 Trial Design • VICTOR-E 3 and 4 were identically designed, randomized, double-blind, placebocontrolled, 48 -week multicenter Phase 3 studies • Subjects were ART-experienced with: – Documented resistance to ≥ 2 then available drug classes (NRTI, NNRTI, or PI) or ART experience of at least 6 months • MITT population = 721 of 857 enrolled subjects – R 5 subjects enrolled based on Trofile – Confirmed by Trofile ES at study conclusion (blinded) Treatmentexperienced R 5 -HIV only Total N =721 VCV 30 mg + OBT Placebo + OBT 2: 1 Week 24 Interim analysis Week 48 Final analysis • Primary endpoint: % HIV RNA <50 copies/m. L at 48 weeks

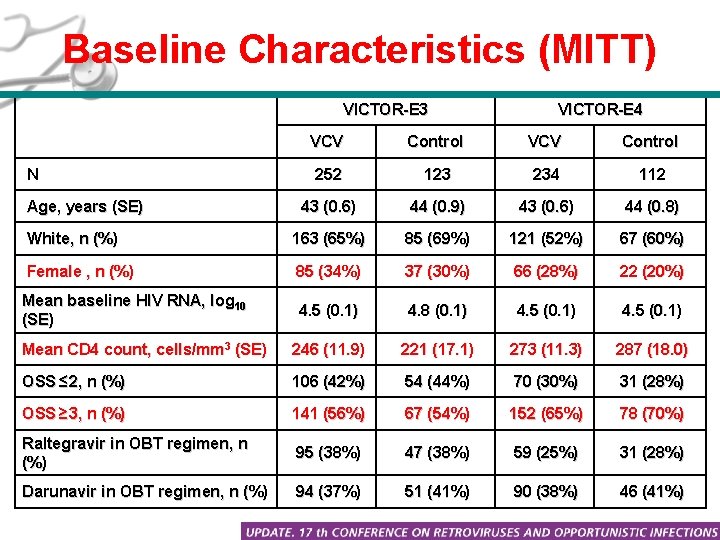
Baseline Characteristics (MITT) VICTOR-E 3 VICTOR-E 4 VCV Control 252 123 234 112 43 (0. 6) 44 (0. 9) 43 (0. 6) 44 (0. 8) White, n (%) 163 (65%) 85 (69%) 121 (52%) 67 (60%) Female , n (%) 85 (34%) 37 (30%) 66 (28%) 22 (20%) Mean baseline HIV RNA, log 10 (SE) 4. 5 (0. 1) 4. 8 (0. 1) 4. 5 (0. 1) Mean CD 4 count, cells/mm 3 (SE) 246 (11. 9) 221 (17. 1) 273 (11. 3) 287 (18. 0) OSS ≤ 2, n (%) 106 (42%) 54 (44%) 70 (30%) 31 (28%) OSS ≥ 3, n (%) 141 (56%) 67 (54%) 152 (65%) 78 (70%) Raltegravir in OBT regimen, n (%) 95 (38%) 47 (38%) 59 (25%) 31 (28%) Darunavir in OBT regimen, n (%) 94 (37%) 51 (41%) 90 (38%) 46 (41%) N Age, years (SE)

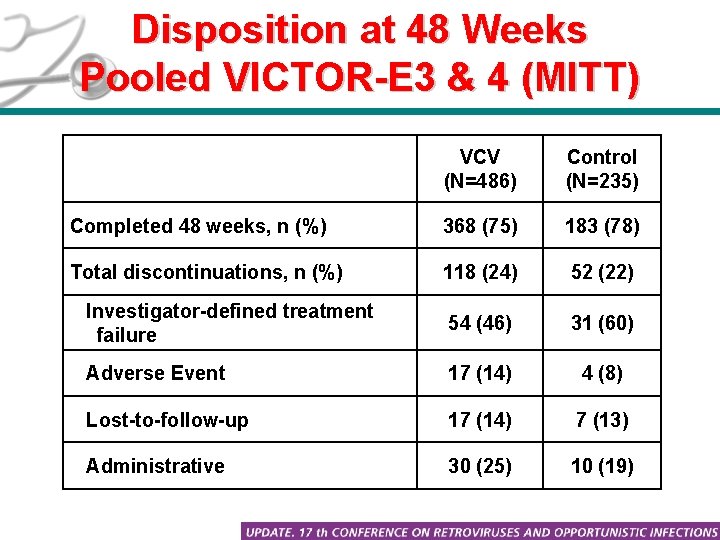
Disposition at 48 Weeks Pooled VICTOR-E 3 & 4 (MITT) VCV (N=486) Control (N=235) Completed 48 weeks, n (%) 368 (75) 183 (78) Total discontinuations, n (%) 118 (24) 52 (22) Investigator-defined treatment failure 54 (46) 31 (60) Adverse Event 17 (14) 4 (8) Lost-to-follow-up 17 (14) 7 (13) Administrative 30 (25) 10 (19)

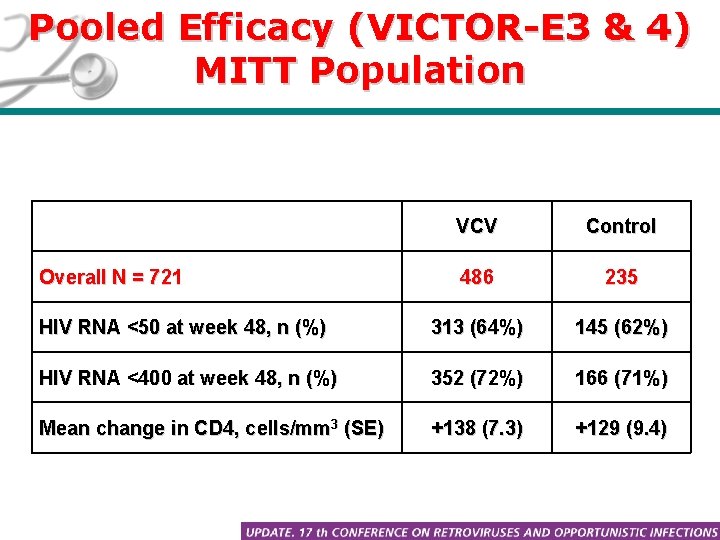
Pooled Efficacy (VICTOR-E 3 & 4) MITT Population VCV Control 486 235 HIV RNA <50 at week 48, n (%) 313 (64%) 145 (62%) HIV RNA <400 at week 48, n (%) 352 (72%) 166 (71%) Mean change in CD 4, cells/mm 3 (SE) +138 (7. 3) +129 (9. 4) Overall N = 721

