New Drug Update 2016 Joe Strain Pharm D
















































- Slides: 93

New Drug Update 2016 Joe Strain, Pharm. D. SDSU College of Pharmacy Rapid City Regional Hospital

Learning Objectives § Upon successful completion of this activity, the audience should be able to: Ø Identify therapeutic indications of drugs Ø Ø recently approved by the FDA. Discuss pharmacological properties of the new medications List side effects, warnings, precautions and significant drug interactions associated with each medication. Identify the normal dose and dosage forms of the drugs presented. Describe limitations to implementing the new medications into clinical practice

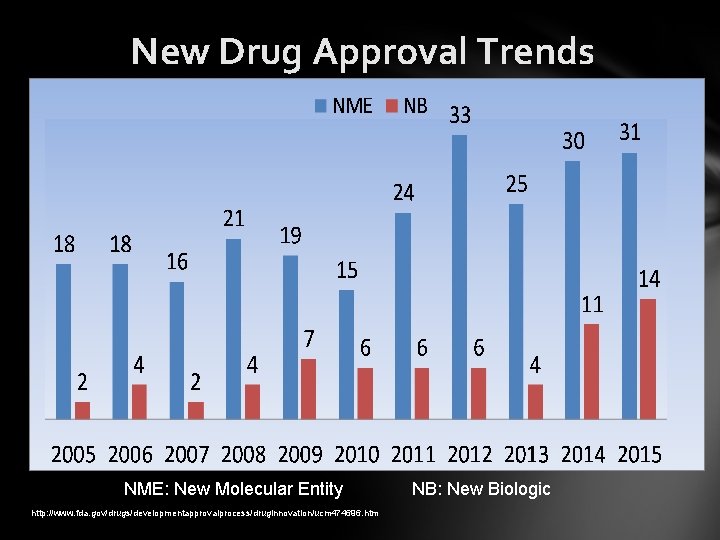
New Drug Approval Trends NME: New Molecular Entity http: //www. fda. gov/drugs/developmentapprovalprocess/druginnovation/ucm 474696. htm NB: New Biologic

Agenda § § § Edoxaban (Savaysa™) Idarucizumab (Praxbind®) Sacubitril/valsartan (Entresto™) Alirocumab (Praluent®) Evolocumab (Repatha™) Flibanserin (Addyi™) Insulin degludec (Tresiba™) Naloxegol (Movantik™) Eluxadoline (Viberzi™) Hepatitis C drug updates Ceftazidime/avibactam (Avycaz®) Ceftolozane/tazobactam (Zerbaxa™)

Edoxaban (Savaysa™) § Indication Ø Reduce stroke and systemic embolism in non-valvular atrial fibrillation Ø Treatment of DVT & PE AFTER 5 -10 days of a parenteral anticoagulant § Pharmacology Ø Factor Xa inhibitor Savaysa PI 2015.

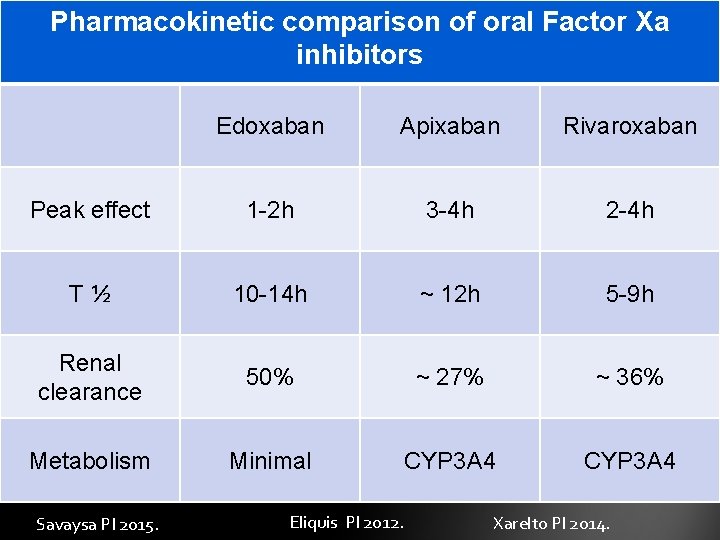
Pharmacokinetic comparison of oral Factor Xa inhibitors Edoxaban Apixaban Rivaroxaban Peak effect 1 -2 h 3 -4 h 2 -4 h T½ 10 -14 h ~ 12 h 5 -9 h Renal clearance 50% ~ 27% ~ 36% Metabolism Minimal CYP 3 A 4 Savaysa PI 2015. Eliquis PI 2012. Xarelto PI 2014.

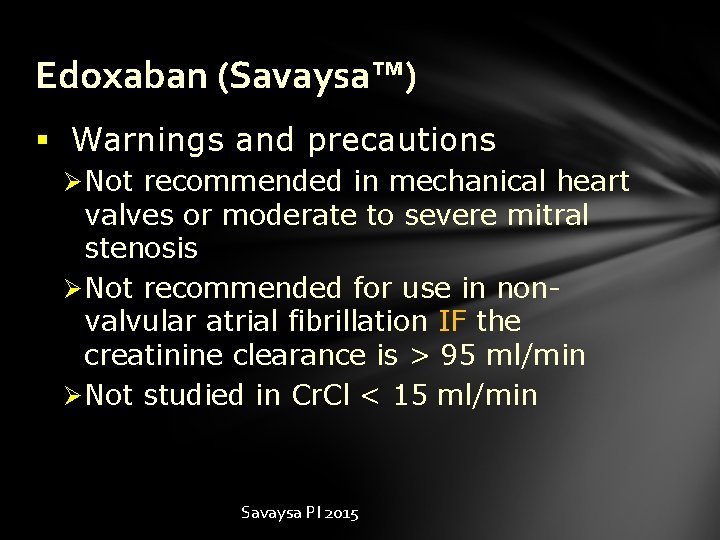
Edoxaban (Savaysa™) § Warnings and precautions Ø Not recommended in mechanical heart valves or moderate to severe mitral stenosis Ø Not recommended for use in nonvalvular atrial fibrillation IF the creatinine clearance is > 95 ml/min Ø Not studied in Cr. Cl < 15 ml/min Savaysa PI 2015

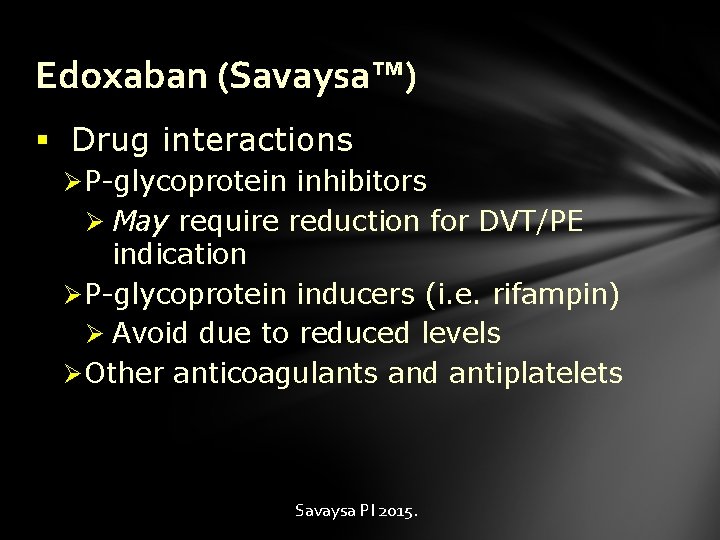
Edoxaban (Savaysa™) § Drug interactions Ø P-glycoprotein inhibitors Ø May require reduction for DVT/PE indication Ø P-glycoprotein inducers (i. e. rifampin) Ø Avoid due to reduced levels Ø Other anticoagulants and antiplatelets Savaysa PI 2015.

