Nuclear Chemistry Chemical reactions involve changes with electrons
- Slides: 10

Nuclear Chemistry Chemical reactions involve changes with electrons. ________ Nuclear reactions involve changes in atomic nuclei. ________ radiation and Spontaneously-changing nuclei emit ____ radioactive are said to be _____. energy and/or particles

Radioactivity nucleons: protons (p+) and neutrons (n 0) mass number: (p+ + n 0) in a given atom isotopes: atoms having the same number of p+, but different numbers of n 0 radioisotopes -- radioactive ones are called ______ nuclide: a nucleus w/a specified number of p+ and n 0 radionuclides -- radioactive ones are called ______ atomic number: (Z); # of p+ these are unstable and emit radiation

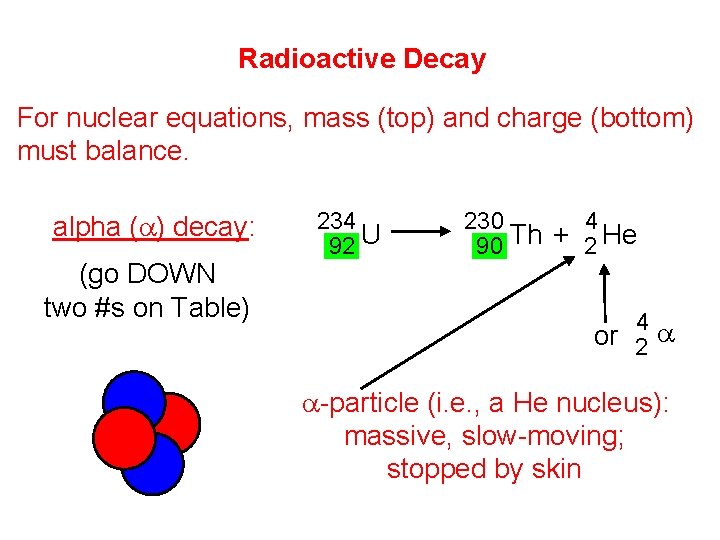
Radioactive Decay For nuclear equations, mass (top) and charge (bottom) must balance. alpha (a) decay: (go DOWN two #s on Table) 234 92 U 230 4 90 Th + 2 He 4 or 2 a a-particle (i. e. , a He nucleus): massive, slow-moving; stopped by skin

234 91 Pa beta (b) decay: (go UP one # on Table) 234 0 92 U + – 1 e 0 or – 1 b b-particle (i. e. , a fast-moving electron): little mass; stops ~1 cm into body In b-decay, the effect is that a n 0 is converted into a p+, ejecting an e– from the nucleus. 1 0 n 1 0 1 p + – 1 e NOTE: There are no e– in the nucleus. The ejected e– is formed when energy released from the E = mc 2 nucleus “congeals” into mass, via _______.

gamma radiation: consists of high-energy photons -- can penetrate to internal organs -- gamma ray: 0 g (or just g) 0 emitted when nucleons rearrange into a more stable configuration -- gamma radiation often accompanies other nuclear decays 234 92 U 230 4 0 90 Th + 2 He + 2 0 g

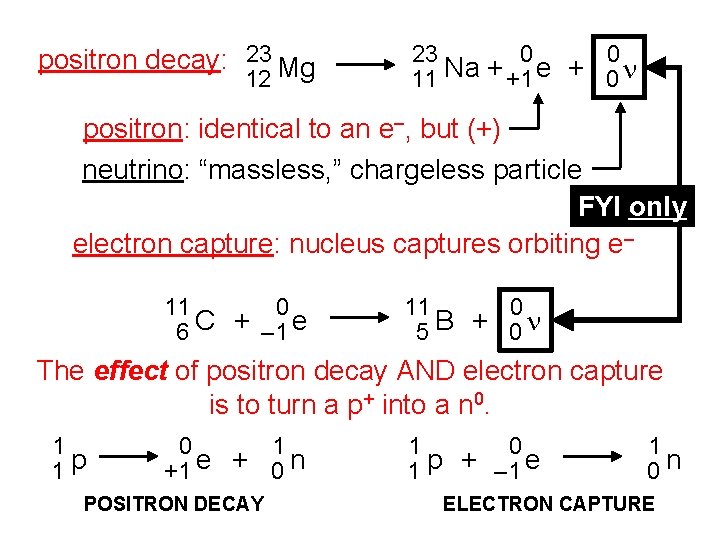
positron decay: 23 Mg 12 23 0 0 11 Na + +1 e + 0 n positron: identical to an e–, but (+) neutrino: “massless, ” chargeless particle FYI only electron capture: nucleus captures orbiting e– 11 0 6 C + – 1 e 11 0 5 B + 0 n The effect of positron decay AND electron capture is to turn a p+ into a n 0. 1 1 p 0 1 +1 e + 0 n POSITRON DECAY 1 0 1 p + – 1 e 1 0 n ELECTRON CAPTURE