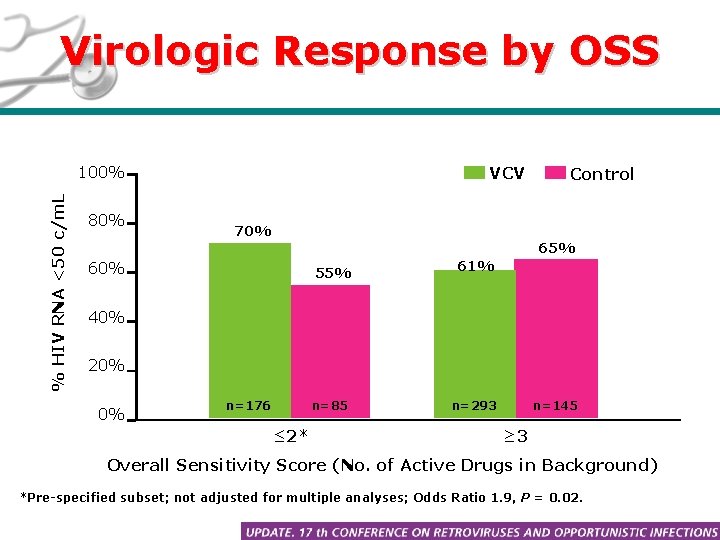
Virologic Response by OSS % HIV RNA <50 c/m. L 100% 80% VCV Control 70% 65% 60% 55% 61% 40% 20% 0% n=176 n=85 ≤ 2* n=293 n=145 ≥ 3 Overall Sensitivity Score (No. of Active Drugs in Background) *Pre-specified subset; not adjusted for multiple analyses; Odds Ratio 1. 9, P = 0. 02.

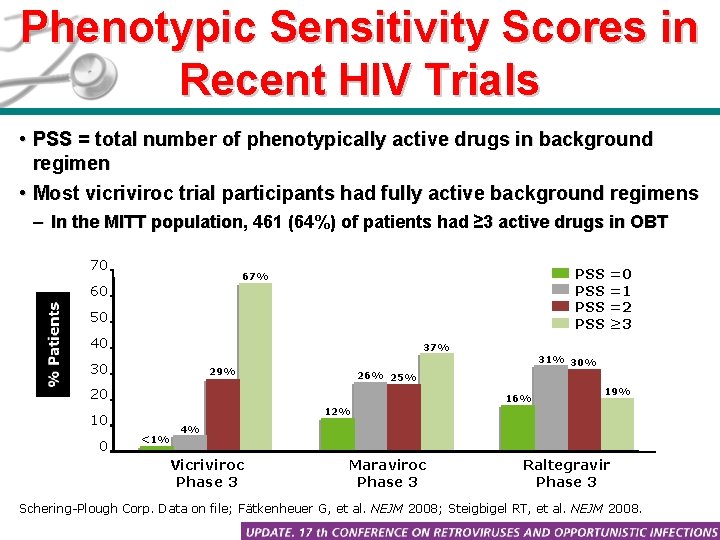
Phenotypic Sensitivity Scores in Recent HIV Trials • PSS = total number of phenotypically active drugs in background regimen • Most vicriviroc trial participants had fully active background regimens – In the MITT population, 461 (64%) of patients had ≥ 3 active drugs in OBT 70 PSS PSS 67% 60 50 40 37% 30 29% 31% 30% 26% 25% 20 16% 19% 12% 10 0 =0 =1 =2 ≥ 3 <1% 4% Vicriviroc Phase 3 Maraviroc Phase 3 Raltegravir Phase 3 Schering-Plough Corp. Data on file; Fätkenheuer G, et al. NEJM 2008; Steigbigel RT, et al. NEJM 2008.

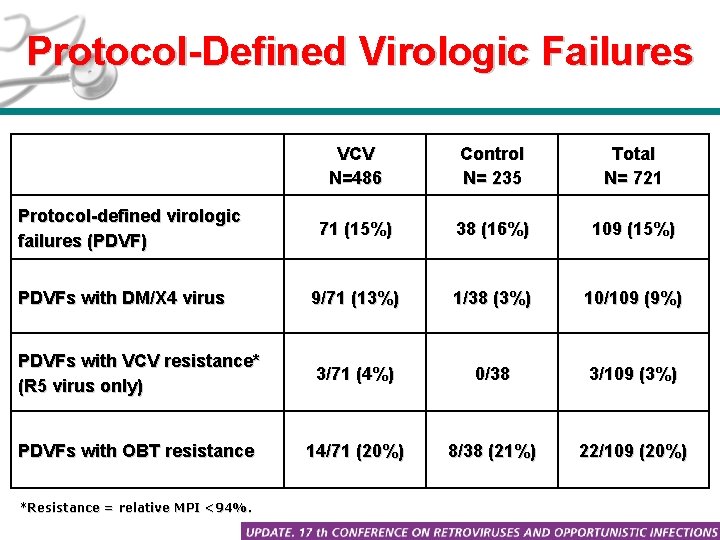
Protocol-Defined Virologic Failures VCV N=486 Control N= 235 Total N= 721 71 (15%) 38 (16%) 109 (15%) PDVFs with DM/X 4 virus 9/71 (13%) 1/38 (3%) 10/109 (9%) PDVFs with VCV resistance* (R 5 virus only) 3/71 (4%) 0/38 3/109 (3%) PDVFs with OBT resistance 14/71 (20%) 8/38 (21%) 22/109 (20%) Protocol-defined virologic failures (PDVF) *Resistance = relative MPI <94%.

Resistance in Virologic Failures • Frequency of OBT resistance similar in VCV and Control arms (~20%) – Affected one or several drugs in OBT – NRTI resistance was most commonly observed – PI/r next most frequent – Raltegravir resistance infrequent (~3%)

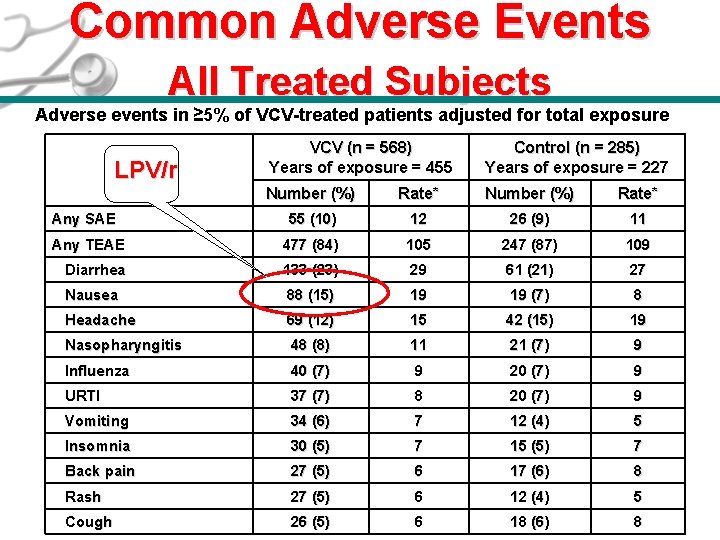
Common Adverse Events All Treated Subjects Adverse events in ≥ 5% of VCV-treated patients adjusted for total exposure VCV (n = 568) Years of exposure = 455 Control (n = 285) Years of exposure = 227 Number (%) Rate* 55 (10) 12 26 (9) 11 Any TEAE 477 (84) 105 247 (87) 109 Diarrhea 133 (23) 29 61 (21) 27 Nausea 88 (15) 19 19 (7) 8 Headache 69 (12) 15 42 (15) 19 Nasopharyngitis 48 (8) 11 21 (7) 9 Influenza 40 (7) 9 20 (7) 9 URTI 37 (7) 8 20 (7) 9 Vomiting 34 (6) 7 12 (4) 5 Insomnia 30 (5) 7 15 (5) 7 Back pain 27 (5) 6 17 (6) 8 Rash 27 (5) 6 12 (4) 5 26 (5) 6 18 (6) 8 LPV/r Any SAE *Per 100 patient years, all treated subjects. Cough