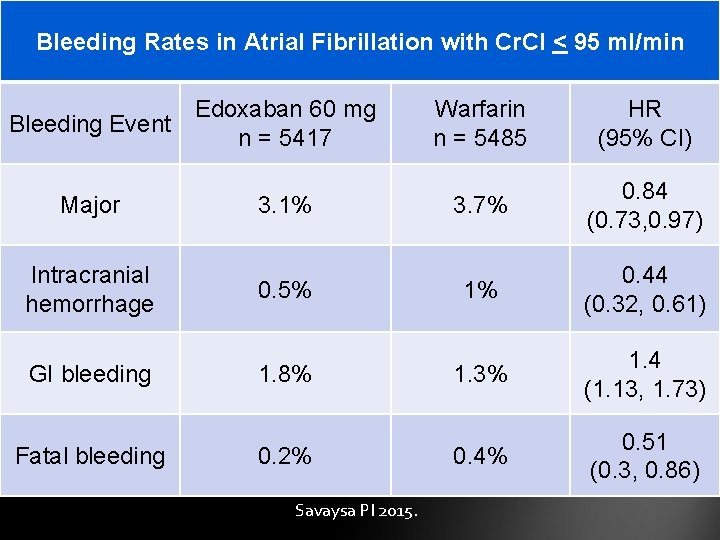
Bleeding Rates in Atrial Fibrillation with Cr. Cl < 95 ml/min Bleeding Event Major Intracranial hemorrhage GI bleeding Fatal bleeding Edoxaban 60 mg n = 5417 3. 1% 0. 5% 1. 8% 0. 2% Savaysa PI 2015. Warfarin n = 5485 HR (95% CI) 3. 7% 0. 84 (0. 73, 0. 97) 1% 0. 44 (0. 32, 0. 61) 1. 3% 1. 4 (1. 13, 1. 73) 0. 4% 0. 51 (0. 3, 0. 86)

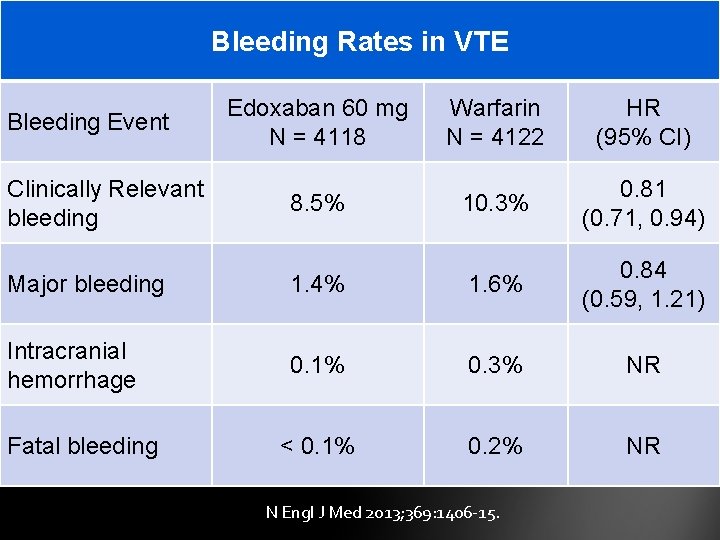
Bleeding Rates in VTE Bleeding Event Clinically Relevant bleeding Edoxaban 60 mg N = 4118 Warfarin N = 4122 HR (95% CI) 8. 5% 10. 3% 0. 81 (0. 71, 0. 94) Major bleeding 1. 4% 1. 6% 0. 84 (0. 59, 1. 21) Intracranial hemorrhage 0. 1% 0. 3% NR < 0. 1% 0. 2% NR Fatal bleeding N Engl J Med 2013; 369: 1406 -15.

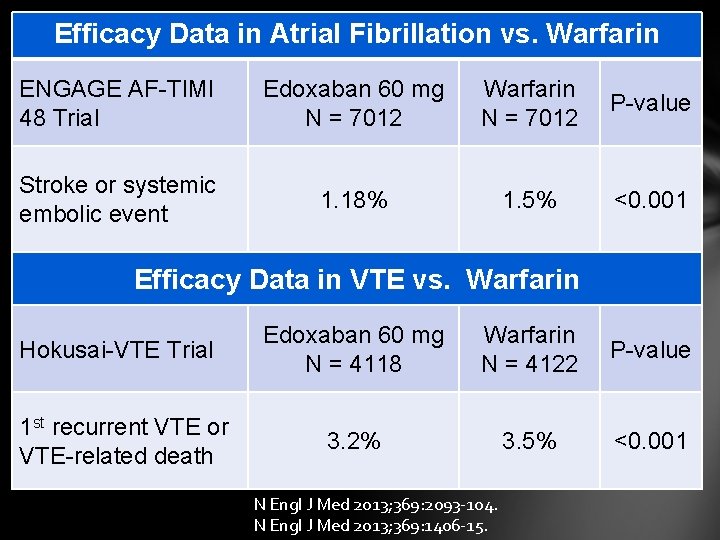
Efficacy Data in Atrial Fibrillation vs. Warfarin ENGAGE AF-TIMI 48 Trial Edoxaban 60 mg N = 7012 Warfarin N = 7012 P-value Stroke or systemic embolic event 1. 18% 1. 5% <0. 001 Efficacy Data in VTE vs. Warfarin Hokusai-VTE Trial 1 st recurrent VTE or VTE-related death Edoxaban 60 mg N = 4118 Warfarin N = 4122 P-value 3. 2% 3. 5% <0. 001 N Engl J Med 2013; 369: 2093 -104. N Engl J Med 2013; 369: 1406 -15.

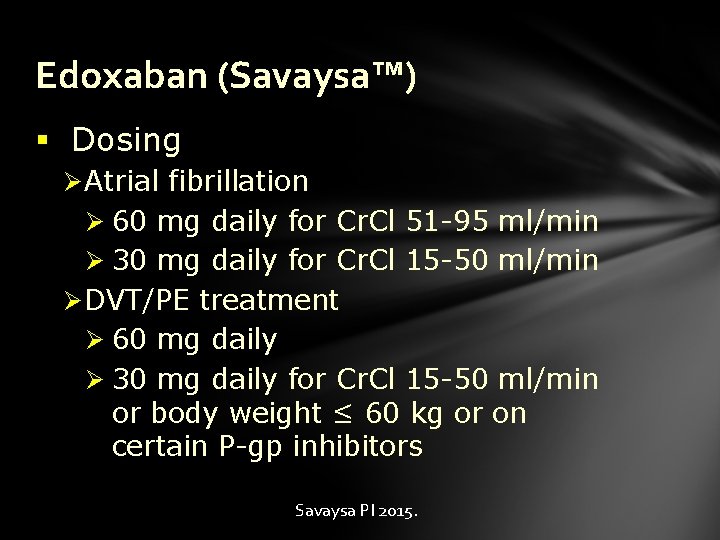
Edoxaban (Savaysa™) § Dosing Ø Atrial fibrillation Ø 60 mg daily for Cr. Cl 51 -95 ml/min Ø 30 mg daily for Cr. Cl 15 -50 ml/min Ø DVT/PE treatment Ø 60 mg daily Ø 30 mg daily for Cr. Cl 15 -50 ml/min or body weight ≤ 60 kg or on certain P-gp inhibitors Savaysa PI 2015.

Edoxaban (Savaysa™) § Transitioning to edoxaban: Ø From warfarin Ø Start when INR < 2. 5 Ø From any other oral anticoagulant or a LMWH Ø Start at next scheduled dose Ø From heparin Ø Turn off heparin and start 4 hours later Savaysa PI 2015.

Edoxaban (Savaysa™) § Transitioning from edoxaban: Ø To warfarin Ø Start warfarin and taper edoxaban Ø If on 60 mg decrease to 30 mg Ø If on 30 mg decrease to 15 mg Ø Draw INR immediately prior to edoxaban dose--when INR is > 2 stop edoxaban Ø To any other oral agent, LMWH, or heparin Ø Start alternative agent at time of next scheduled edoxaban dose Savaysa PI 2015.

Edoxaban (Savaysa™) § Bottom line Ø Non-inferior to warfarin for stroke prevention in atrial fibrillation and for DVT/PE treatment Ø Use of heparin/LWMH for 5 -10 days prior to DVT/PE tx may limit use Ø Watch renal function—high and low! Ø No specific reversal agent…. yet…. § Additional review Ø Mekaj YH et al. Ther Clin Risk Manag 2015; 11: 967 -77.

