Nucleons Protons 1 each determines identity of element







- Slides: 27

Nucleons • Protons: +1 each, determines identity of element, mass of 1 amu, determined using atomic number, nuclear charge • Neutrons: no charge, determines identity of isotope of an element, 1 amu, determined using mass number - atomic number (amu = atomic mass unit) • 32 S and 33 S are both isotopes of S 16 16 • S-32 has 16 protons and 16 neutrons • S-33 has 16 protons and 17 neutrons • All atoms of S have a nuclear charge of +16 due to the 16 protons.


Isotopes • Atoms of the same element MUST have the same number of protons. • Atoms of the same element can vary in their numbers of neutrons. therefore have different atomic masses and are called isotopes. • The atomic mass on the Periodic Table is the weight-average atomic mass, taking into account the different isotope masses and their relative abundance. • Rounding off the atomic mass on the Periodic Table will tell you what the most common isotope of that element is.

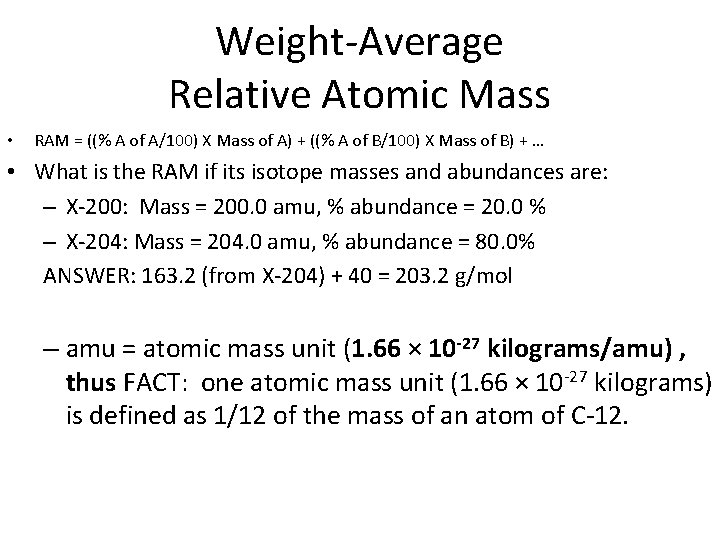
Weight-Average Relative Atomic Mass • RAM = ((% A of A/100) X Mass of A) + ((% A of B/100) X Mass of B) + … • What is the RAM if its isotope masses and abundances are: – X-200: Mass = 200. 0 amu, % abundance = 20. 0 % – X-204: Mass = 204. 0 amu, % abundance = 80. 0% ANSWER: 163. 2 (from X-204) + 40 = 203. 2 g/mol – amu = atomic mass unit (1. 66 × 10 -27 kilograms/amu) , thus FACT: one atomic mass unit (1. 66 × 10 -27 kilograms) is defined as 1/12 of the mass of an atom of C-12.

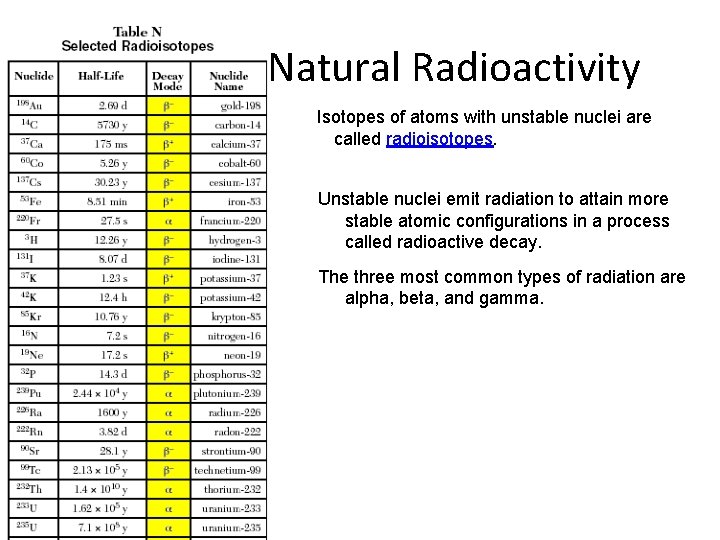
Natural Radioactivity Isotopes of atoms with unstable nuclei are called radioisotopes. Unstable nuclei emit radiation to attain more stable atomic configurations in a process called radioactive decay. The three most common types of radiation are alpha, beta, and gamma.