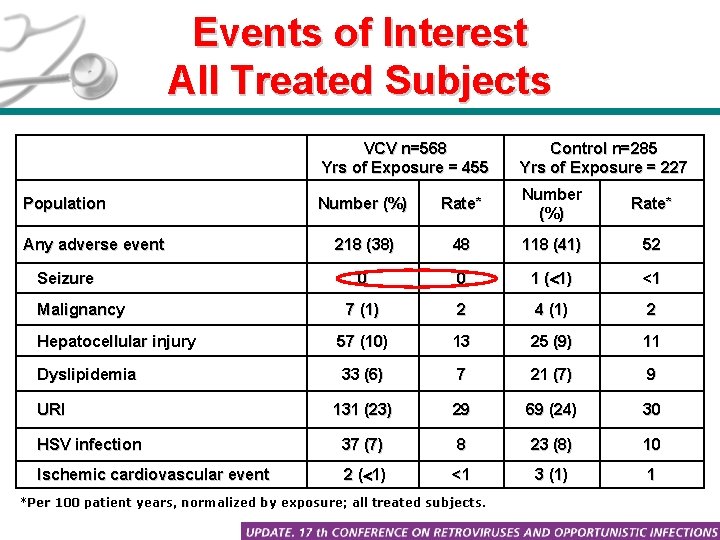
Events of Interest All Treated Subjects VCV n=568 Yrs of Exposure = 455 Control n=285 Yrs of Exposure = 227 Number (%) Rate* 218 (38) 48 118 (41) 52 0 0 1 ( 1) <1 7 (1) 2 4 (1) 2 Hepatocellular injury 57 (10) 13 25 (9) 11 Dyslipidemia 33 (6) 7 21 (7) 9 131 (23) 29 69 (24) 30 HSV infection 37 (7) 8 23 (8) 10 Ischemic cardiovascular event 2 ( 1) <1 3 (1) 1 Population Any adverse event Seizure Malignancy URI *Per 100 patient years, normalized by exposure; all treated subjects.

Summary and Conclusions • VCV was well tolerated in both phase 3 studies • VCV did not meet the primary efficacy endpoint in these trials – OBT in these trials included more potent antiretroviral drugs than in the VCV Phase 2 trial or Phase 3 trials of recently approved HIV drugs – 64% of subjects had ≥ 3 fully active drugs in their OBT • Resistance to VCV was observed very infrequently

Implications • For subjects with 2 or fewer available active drugs, VCV provided additional opportunity to achieve full viral suppression (<50 copies/m. L) • With the success of recently available therapies for treatment-experienced subjects, new agents will require novel study designs to demonstrate efficacy

Inhibidores de la Integrasa

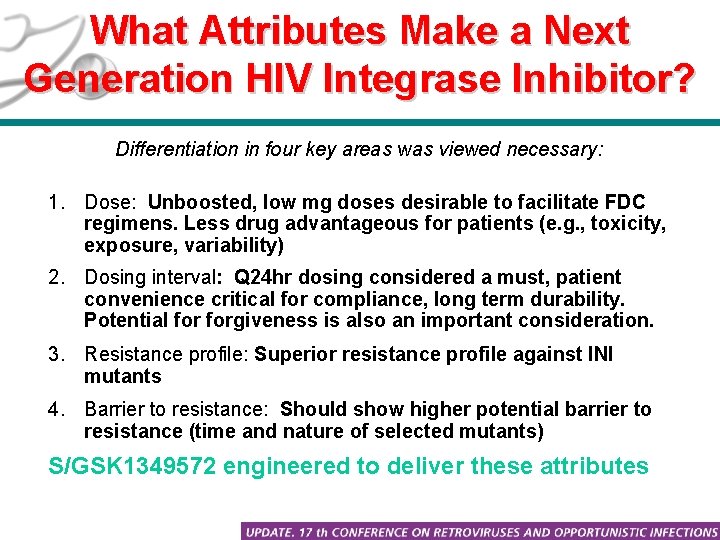
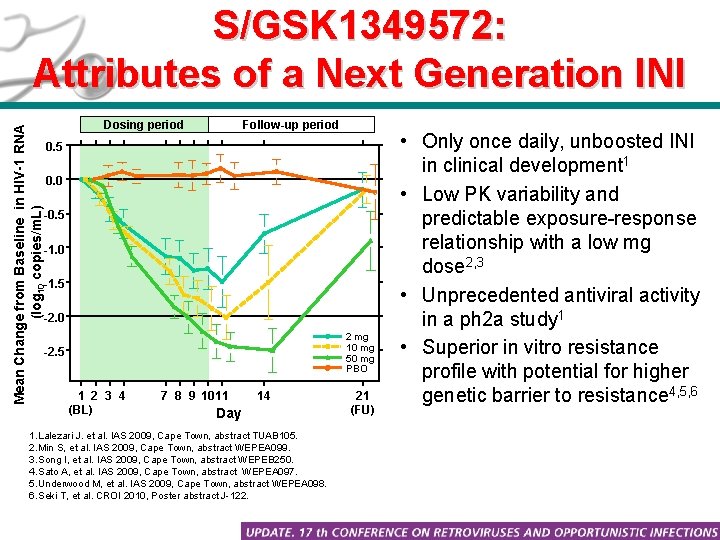
What Attributes Make a Next Generation HIV Integrase Inhibitor? Differentiation in four key areas was viewed necessary: 1. Dose: Unboosted, low mg doses desirable to facilitate FDC regimens. Less drug advantageous for patients (e. g. , toxicity, exposure, variability) 2. Dosing interval: Q 24 hr dosing considered a must, patient convenience critical for compliance, long term durability. Potential forgiveness is also an important consideration. 3. Resistance profile: Superior resistance profile against INI mutants 4. Barrier to resistance: Should show higher potential barrier to resistance (time and nature of selected mutants) S/GSK 1349572 engineered to deliver these attributes

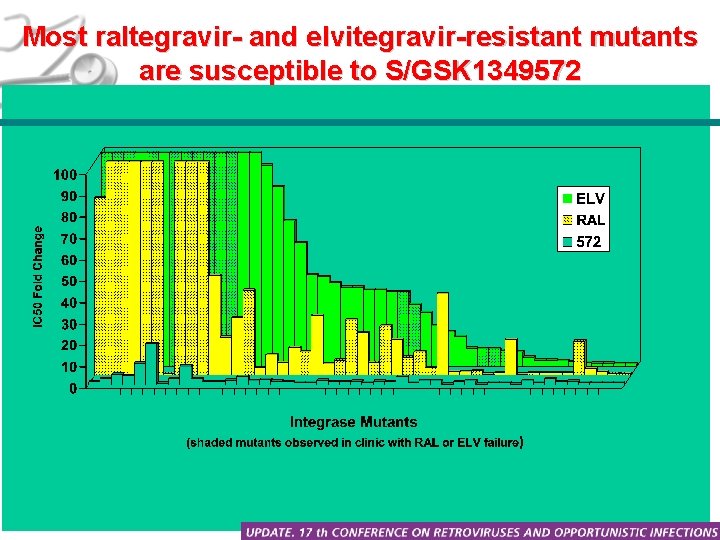
Most raltegravir- and elvitegravir-resistant mutants are susceptible to S/GSK 1349572 Seki T, et al. CROI 2010, Friday poster abstract J-122.