Idarucizumab (Praxbind®) § Indication Ø Reversal of dabigatran Ø Emergency surgery/urgent procedures Ø Life-threatening or uncontrolled bleeding § Pharmacology Ø Humanized monoclonal antibody specific for binding dabigatran Praxbind PI 2015.

Idarucizumab (Praxbind®) § Pharmacokinetics Ø Onset is immediate Ø Effect usually sustained for 24 hours Ø Half-life Ø 47 minutes (initial) Ø 10. 3 hours (terminal) Ø Elimination Ø Urine (32% in first 6 hours) Ø Likely biodegradation Praxbind PI 2015.

Idarucizumab (Praxbind®) § Warnings and precautions Ø Thromboembolic risk Ø Re-elevation of coagulation parameters in 12 -24 hours Ø Hypersensitivity reactions Ø Sorbitol excipient § Drug interactions Ø None Praxbind PI 2015.

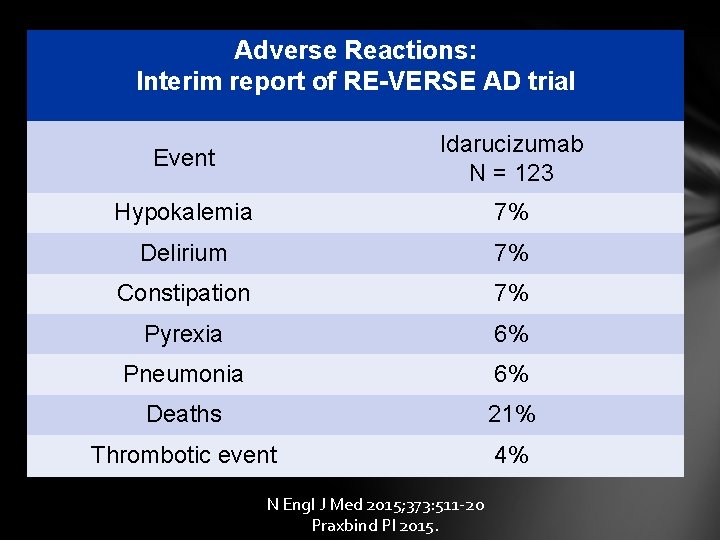
Adverse Reactions: Interim report of RE-VERSE AD trial Event Idarucizumab N = 123 Hypokalemia 7% Delirium 7% Constipation 7% Pyrexia 6% Pneumonia 6% Deaths 21% Thrombotic event 4% N Engl J Med 2015; 373: 511 -20 Praxbind PI 2015.

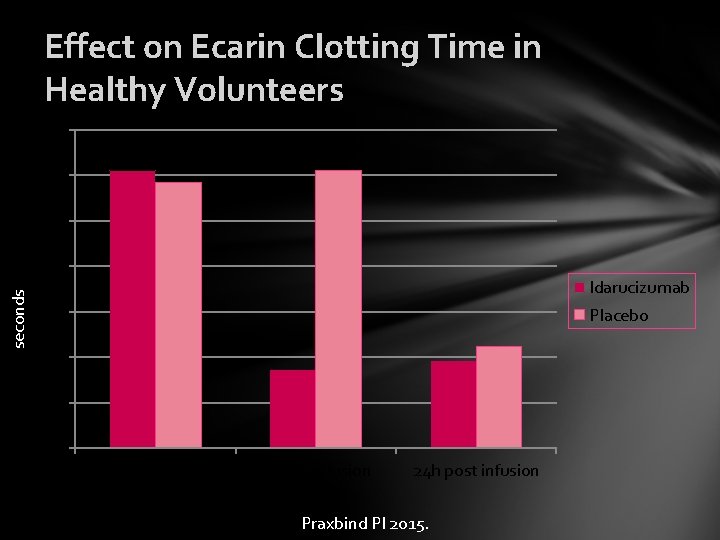
Effect on Ecarin Clotting Time in Healthy Volunteers 140 120 100 seconds 80 Idarucizumab 60 Placebo 40 20 0 Pre-infusion End of infusion 24 h post infusion Praxbind PI 2015.

Idarucizumab (Praxbind®) § Dosing Ø 5 grams Ø Available in 2. 5 g/50 ml vials Ø Give as two consecutive doses Ø Infuse both vials or give as 2 injections Ø Must administer within 1 hour of removing from the vial Ø May restart dabigatran in 24 hours if indicated Praxbind PI 2015.

Idarucizumab (Praxbind®) § Bottom line Ø Effective reversal agent for dabigatran Ø Usually will be a one time dose Ø Will this increase the comfort level for providers using dabigatran? Ø Anti-Xa reversal agent expected soon § Additional review Ø Mo Y, Yam F. Pharmacotherapy 2015; 35: 198 -207.

Sacubitril/valsartan (Entresto™) § Indication Ø NYHA Class II-IV to reduce risk of CV death and heart failure hospitalization § Pharmacology Ø Neprilysin inhibitor Ø Blocks the break down of natriuretic peptides Entresto PI 2015

Sacubitril/valsartan (Entresto™) § Pharmacokinetics Ø Peaks in ~ 2 hours Ø T ½ ~ 10 hours Ø Valsartan has a higher bioavailability in Entresto vs. other formulations Ø Sacubitril rapidly metabolized by esterases to active metabolite LBQ 657 Ø Majority excreted via kidneys Entresto PI 2015

Sacubitril/valsartan (Entresto™) § Contraindications Ø History of angioedema due to ACEI or ARB Ø Concomitant use with an ACEI Ø Avoid within 36 hours Ø Concomitant use with aliskiren in diabetics § Warnings and precautions Ø Hyperkalemia Ø Pregnancy Ø Hypotension Entresto PI 2015

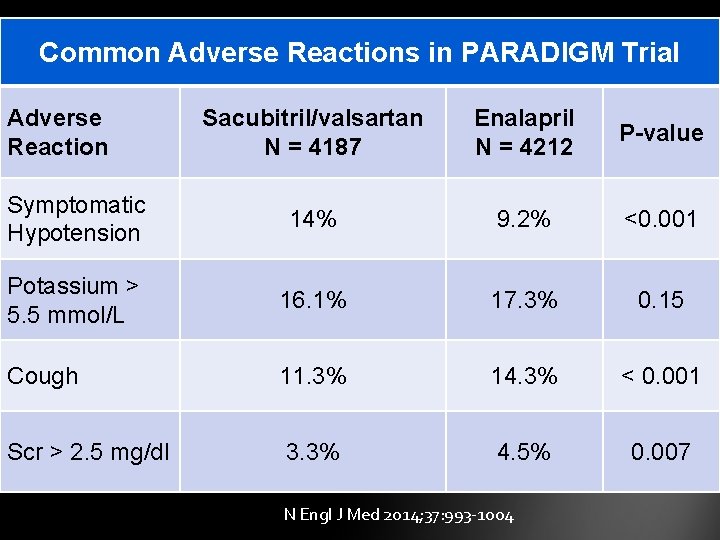
Common Adverse Reactions in PARADIGM Trial Adverse Reaction Sacubitril/valsartan N = 4187 Enalapril N = 4212 P-value Symptomatic Hypotension 14% 9. 2% <0. 001 Potassium > 5. 5 mmol/L 16. 1% 17. 3% 0. 15 Cough 11. 3% 14. 3% < 0. 001 Scr > 2. 5 mg/dl 3. 3% 4. 5% 0. 007 N Engl J Med 2014; 37: 993 -1004