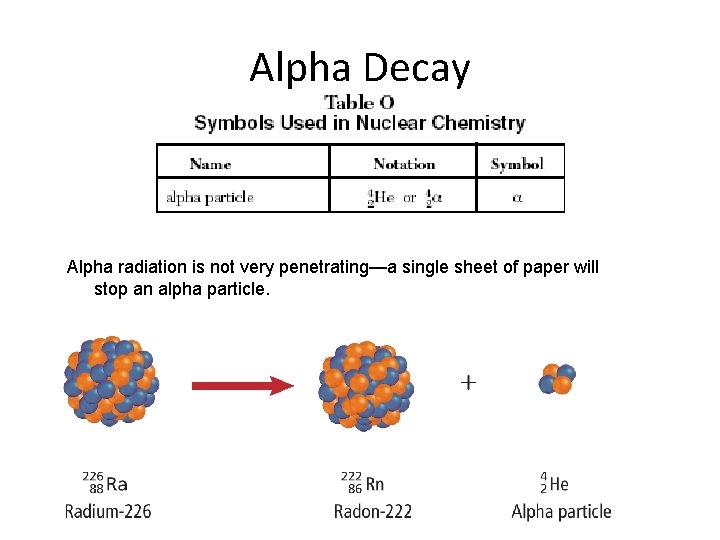
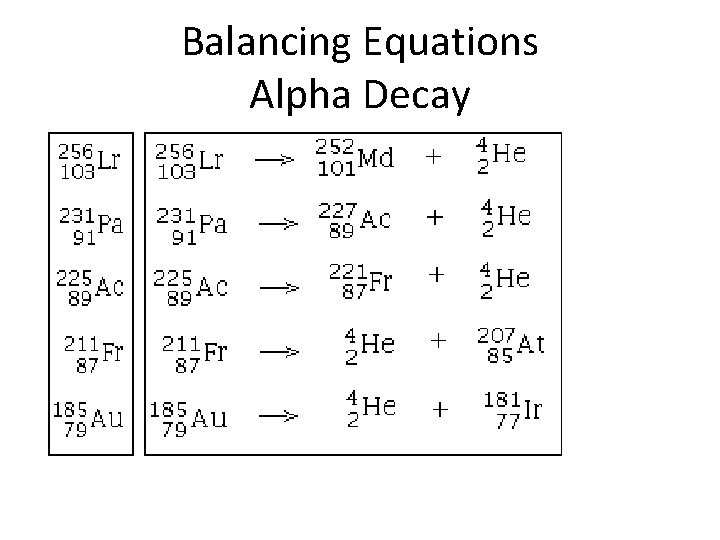
Alpha Decay Alpha radiation is not very penetrating—a single sheet of paper will stop an alpha particle.