Mean Change from Baseline in HIV-1 RNA (log 10 copies/m. L) S/GSK 1349572: Attributes of a Next Generation INI Dosing period Follow-up period 0. 5 0. 0 -0. 5 -1. 0 -1. 5 -2. 0 2 mg 10 mg 50 mg PBO -2. 5 1 2 3 4 (BL) 7 8 9 1011 14 Day 1. Lalezari J. et al. IAS 2009, Cape Town, abstract TUAB 105. 2. Min S, et al. IAS 2009, Cape Town, abstract WEPEA 099. 3. Song I, et al. IAS 2009, Cape Town, abstract WEPEB 250. 4. Sato A, et al. IAS 2009, Cape Town, abstract WEPEA 097. 5. Underwood M, et al. IAS 2009, Cape Town, abstract WEPEA 098. 6. Seki T, et al. CROI 2010, Poster abstract J-122. 21 (FU) • Only once daily, unboosted INI in clinical development 1 • Low PK variability and predictable exposure-response relationship with a low mg dose 2, 3 • Unprecedented antiviral activity in a ph 2 a study 1 • Superior in vitro resistance profile with potential for higher genetic barrier to resistance 4, 5, 6

The Single-Tablet, Fixed-Dose Regimen of Elvitegravir/GS-9350/Emtricitabine/Tenofovir DF (Quad) Achieves a High Rate of Virologic Suppression and Calvin Cohen , David Shamblaw , Peter Ruane , Richard Elion , Edwin De. Jesus , is. Warren an Effective Booster Hui C. Liu , GS-9350 Lijie Zhong , David , Brian P. Kearney , and Steven L. Chuck 1 6 2 6 3 6 1 Community 4 6 Research Initiative of New England, Boston, MA; 2 San Diego, CA; 3 Los Angeles, CA; 4 Whitman Walker Clinic, Washington, D. C. ; 5 Orlando Immunology Center, Orlando, FL; and 6 Gilead Sciences, Foster City, CA CROI 2010 5 6

Background • Boosted elvitegravir 150 mg is a potent, once -daily HIV integrase inhibitor • GS-9350 is a CYP 3 A inhibitor that lacks anti. HIV activity • GS-9350 150 mg boosts EVG or atazanavir (ATV) equivalently to ritonavir 100 mg • Elvitegravir (EVG)/GS-9350/Emtricitabine (FTC)/Tenofovir Disoproxil Fumarate (TDF) has been co-formulated in one tablet (“Quad”)

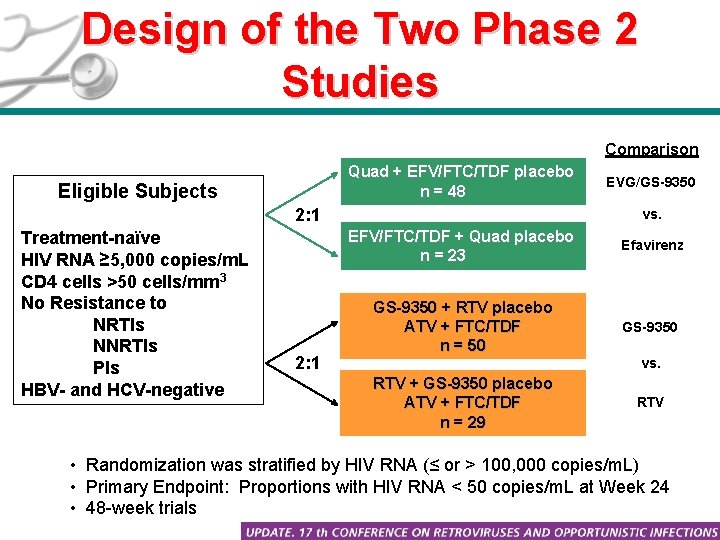
Design of the Two Phase 2 Studies Comparison Quad + EFV/FTC/TDF placebo n = 48 Eligible Subjects 2: 1 Treatment-naïve HIV RNA ≥ 5, 000 copies/m. L CD 4 cells >50 cells/mm 3 No Resistance to NRTIs NNRTIs PIs HBV- and HCV-negative 2: 1 EVG/GS-9350 vs. EFV/FTC/TDF + Quad placebo n = 23 Efavirenz GS-9350 + RTV placebo ATV + FTC/TDF n = 50 GS-9350 vs. RTV + GS-9350 placebo ATV + FTC/TDF n = 29 RTV • Randomization was stratified by HIV RNA (≤ or > 100, 000 copies/m. L) • Primary Endpoint: Proportions with HIV RNA < 50 copies/m. L at Week 24 • 48 -week trials

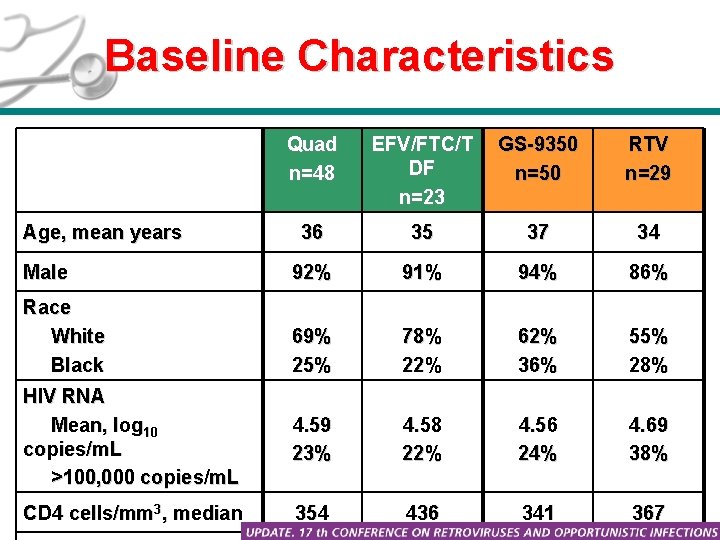
Baseline Characteristics Quad n=48 EFV/FTC/T DF n=23 GS-9350 n=50 RTV n=29 36 35 37 34 Male 92% 91% 94% 86% Race White Black 69% 25% 78% 22% 62% 36% 55% 28% HIV RNA Mean, log 10 copies/m. L >100, 000 copies/m. L 4. 59 23% 4. 58 22% 4. 56 24% 4. 69 38% CD 4 cells/mm 3, median 354 436 341 367 Age, mean years