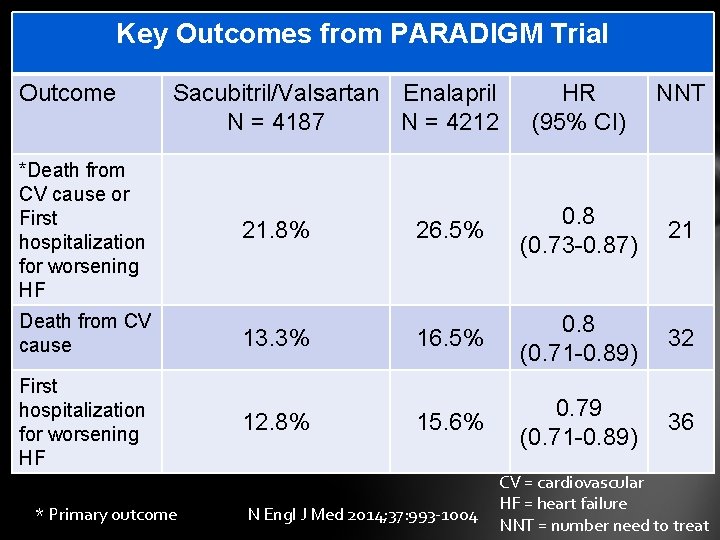
Key Outcomes from PARADIGM Trial Outcome Sacubitril/Valsartan Enalapril N = 4187 N = 4212 *Death from CV cause or First hospitalization for worsening HF 21. 8% HR (95% CI) 26. 5% 0. 8 (0. 73 -0. 87) 21 32 36 Death from CV cause 13. 3% 16. 5% 0. 8 (0. 71 -0. 89) First hospitalization for worsening HF 12. 8% 15. 6% 0. 79 (0. 71 -0. 89) * Primary outcome NNT N Engl J Med 2014; 37: 993 -1004 CV = cardiovascular HF = heart failure NNT = number need to treat

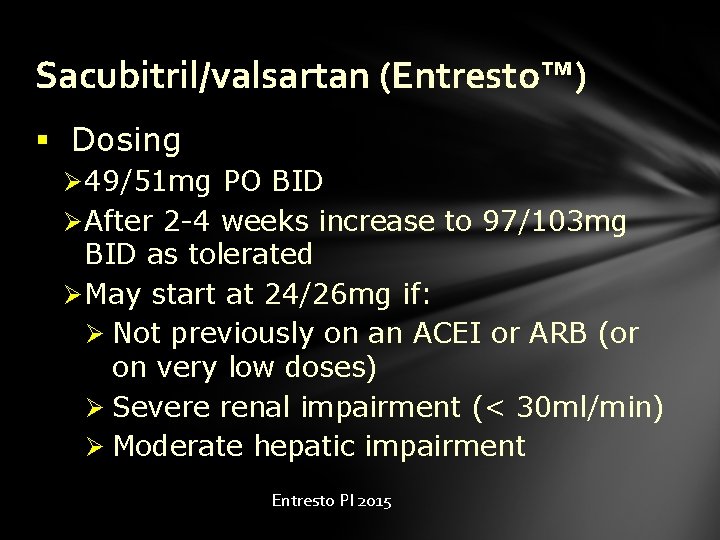
Sacubitril/valsartan (Entresto™) § Dosing Ø 49/51 mg PO BID Ø After 2 -4 weeks increase to 97/103 mg BID as tolerated Ø May start at 24/26 mg if: Ø Not previously on an ACEI or ARB (or on very low doses) Ø Severe renal impairment (< 30 ml/min) Ø Moderate hepatic impairment Entresto PI 2015

Sacubitril/valsartan (Entresto™) § Availability Ø Tablets Ø 24 -26 mg Ø 49 -51 mg Ø 97 -103 mg § Cost Ø ~ $375 for #60 tabs Entresto PI 2015

Sacubitril/valsartan (Entresto™) § Bottom line Ø Impressive trial results Ø Potential to become new standard for HF patients but some criticize trial design Ø On-going trials will further define role Ø Long-term safety? Ø Do not use with an ACE inhibitor § Additional review Ø Singh JS, Land CC. Vasc Health Risk Manag. 2015; 11: 283 -95.

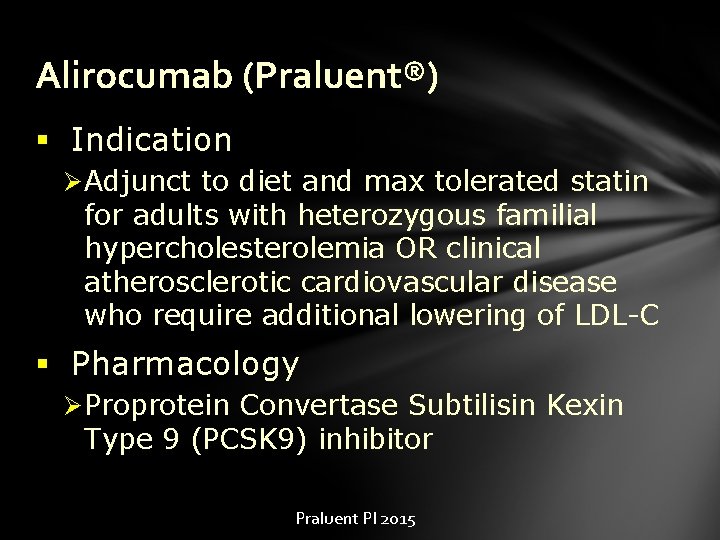
Alirocumab (Praluent®) § Indication Ø Adjunct to diet and max tolerated statin for adults with heterozygous familial hypercholesterolemia OR clinical atherosclerotic cardiovascular disease who require additional lowering of LDL-C § Pharmacology Ø Proprotein Convertase Subtilisin Kexin Type 9 (PCSK 9) inhibitor Praluent PI 2015

Alirocumab (Praluent®) § Pharmacokinetics Ø Tmax 3 -7 days Ø Max PCSK 9 inhibition in 4 -9 hours Ø LDL nadir in ~ 15 days Ø No data in severe renal or hepatic impairment Praluent PI 2015

Alirocumab (Praluent®) § Contraindication/warnings/precautions Ø Hypersensitivity reactions Ø Some have required hospitalization § Drug interactions Ø None identified Praluent PI 2015

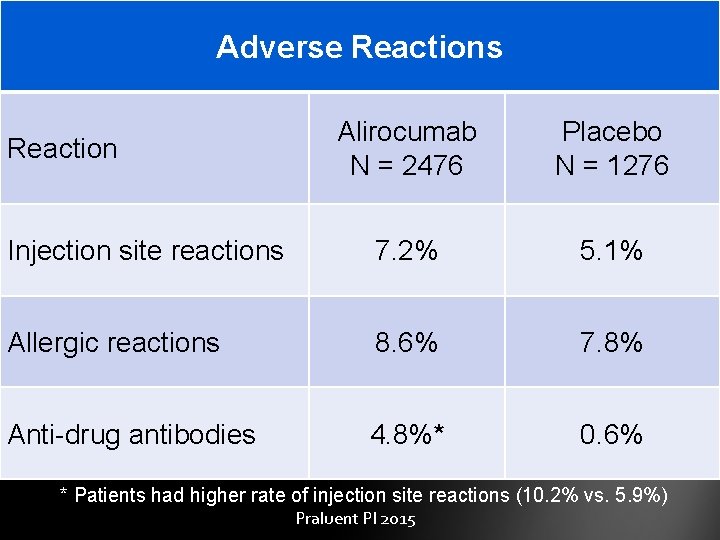
Adverse Reactions Alirocumab N = 2476 Placebo N = 1276 Injection site reactions 7. 2% 5. 1% Allergic reactions 8. 6% 7. 8% Anti-drug antibodies 4. 8%* 0. 6% Reaction * Patients had higher rate of injection site reactions (10. 2% vs. 5. 9%) Praluent PI 2015

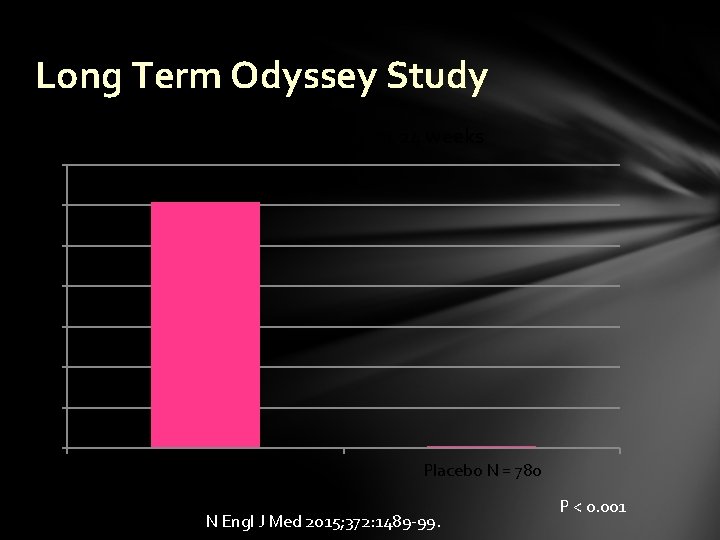
Long Term Odyssey Study % reduction in LDL-C at 24 weeks 70 60 50 40 30 20 10 0 Alirocumab N = 1530 Placebo N = 780 N Engl J Med 2015; 372: 1489 -99. P < 0. 001