Balancing Equations Alpha Decay

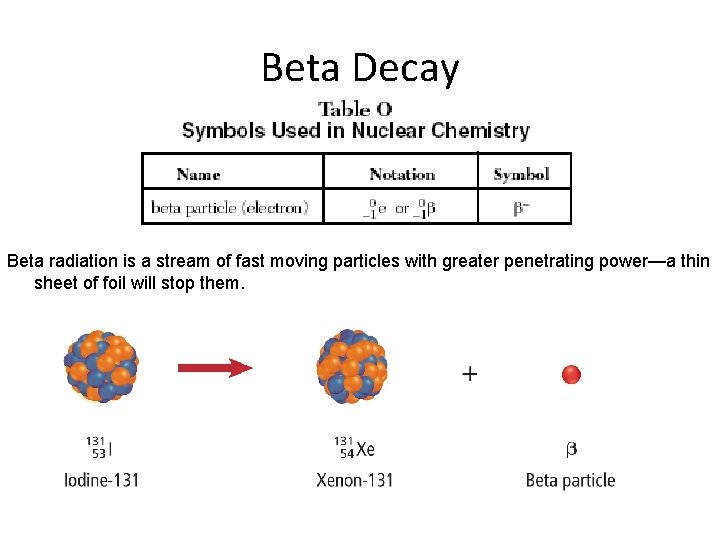
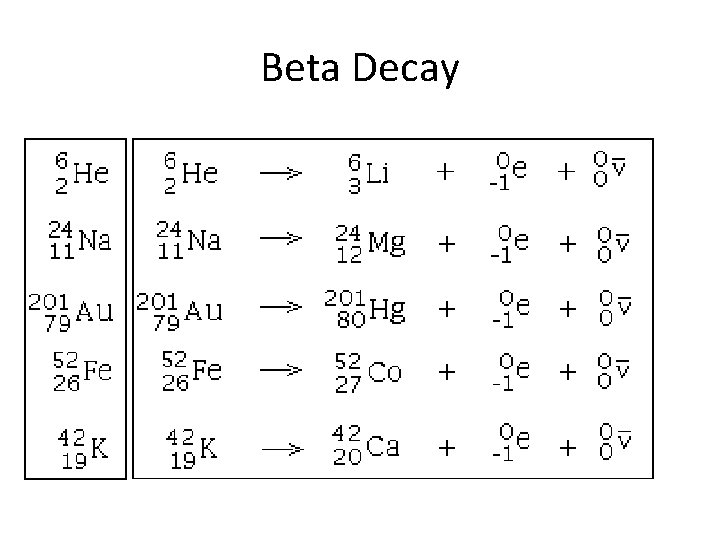
Beta Decay Beta radiation is a stream of fast moving particles with greater penetrating power—a thin sheet of foil will stop them.

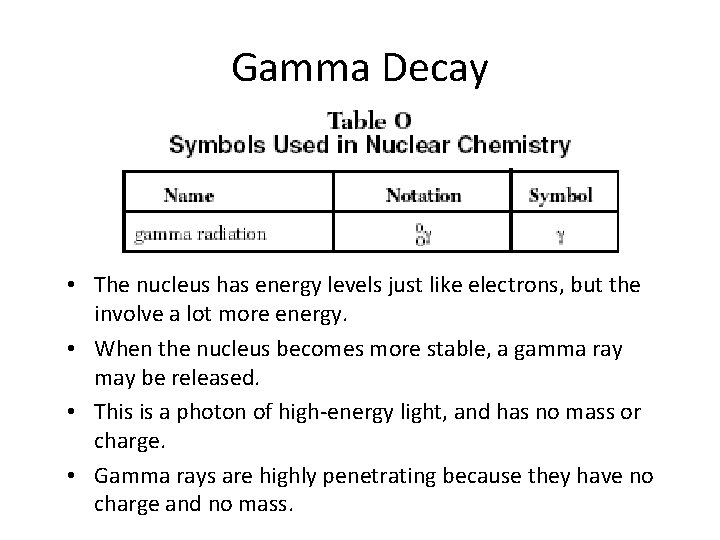
Gamma Decay • The nucleus has energy levels just like electrons, but the involve a lot more energy. • When the nucleus becomes more stable, a gamma ray may be released. • This is a photon of high-energy light, and has no mass or charge. • Gamma rays are highly penetrating because they have no charge and no mass.