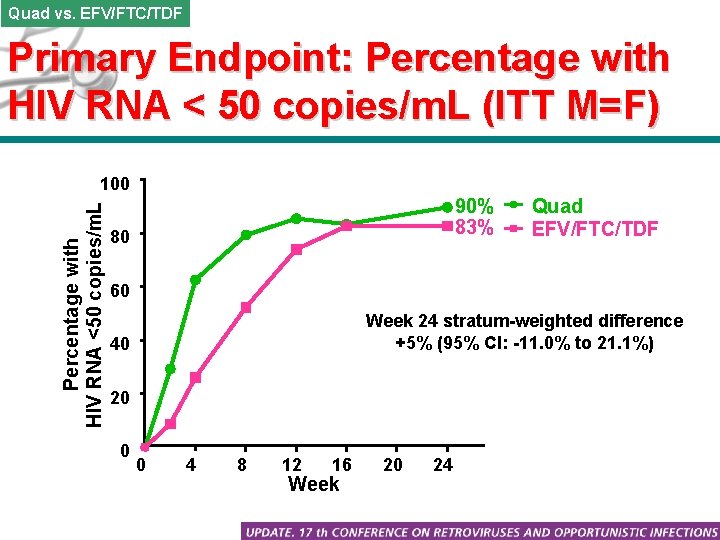
Quad vs. EFV/FTC/TDF Primary Endpoint: Percentage with HIV RNA < 50 copies/m. L (ITT M=F) Percentage with HIV RNA <50 copies/m. L 100 90% 83% 80 Quad EFV/FTC/TDF 60 Week 24 stratum-weighted difference +5% (95% CI: -11. 0% to 21. 1%) 40 20 0 0 4 8 12 16 Week 20 24

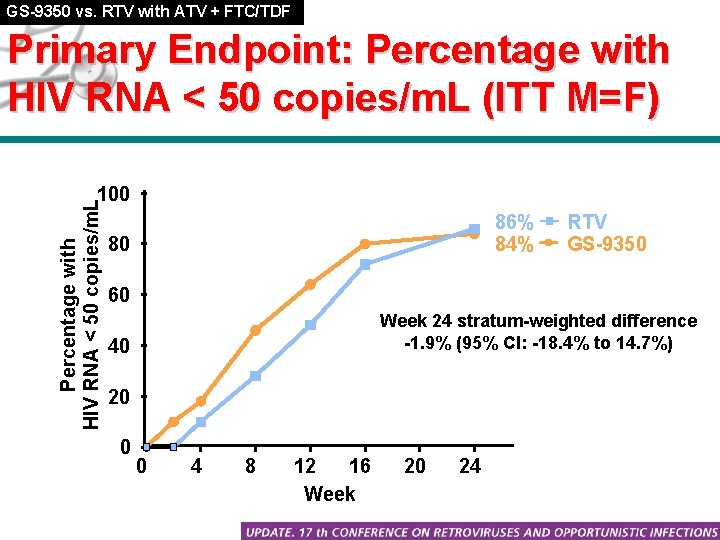
GS-9350 vs. RTV with ATV + FTC/TDF Primary Endpoint: Percentage with HIV RNA < 50 copies/m. L (ITT M=F) Percentage with HIV RNA < 50 copies/m. L 100 86% 84% 80 RTV GS-9350 60 Week 24 stratum-weighted difference -1. 9% (95% CI: -18. 4% to 14. 7%) 40 20 0 0 4 8 12 16 Week 20 24

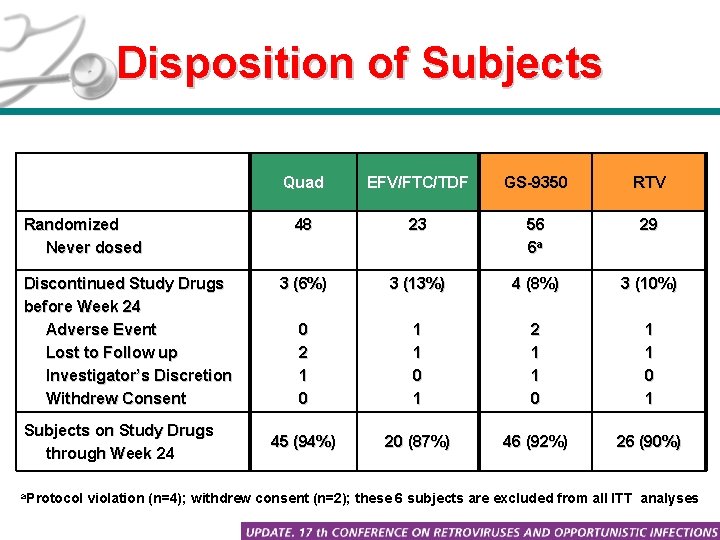
Disposition of Subjects Randomized Never dosed Discontinued Study Drugs before Week 24 Adverse Event Lost to Follow up Investigator’s Discretion Withdrew Consent Subjects on Study Drugs through Week 24 a. Protocol Quad EFV/FTC/TDF GS-9350 RTV 48 23 56 6 a 29 3 (6%) 3 (13%) 4 (8%) 3 (10%) 0 2 1 0 1 2 1 1 0 1 45 (94%) 20 (87%) 46 (92%) 26 (90%) violation (n=4); withdrew consent (n=2); these 6 subjects are excluded from all ITT analyses

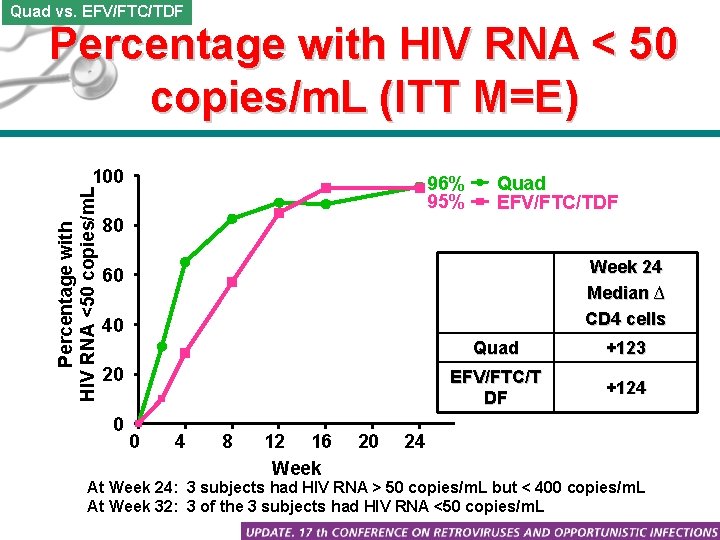
Quad vs. EFV/FTC/TDF Percentage with HIV RNA < 50 copies/m. L (ITT M=E) Percentage with HIV RNA <50 copies/m. L 100 96% 95% Quad EFV/FTC/TDF 80 Week 24 Median ∆ CD 4 cells 60 40 20 0 0 4 8 12 16 Week 20 Quad +123 EFV/FTC/T DF +124 24 At Week 24: 3 subjects had HIV RNA > 50 copies/m. L but < 400 copies/m. L At Week 32: 3 of the 3 subjects had HIV RNA <50 copies/m. L