Alirocumab (Praluent®) § Dosing Ø 75 mg SQ every 2 weeks Ø If inadequate response after 4 -8 weeks may increase to 150 mg SQ every 2 weeks § Cost Ø ~ $14, 600 per year Praluent PI 2015

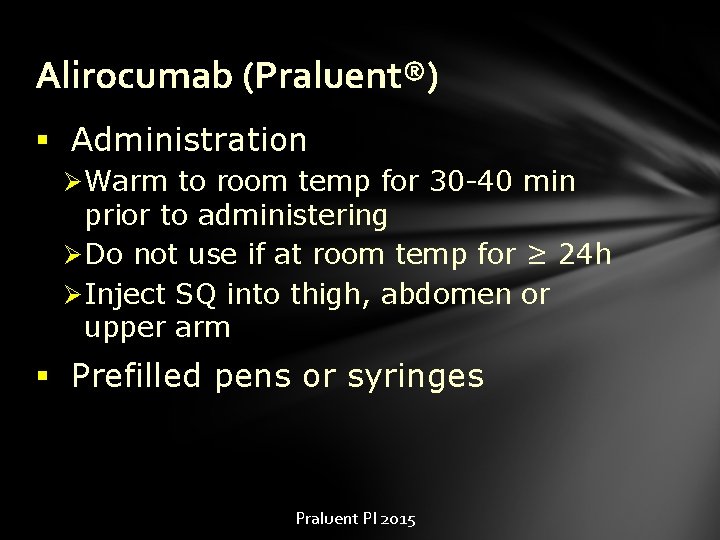
Alirocumab (Praluent®) § Administration Ø Warm to room temp for 30 -40 min prior to administering Ø Do not use if at room temp for ≥ 24 h Ø Inject SQ into thigh, abdomen or upper arm § Prefilled pens or syringes Praluent PI 2015

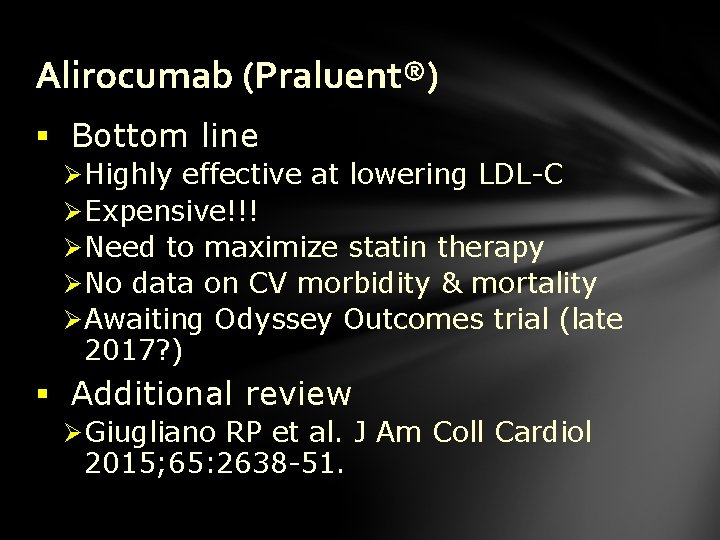
Alirocumab (Praluent®) § Bottom line Ø Highly effective at lowering LDL-C Ø Expensive!!! Ø Need to maximize statin therapy Ø No data on CV morbidity & mortality Ø Awaiting Odyssey Outcomes trial (late 2017? ) § Additional review Ø Giugliano RP et al. J Am Coll Cardiol 2015; 65: 2638 -51.

Evolocumab (Repatha™) § Indication Ø Adjunct to diet and max tolerated statin for adults with homozygous or heterozygous familial hypercholesterolemia OR clinical atherosclerotic cardiovascular disease who require additional lowering of LDL -C § Pharmacology Ø PCSK 9 inhibitor Repatha PI 2015

Evolocumab (Repatha™) § Dosing Ø 140 mg SQ every 2 weeks Ø 420 mg SQ once monthly § Cost Ø Estimated at ~ $14, 100 per year § Fourier Trial is evaluating mortality Ø Expected results in 2018 Repatha PI 2015 https: //www. clinicaltrials. gov/ct 2/show/NCT 01764633? term=evolocumab&rank=18

Flibanserin (Addyi™) § Indication Ø Hypoactive sexual desire disorder (HSDD) in premenopausal women § Pharmacology Ø Unknown mechanism for HSDD Ø 5 -HT 1 A agonist Ø 5 -HT 2 A, 5 HT 2 B, 5 -HT 2 C, D 4 antagonist Addyi PI 2015

Flibanserin (Addyi™) § Contraindications Ø Alcohol Ø 17 -25% incidence of hypotension or syncope Ø Moderate to strong CYP 3 A 4 inhibitors Ø Hepatic impairment (4 subjects) Ø increased 4. 5 x in mild impairment Ø T ½ extended from 10 h to 26 h § Warnings & precautions Ø Hypotension & syncope Ø Somnolence & sedation Addyi PI 2015

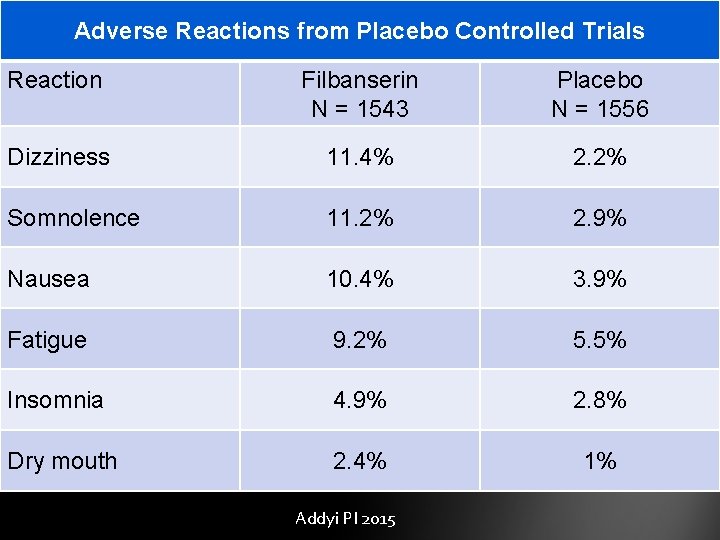
Adverse Reactions from Placebo Controlled Trials Reaction Filbanserin N = 1543 Placebo N = 1556 Dizziness 11. 4% 2. 2% Somnolence 11. 2% 2. 9% Nausea 10. 4% 3. 9% Fatigue 9. 2% 5. 5% Insomnia 4. 9% 2. 8% Dry mouth 2. 4% 1% Addyi PI 2015

Flibanserin (Addyi™) § Clinical efficacy Ø Three phase 3, 24 -week trials; 2375 women Ø Increased the number of “satisfying sexual events” per 28 days by 0. 5 -1 over placebo Ø In two studies the co-primary endpoint (sexual desire) was not significantly better than placebo Addyi PI 2015

Flibanserin (Addyi™) § Dosing Ø 100 mg at night Ø May cause drowsiness Ø Stop after 8 weeks if no improvement § Prescribers and pharmacies must be certified through the Addyi REMS program § www. addyirems. com Addyi PI 2015

Flibanserin (Addyi™) § Bottom line Ø First drug approved for HSDD Ø Controversial approval Ø Watch for drug interactions and remind patients to avoid alcohol § Additional review Ø Gellad WF, et al. JAMA; published on- line July 6, 2015.