Beta Decay

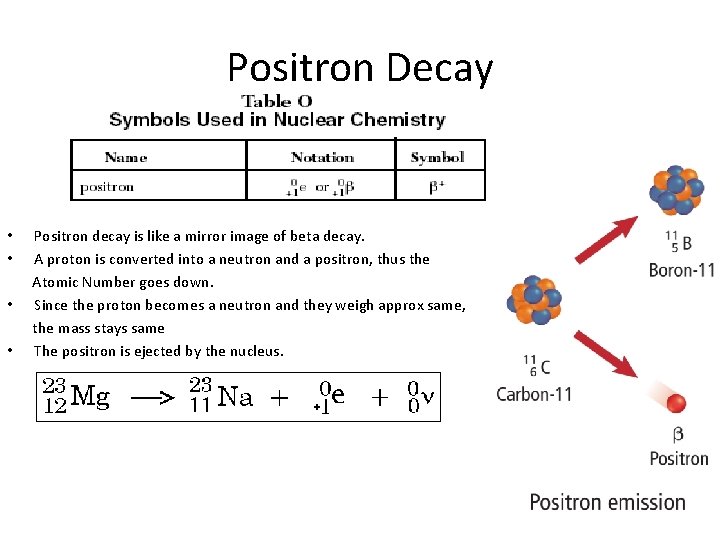
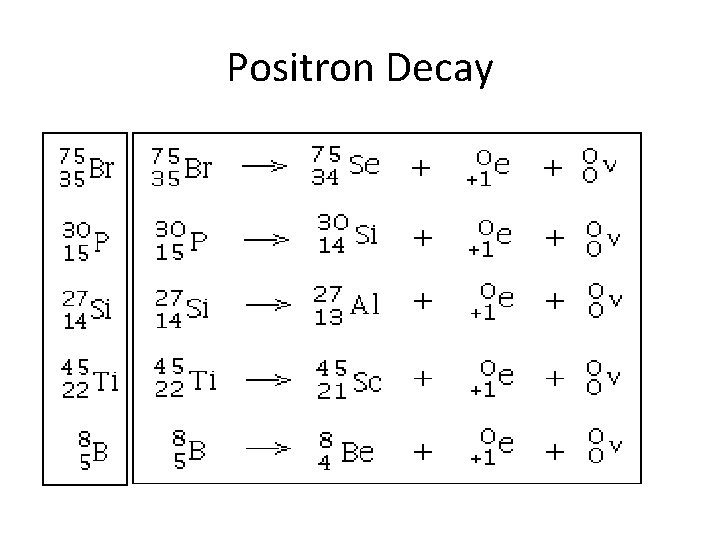
Positron Decay • • Positron decay is like a mirror image of beta decay. A proton is converted into a neutron and a positron, thus the Atomic Number goes down. Since the proton becomes a neutron and they weigh approx same, the mass stays same The positron is ejected by the nucleus.

Positron Decay

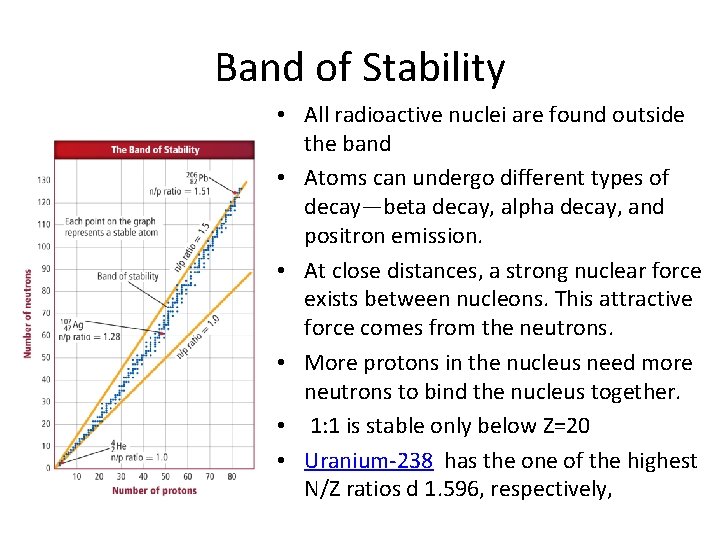
Band of Stability • All radioactive nuclei are found outside the band • Atoms can undergo different types of decay—beta decay, alpha decay, and positron emission. • At close distances, a strong nuclear force exists between nucleons. This attractive force comes from the neutrons. • More protons in the nucleus need more neutrons to bind the nucleus together. • 1: 1 is stable only below Z=20 • Uranium-238 has the one of the highest N/Z ratios d 1. 596, respectively,

Going Forwards in Time A half-life is the time required for one-half of a radioisotope to decay into its products. • How many grams of a 10. 0 gram sample of I-131 (half-life of 8 days) will remain in 24 days? • #HL = 24/8 = 3 • Cut 10. 0 g in half 3 times: 5. 00, 2. 50, 1. 25 g