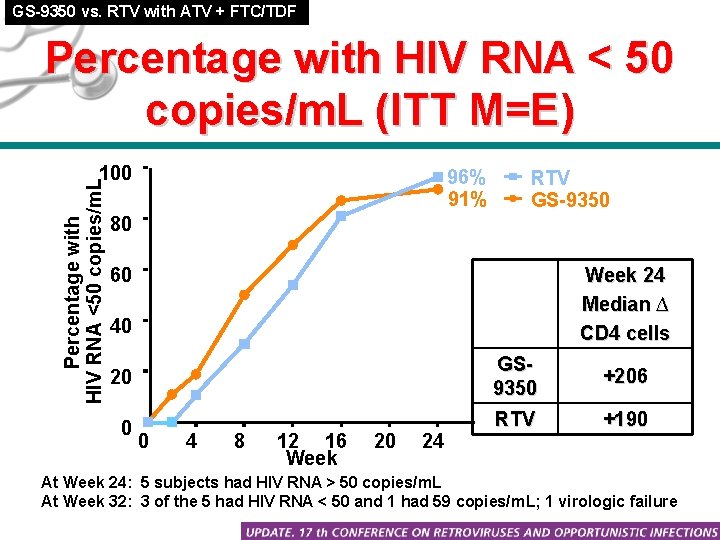
GS-9350 vs. RTV with ATV + FTC/TDF Percentage with HIV RNA < 50 copies/m. L (ITT M=E) Percentage with HIV RNA <50 copies/m. L 100 96% 91% RTV GS-9350 80 60 Week 24 Median ∆ CD 4 cells 40 20 GS 9350 +206 0 RTV +190 0 4 8 12 16 Week 20 24 At Week 24: 5 subjects had HIV RNA > 50 copies/m. L At Week 32: 3 of the 5 had HIV RNA < 50 and 1 had 59 copies/m. L; 1 virologic failure

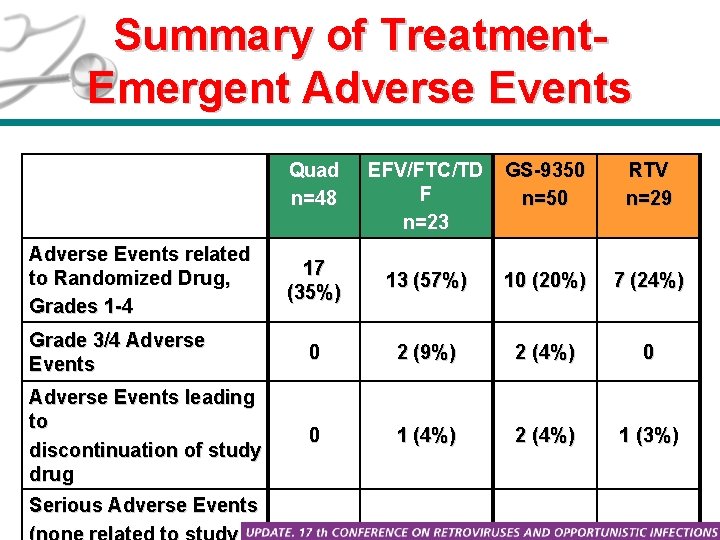
Summary of Treatment. Emergent Adverse Events Quad n=48 EFV/FTC/TD F n=23 GS-9350 n=50 RTV n=29 17 (35%) 13 (57%) 10 (20%) 7 (24%) Grade 3/4 Adverse Events 0 2 (9%) 2 (4%) 0 Adverse Events leading to discontinuation of study drug 0 1 (4%) 2 (4%) 1 (3%) 1 (2%) 1 (4%) 0 1 (3%) Adverse Events related to Randomized Drug, Grades 1 -4 Serious Adverse Events

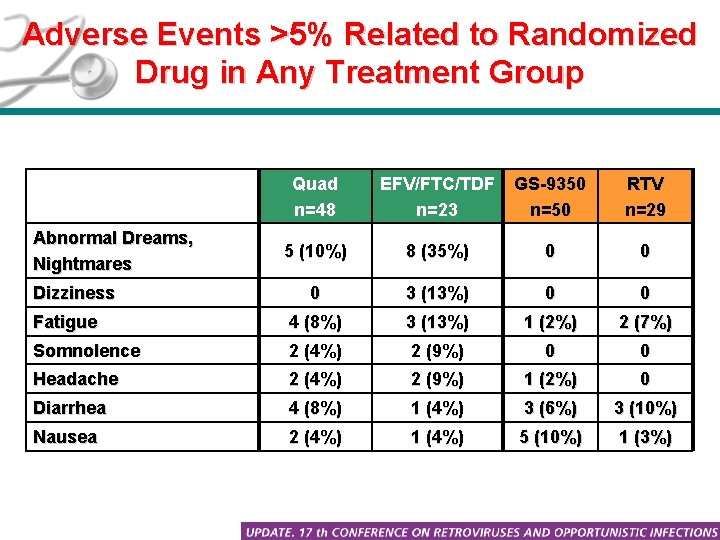
Adverse Events >5% Related to Randomized Drug in Any Treatment Group Quad n=48 EFV/FTC/TDF n=23 GS-9350 n=50 RTV n=29 5 (10%) 8 (35%) 0 0 0 3 (13%) 0 0 Fatigue 4 (8%) 3 (13%) 1 (2%) 2 (7%) Somnolence 2 (4%) 2 (9%) 0 0 Headache 2 (4%) 2 (9%) 1 (2%) 0 Diarrhea 4 (8%) 1 (4%) 3 (6%) 3 (10%) Nausea 2 (4%) 1 (4%) 5 (10%) 1 (3%) Abnormal Dreams, Nightmares Dizziness