Insulin degludec (Tresiba™) § Indication Ø Adults with diabetes mellitus § Pharmacology Ø “Ultra long-acting” Ø Forms soluble, stable, multi-hexamers Ø Insulin slowly, and continuously absorbed Tresiba PI 2015 Pharmacotherapy 2014; 34: 291 -302.

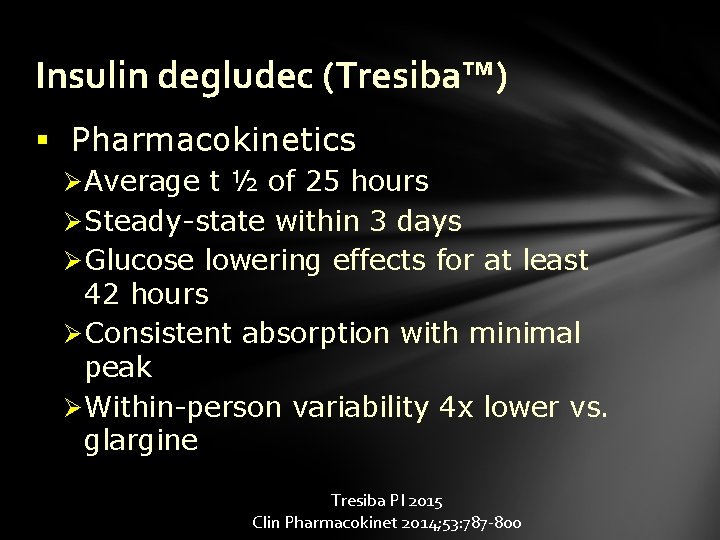
Insulin degludec (Tresiba™) § Pharmacokinetics Ø Average t ½ of 25 hours Ø Steady-state within 3 days Ø Glucose lowering effects for at least 42 hours Ø Consistent absorption with minimal peak Ø Within-person variability 4 x lower vs. glargine Tresiba PI 2015 Clin Pharmacokinet 2014; 53: 787 -800

Proposed Clinical Advantages § More flexible dosing regimens Ø Improved compliance § Flatter glucose-lowering profile Ø Less hypoglycemia Ø Less nocturnal hypoglycemia Ø Tighter glycemic control Pharmacotherapy 2014; 34: 291 -302.

Insulin degludec (Tresiba™) § Dosing Ø Once daily at any time Ø At least 8 hours between doses Ø Abdomen, upper arm or thigh Ø Rotating sites Ø Dose titrations every 3 -4 days § Dose conversion Ø 1: 1 conversion from other long-acting insulin Tresiba PI 2015

Insulin degludec (Tresiba™) § Dosing forms Ø Flex. Touch Pens (3 ml) Ø U-100 (max of 80 units/dose) Ø U-200 (max of 160 units/dose) § Combined with insulin aspart (Ryzodeg®) Ø 70/30 Ø Availability? Tresiba PI 2015

Insulin degludec (Tresiba™) § Bottom line Ø Long-acting insulin Ø Once-daily, anytime dosing Ø Will be available with aspart § Additional review Ø Drab S, Philis-Tsimikas A. Pharmacotherapy 2014; 34: 291 -302.

Sugammadex (Bridion®) § Indication Ø Reversal of rocuronium and vecuronium § Pharmacology Ø Gamma-cyclodextrin Ø 8 sugar ring with negatively charged side chains Ø Binds free rocuronium & vecuronium in plasma �remaining NMBA shifts from NMJ back to plasma Bridion PI 2015

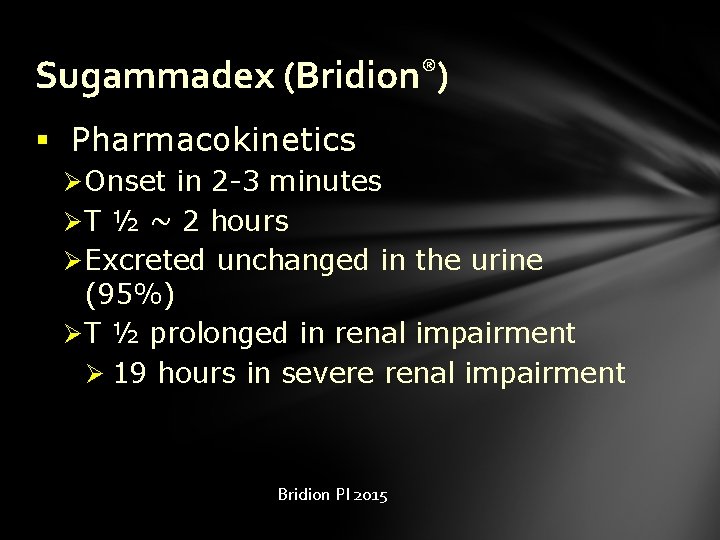
Sugammadex (Bridion®) § Pharmacokinetics Ø Onset in 2 -3 minutes Ø T ½ ~ 2 hours Ø Excreted unchanged in the urine (95%) Ø T ½ prolonged in renal impairment Ø 19 hours in severe renal impairment Bridion PI 2015

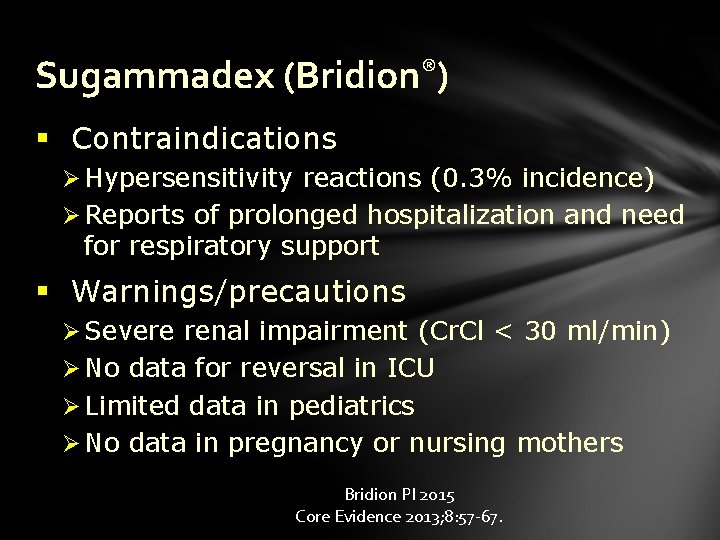
Sugammadex (Bridion®) § Contraindications Ø Hypersensitivity reactions (0. 3% incidence) Ø Reports of prolonged hospitalization and need for respiratory support § Warnings/precautions Ø Severe renal impairment (Cr. Cl < 30 ml/min) Ø No data for reversal in ICU Ø Limited data in pediatrics Ø No data in pregnancy or nursing mothers Bridion PI 2015 Core Evidence 2013; 8: 57 -67.

Sugammadex (Bridion®) § Warnings/precautions Ø Bradycardia �cardiac arrest within minutes of administration Ø Treat with atropine § Drug interactions Ø Progestin-containing contraceptives Ø Must use alternative contraception for 7 days Bridion PI 2015

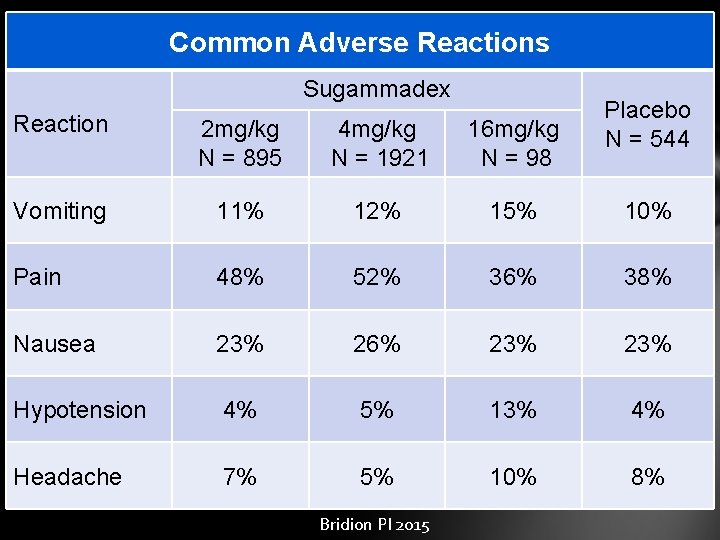
Common Adverse Reactions Sugammadex Placebo N = 544 Reaction 2 mg/kg N = 895 4 mg/kg N = 1921 16 mg/kg N = 98 Vomiting 11% 12% 15% 10% Pain 48% 52% 36% 38% Nausea 23% 26% 23% Hypotension 4% 5% 13% 4% Headache 7% 5% 10% 8% Bridion PI 2015