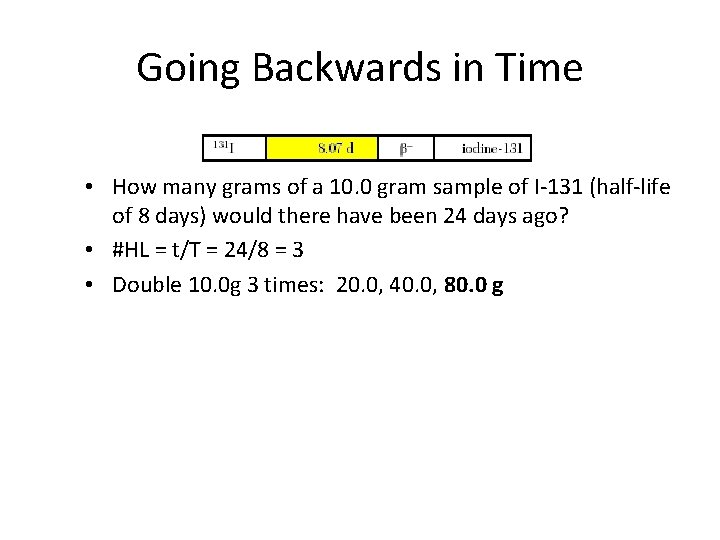
Going Backwards in Time • How many grams of a 10. 0 gram sample of I-131 (half-life of 8 days) would there have been 24 days ago? • #HL = t/T = 24/8 = 3 • Double 10. 0 g 3 times: 20. 0, 40. 0, 80. 0 g

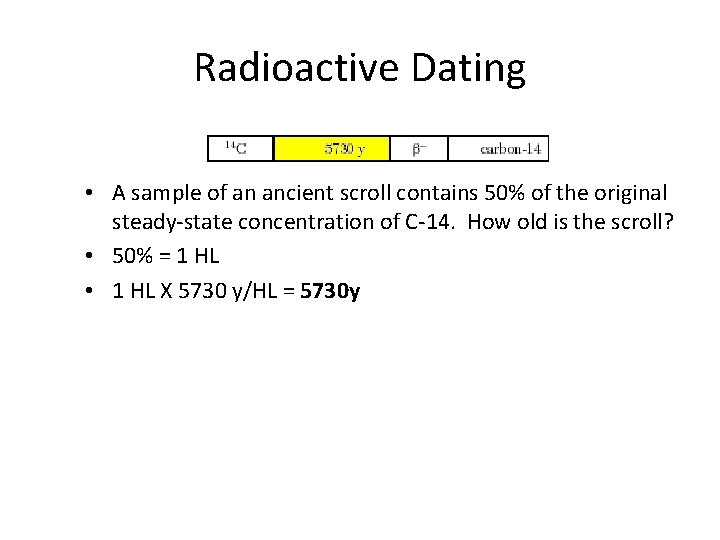
Radioactive Dating • A sample of an ancient scroll contains 50% of the original steady-state concentration of C-14. How old is the scroll? • 50% = 1 HL • 1 HL X 5730 y/HL = 5730 y

Nuclear Transmutation • New elements or isotopes can be created by adding particles to elements or splitting atoms. • These are generally done using particle accelerators. • These speed particles at each other near the speed of light to overcome electrostatic repulsion.

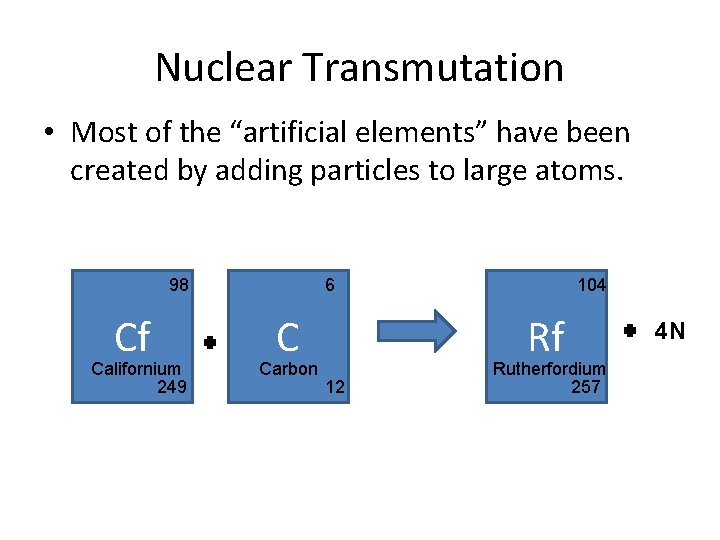
Nuclear Transmutation • Most of the “artificial elements” have been created by adding particles to large atoms. 98 Cf Californium 249 6 C Carbon 104 Rf 12 Rutherfordium 257 4 N