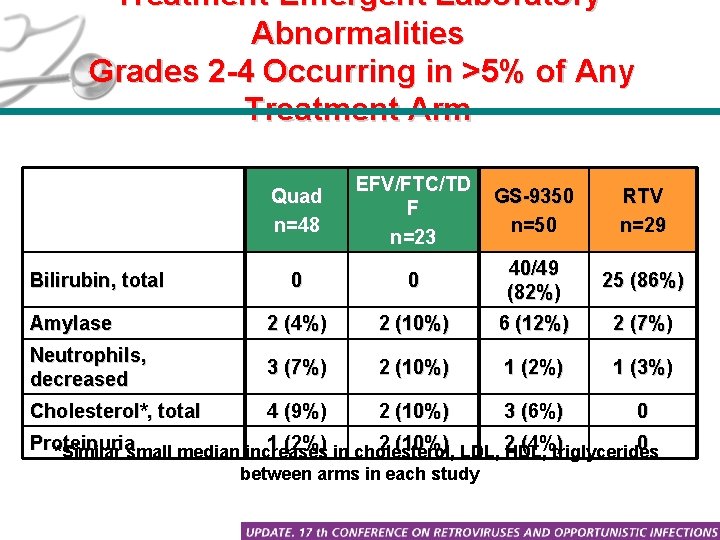
Treatment-Emergent Laboratory Abnormalities Grades 2 -4 Occurring in >5% of Any Treatment Arm Quad n=48 EFV/FTC/TD F n=23 GS-9350 n=50 RTV n=29 0 0 40/49 (82%) 25 (86%) Amylase 2 (4%) 2 (10%) 6 (12%) 2 (7%) Neutrophils, decreased 3 (7%) 2 (10%) 1 (2%) 1 (3%) Cholesterol*, total 4 (9%) 2 (10%) 3 (6%) 0 Bilirubin, total Proteinuria 1 (2%) in cholesterol, 2 (10%) LDL, 2 (4%) 0 *Similar small median increases HDL, triglycerides between arms in each study

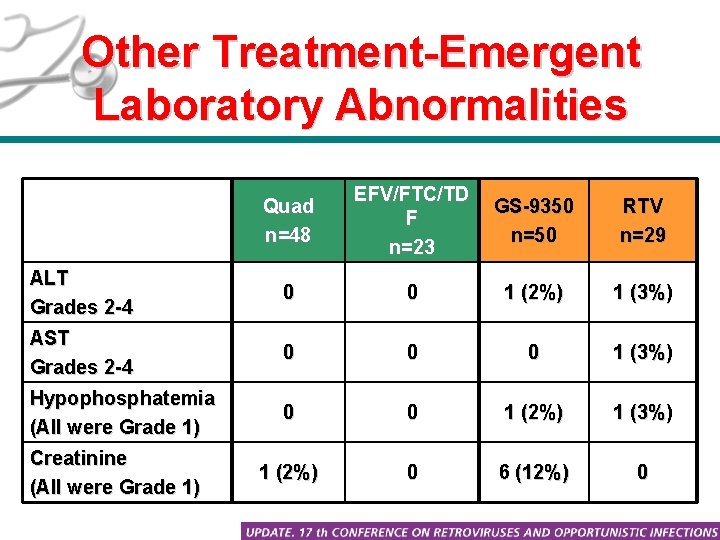
Other Treatment-Emergent Laboratory Abnormalities Quad n=48 EFV/FTC/TD F n=23 GS-9350 n=50 RTV n=29 ALT Grades 2 -4 0 0 1 (2%) 1 (3%) AST Grades 2 -4 0 0 0 1 (3%) Hypophosphatemia (All were Grade 1) 0 0 1 (2%) 1 (3%) 1 (2%) 0 6 (12%) 0 Creatinine (All were Grade 1)

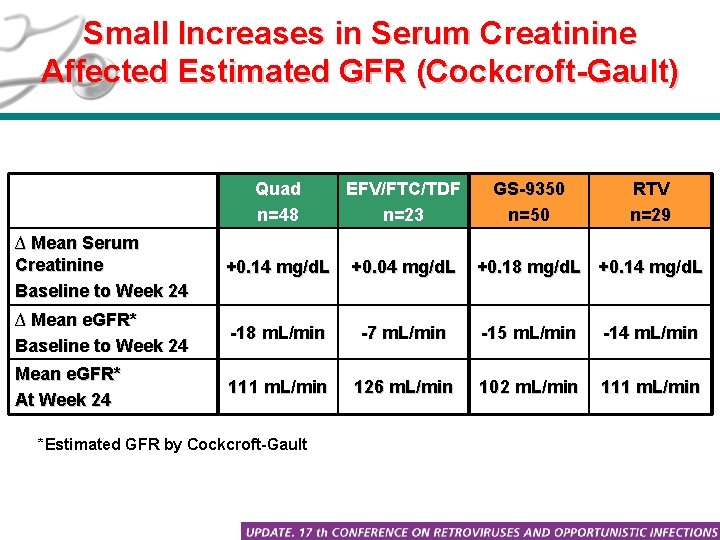
Small Increases in Serum Creatinine Affected Estimated GFR (Cockcroft-Gault) Quad n=48 EFV/FTC/TDF n=23 ∆ Mean Serum Creatinine Baseline to Week 24 +0. 14 mg/d. L +0. 04 mg/d. L ∆ Mean e. GFR* Baseline to Week 24 -18 m. L/min -7 m. L/min -15 m. L/min -14 m. L/min Mean e. GFR* At Week 24 111 m. L/min 126 m. L/min 102 m. L/min 111 m. L/min *Estimated GFR by Cockcroft-Gault GS-9350 n=50 RTV n=29 +0. 18 mg/d. L +0. 14 mg/d. L

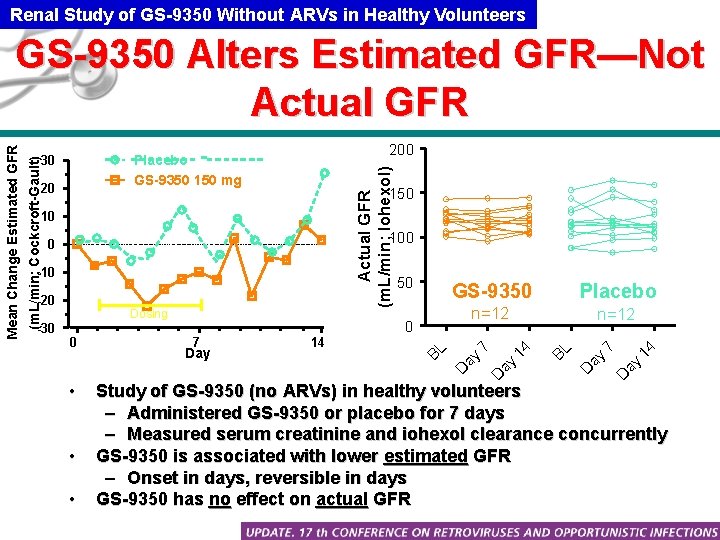
Renal Study of GS-9350 Without ARVs in Healthy Volunteers 200 20 Actual GFR (m. L/min; Iohexol) Placebo GS-9350 150 mg 30 150 10 100 • • 14 ay 7 D • ay Day 14 D 0 7 BL n=12 14 n=12 7 0 Placebo D -30 Dosing GS-9350 ay -20 50 ay -10 D 0 BL (m. L/min; Cockcroft-Gault) Mean Change Estimated GFR GS-9350 Alters Estimated GFR—Not Actual GFR Study of GS-9350 (no ARVs) in healthy volunteers – Administered GS-9350 or placebo for 7 days – Measured serum creatinine and iohexol clearance concurrently GS-9350 is associated with lower estimated GFR – Onset in days, reversible in days GS-9350 has no effect on actual GFR