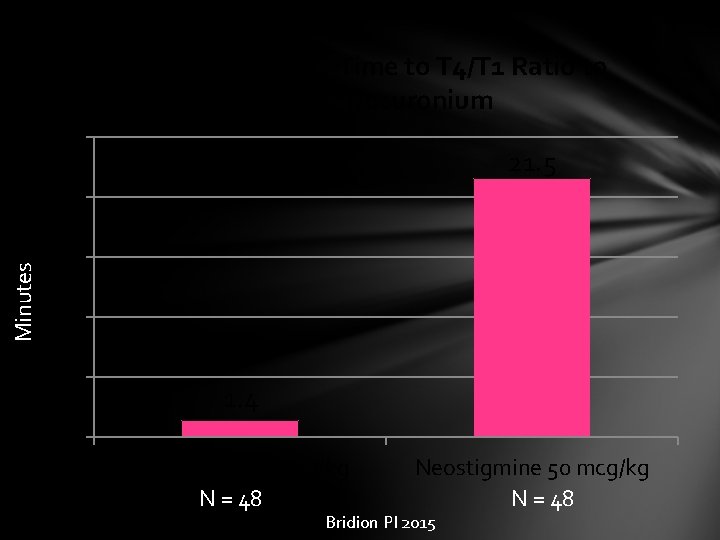
Median Recovery Time to T 4/T 1 Ratio to 0. 9 after Rocuronium 25 21. 5 Minutes 20 15 10 5 1. 4 0 Sugammadex 2 mg/kg N = 48 Neostigmine 50 mcg/kg N = 48 Bridion PI 2015

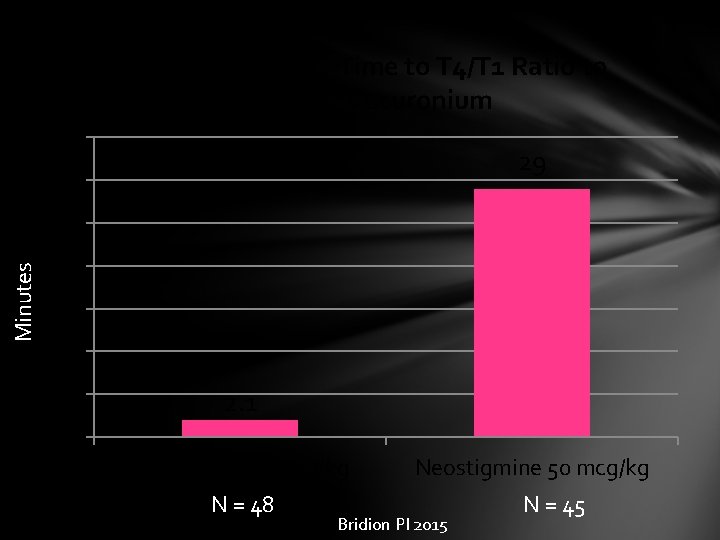
Median Recovery Time to T 4/T 1 Ratio to 0. 9 after Vecuronium 35 29 30 Minutes 25 20 15 10 5 2. 1 0 Sugammadex 2 mg/kg N = 48 Neostigmine 50 mcg/kg Bridion PI 2015 N = 45

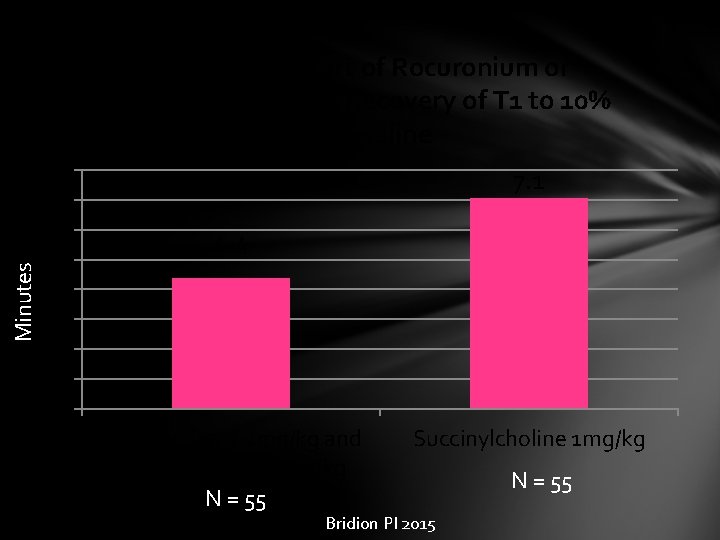
Minutes Time from Start of Rocuronium or Succinylcholine to Recovery of T 1 to 10% of Baseline 8 7 6 5 4 3 2 1 0 7. 1 4. 4 Rocuronium 1. 2 mg/kg and sugammadex 16 mg/kg N = 55 Succinylcholine 1 mg/kg Bridion PI 2015 N = 55

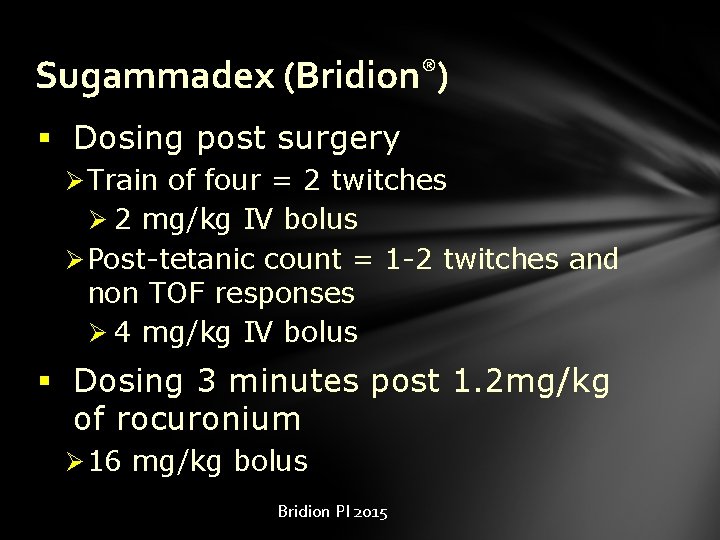
Sugammadex (Bridion®) § Dosing post surgery Ø Train of four = 2 twitches Ø 2 mg/kg IV bolus Ø Post-tetanic count = 1 -2 twitches and non TOF responses Ø 4 mg/kg IV bolus § Dosing 3 minutes post 1. 2 mg/kg of rocuronium Ø 16 mg/kg bolus Bridion PI 2015

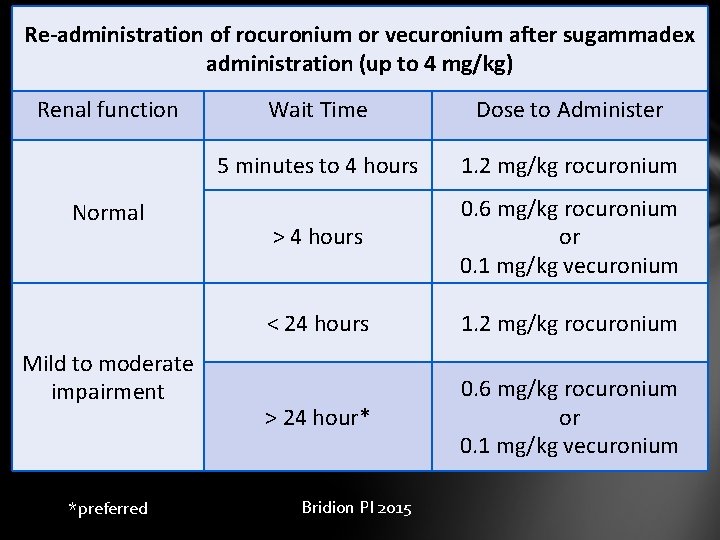
Re-administration of rocuronium or vecuronium after sugammadex administration (up to 4 mg/kg) Renal function Normal Mild to moderate impairment *preferred Wait Time Dose to Administer 5 minutes to 4 hours 1. 2 mg/kg rocuronium > 4 hours 0. 6 mg/kg rocuronium or 0. 1 mg/kg vecuronium < 24 hours 1. 2 mg/kg rocuronium > 24 hour* 0. 6 mg/kg rocuronium or 0. 1 mg/kg vecuronium Bridion PI 2015