Magnets Are Used to Move Particles

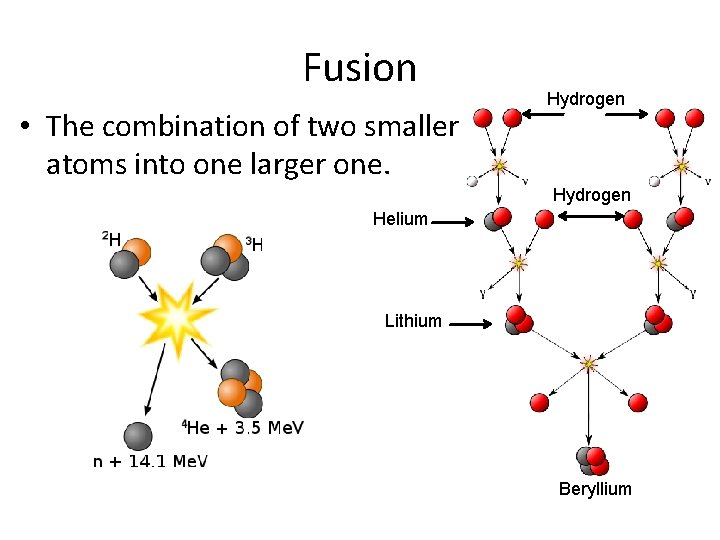
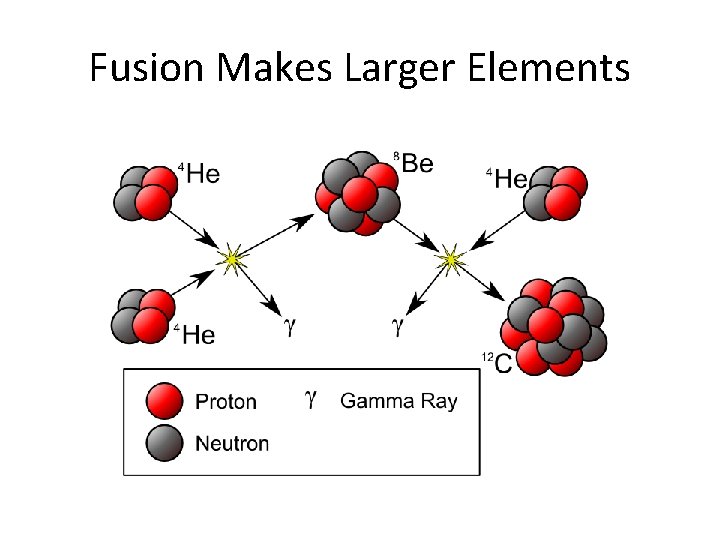
Fusion • The combination of two smaller atoms into one larger one. Hydrogen Helium Lithium Beryllium