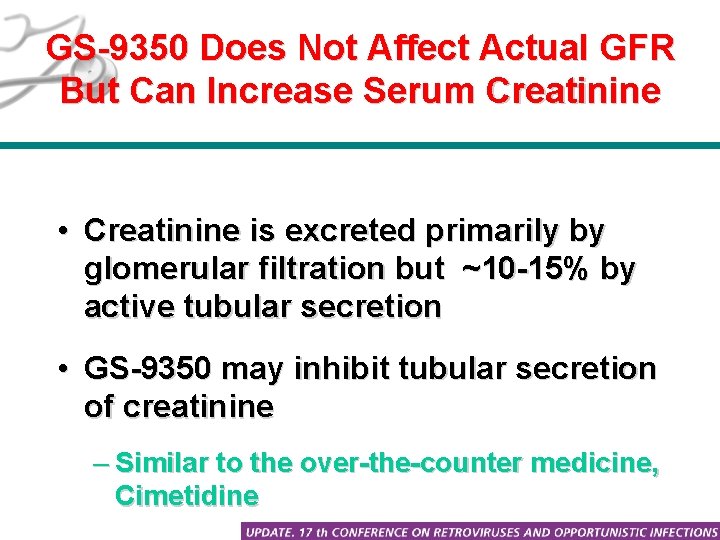
GS-9350 Does Not Affect Actual GFR But Can Increase Serum Creatinine • Creatinine is excreted primarily by glomerular filtration but ~10 -15% by active tubular secretion • GS-9350 may inhibit tubular secretion of creatinine – Similar to the over-the-counter medicine, Cimetidine

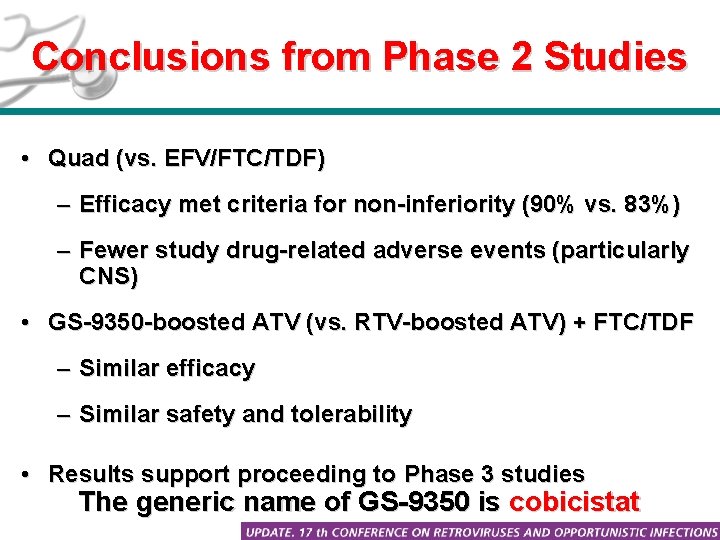
Conclusions from Phase 2 Studies • Quad (vs. EFV/FTC/TDF) – Efficacy met criteria for non-inferiority (90% vs. 83%) – Fewer study drug-related adverse events (particularly CNS) • GS-9350 -boosted ATV (vs. RTV-boosted ATV) + FTC/TDF – Similar efficacy – Similar safety and tolerability • Results support proceeding to Phase 3 studies The generic name of GS-9350 is cobicistat

Funny how the new things are the old things Rudyard Kipling (1865 -1936)
 Pere pere
Pere pere Frmacos
Frmacos Criterios de beers
Criterios de beers Frmacos
Frmacos Malalties agudes exemples
Malalties agudes exemples Laudate ao domingo
Laudate ao domingo Location and layout of hospital pharmacy
Location and layout of hospital pharmacy Cuentos nuevos
Cuentos nuevos Paradigma conductista
Paradigma conductista Ella necesita ir de compras para comprar calcetines nuevos.
Ella necesita ir de compras para comprar calcetines nuevos. Etapas de desarrollo de nuevos productos
Etapas de desarrollo de nuevos productos Nuevos anticonvulsivantes
Nuevos anticonvulsivantes La verdad yo no comparto ese desprecio a los nuevos ricos
La verdad yo no comparto ese desprecio a los nuevos ricos Paradigma conductista
Paradigma conductista Nuevos inhibidores de betalactamasas
Nuevos inhibidores de betalactamasas Nuevos materiales de la edad antigua
Nuevos materiales de la edad antigua Cambios culturales
Cambios culturales Nuevos comienzos en la biblia
Nuevos comienzos en la biblia Nuevos retos nuevas oportunidades
Nuevos retos nuevas oportunidades Nuevos ambientes de aprendizaje
Nuevos ambientes de aprendizaje Veo de ver
Veo de ver Nuevos inhibidores de betalactamasas
Nuevos inhibidores de betalactamasas Las tic y los nuevos paradigmas educativos
Las tic y los nuevos paradigmas educativos Nuevos paradigmas educativos
Nuevos paradigmas educativos Los productos no buscados
Los productos no buscados Odres nuevos vino nuevo
Odres nuevos vino nuevo Nuevos grados
Nuevos grados Družba pere kvržice film youtube
Družba pere kvržice film youtube Ouvre mes yeux seigneur fais que je vois
Ouvre mes yeux seigneur fais que je vois Van gogh ritratto di pere tanguy
Van gogh ritratto di pere tanguy Johann strauss père marche de radetzky
Johann strauss père marche de radetzky Druzba pere kvrzice analiza
Druzba pere kvrzice analiza Claude cahu
Claude cahu Notre père
Notre père Ins pere borrell
Ins pere borrell Allez par toute la terre annoncer l'évangile aux nations
Allez par toute la terre annoncer l'évangile aux nations Clic clac chanson saint nicolas
Clic clac chanson saint nicolas Družba pere kvržice pero kvrzica
Družba pere kvržice pero kvrzica Oh pere je suis ton enfant
Oh pere je suis ton enfant Vincent lafargue homélies
Vincent lafargue homélies Ins pere borrell
Ins pere borrell Portes obertes pere calders
Portes obertes pere calders Vangelis pere lachaise
Vangelis pere lachaise Credac pere barnils
Credac pere barnils Jean 15,1-8
Jean 15,1-8 Escola les roquetes sant pere de ribes
Escola les roquetes sant pere de ribes Pourquoi le pere noel rit tout le temps
Pourquoi le pere noel rit tout le temps Sant pere del vatica
Sant pere del vatica Primjeri dobre prakse u nastavi
Primjeri dobre prakse u nastavi Notre père
Notre père Pilar cruciforme romanico
Pilar cruciforme romanico Prière canonisation père caffarel
Prière canonisation père caffarel Père soubise
Père soubise Povrsinski aktivne supstance
Povrsinski aktivne supstance Milo dijete osobine
Milo dijete osobine Hobbeis
Hobbeis Acromégalie bogdanov
Acromégalie bogdanov Horaireq
Horaireq Družba pere kvržice kviz
Družba pere kvržice kviz Connaitredieu.com la lettre d'amour du père
Connaitredieu.com la lettre d'amour du père Pere marquès graells
Pere marquès graells