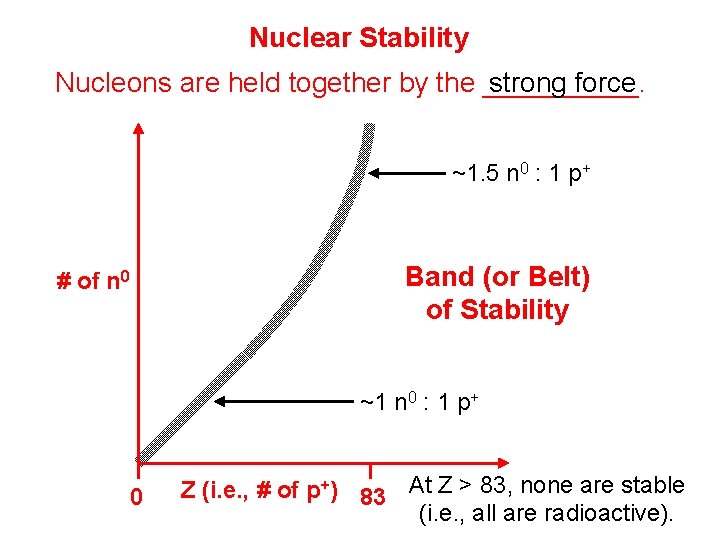
Nuclear Stability strong force Nucleons are held together by the _____. ~1. 5 n 0 : 1 p+ Band (or Belt) of Stability # of n 0 ~1 n 0 : 1 p+ 0 Z (i. e. , # of p+) 83 At Z > 83, none are stable (i. e. , all are radioactive).

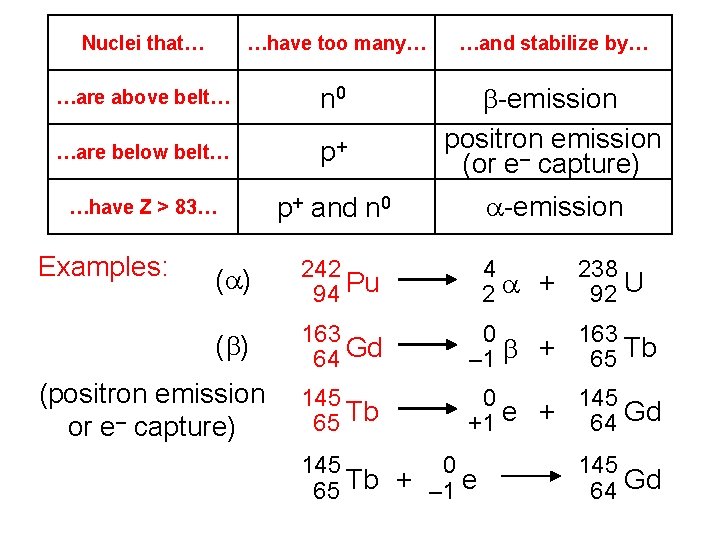
Nuclei that… …have too many… …and stabilize by… …are above belt… n 0 …are below belt… p+ b-emission positron emission (or e– capture) …have Z > 83… p+ and n 0 a-emission Examples: (a) 242 94 Pu 4 238 2 a + 92 U (b) 163 64 Gd 0 163 – 1 b + 65 Tb 145 65 Tb 0 145 +1 e + 64 Gd (positron emission or e– capture) 145 0 65 Tb + – 1 e 145 64 Gd

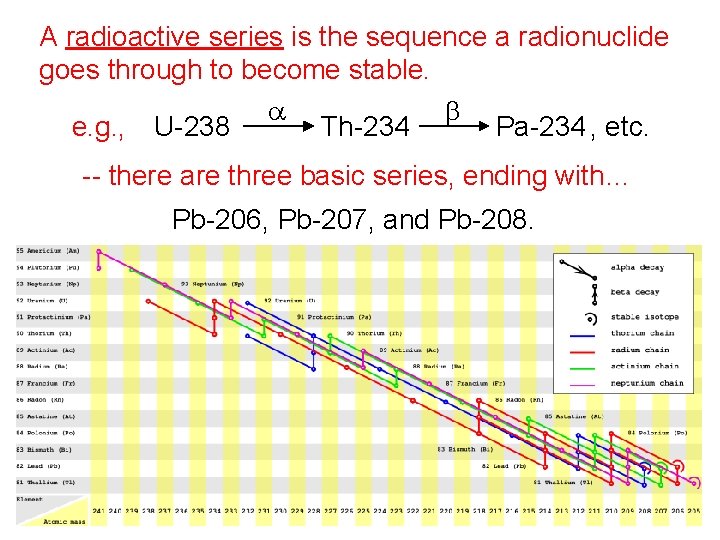
A radioactive series is the sequence a radionuclide goes through to become stable. a b e. g. , U-238 Th-234 Pa-234 , etc. -- there are three basic series, ending with… Pb-206, Pb-207, and Pb-208.

Also, nuclei having “magic numbers” of p+ or n 0 tend to be more stable than those that don’t. p+: 2, 8, 20, 28, 50, 82 n 0: 2, 8, 20, 28, 50, 82, 126 The shell model of the nucleus, which says that the nucleons reside in shells, has been proposed to explain these observations. This theory is analogous to the “shells of e–s” theory. Finally, nuclides with an even number of p+ AND an even number of n 0 tend to be stable.