Fusion Makes Larger Elements

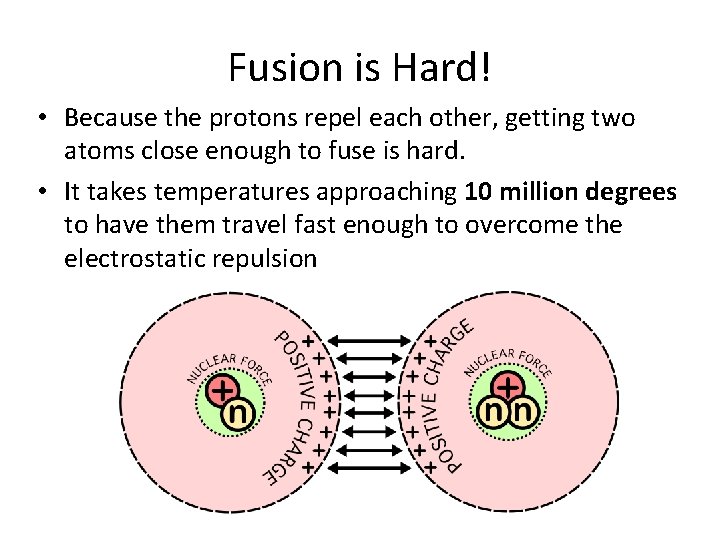
Fusion is Hard! • Because the protons repel each other, getting two atoms close enough to fuse is hard. • It takes temperatures approaching 10 million degrees to have them travel fast enough to overcome the electrostatic repulsion

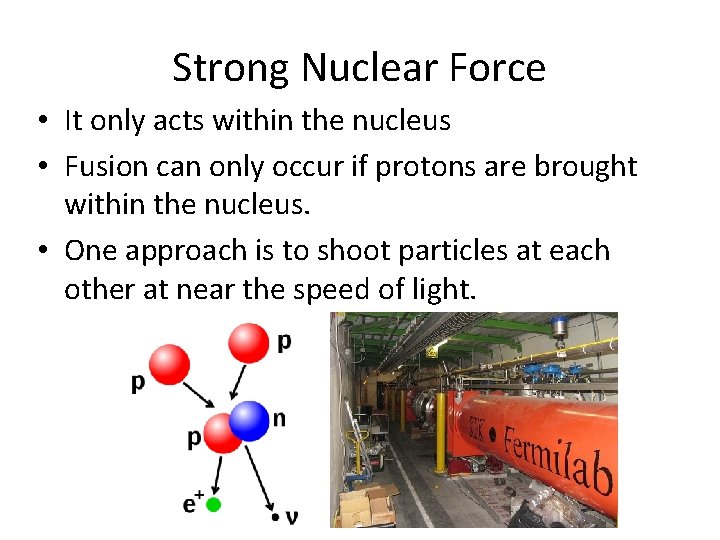
Strong Nuclear Force • It only acts within the nucleus • Fusion can only occur if protons are brought within the nucleus. • One approach is to shoot particles at each other at near the speed of light.

Fusion Releases Energy • When fusion occurs, some of the matter is converted to energy and that energy is released as heat.

Stars are Element Makers • Medium stars like our sun mostly convert Hydrogen to Helium. • Later, it will convert Helium to Carbon. • Giant stars can fuse higher atoms to larger elements.

Human Induced Fusion • Hydrogen Bomb

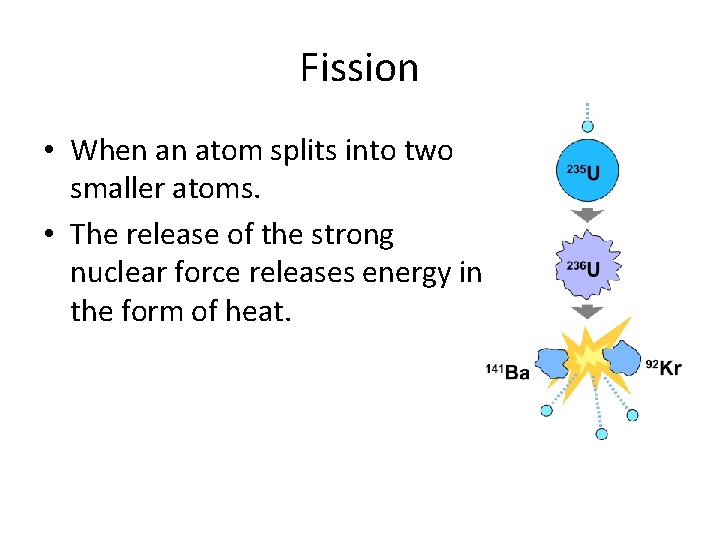
Fission • When an atom splits into two smaller atoms. • The release of the strong nuclear force releases energy in the form of heat.