Sugammadex (Bridion®) § Cost Ø 200 mg ~ $90 Ø 500 mg ~ $166 § Availability Ø 200 mg and 500 mg vials (100 mg/ml)

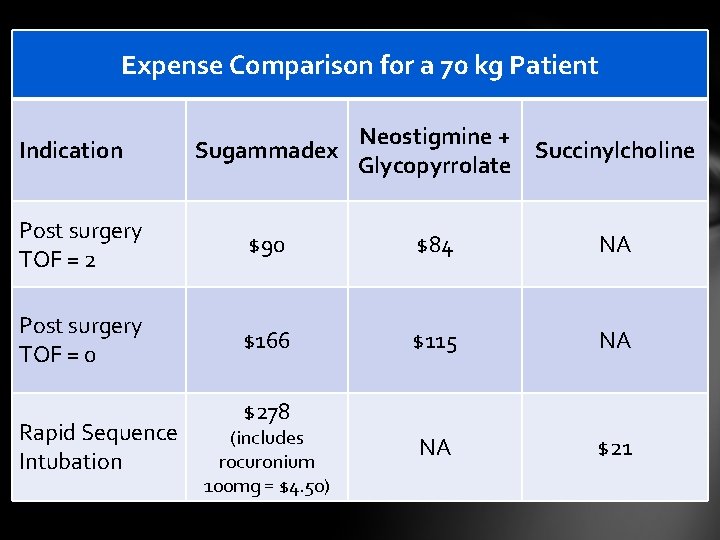
Expense Comparison for a 70 kg Patient Indication Neostigmine + Sugammadex Succinylcholine Glycopyrrolate Post surgery TOF = 2 $90 $84 NA Post surgery TOF = 0 $166 $115 NA NA $21 Rapid Sequence Intubation $278 (includes rocuronium 100 mg = $4. 50)

Sugammadex (Bridion®) § Bottom line Ø Fast, effective, safe reversal agent for rocuronium and vecuronium Ø Will NOT reverse other NMB’s Ø Watch for rare allergic reactions Ø ? Potential to replace succinylcholine § Additional review Ø Schaller SJ, Fink H. Core Evidence 2013; 8: 57 -67.

Naloxegol (Movantik™) § Indication Ø Treatment of opioid-induced constipation in adults with chronic noncancer pain Ø Pharmacology Ø Pegylated naloxone Ø Minimal CNS penetration Ø Displaces opioids from mu receptors in the GI tract Movantik PI 2015

Naloxegol (Movantik™) § Pharmacokinetics Ø Peaks within 2 hours Ø T ½ 6 -11 hours Ø CYP 3 A 4 metabolism Ø Renal excretion ~ 16% § Contraindications Ø GI obstruction Ø Strong CYP 3 A 4 inhibitors (i. e. ketoconazole or clarithromycin) Movantik PI 2015

Naloxegol (Movantik™) § Warnings and precautions Ø GI perforation Ø Opioid withdrawal Ø No data in severe hepatic impairment § Drug interactions Ø Avoid moderate CYP 3 A 4 inhibitors or reduce the dose (avoid grapefruit juice) Ø Avoid strong inducers of CYP 3 A 4 Movantik PI 2015

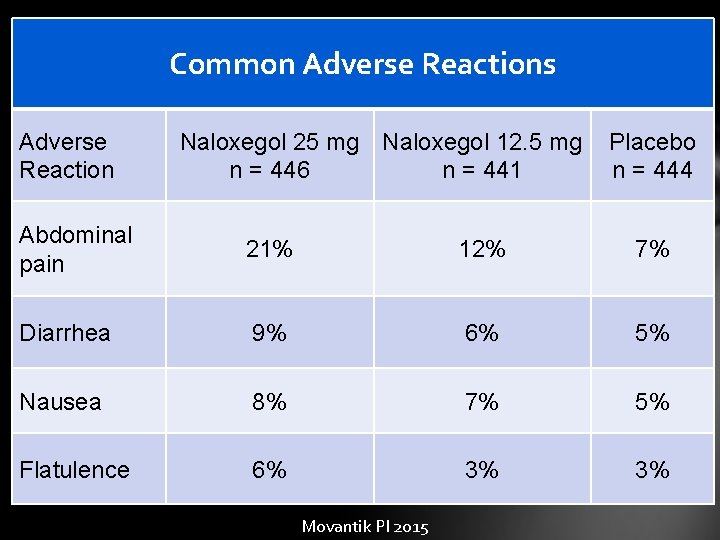
Common Adverse Reactions Adverse Reaction Naloxegol 25 mg Naloxegol 12. 5 mg n = 446 n = 441 Placebo n = 444 Abdominal pain 21% 12% 7% Diarrhea 9% 6% 5% Nausea 8% 7% 5% Flatulence 6% 3% 3% Movantik PI 2015

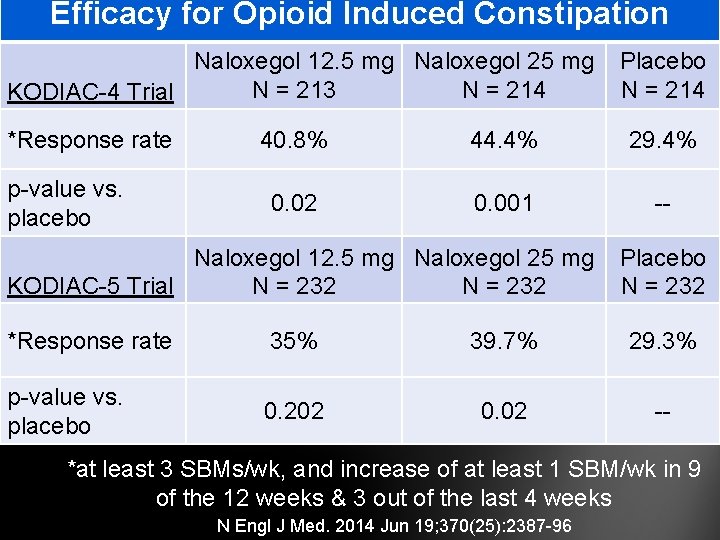
Efficacy for Opioid Induced Constipation Naloxegol 12. 5 mg Naloxegol 25 mg N = 213 N = 214 KODIAC-4 Trial *Response rate p-value vs. placebo Placebo N = 214 40. 8% 44. 4% 29. 4% 0. 02 0. 001 -- Naloxegol 12. 5 mg Naloxegol 25 mg KODIAC-5 Trial N = 232 Placebo N = 232 *Response rate 35% 39. 7% 29. 3% p-value vs. placebo 0. 202 0. 02 -- *at least 3 SBMs/wk, and increase of at least 1 SBM/wk in 9 of the 12 weeks & 3 out of the last 4 weeks N Engl J Med. 2014 Jun 19; 370(25): 2387 -96

Naloxegol (Movantik™) § Dosing Ø Normal renal function Ø 25 mg daily in the AM on an empty stomach Ø Cr. Cl < 60 ml/min Ø Start at 12. 5 mg daily in the AM on an empty stomach Ø Moderate CYP 3 A 4 inhibitors Ø 12. 5 mg daily in the AM on an empty stomach Ø Discontinue maintenance laxatives; may resume in 3 days Movantik PI 2015

Naloxegol (Movantik™) § Cost Ø #30 = ~$300 Ø Same price for 12. 5 mg or 25 mg Ø Advised not to split or crush Pharmacists Letter June 2015; 31.

Naloxegol (Movantik™) § Bottom line Ø Likely used when osmotic laxatives and stimulants not effective Ø No direct comparison vs. other agents Ø Stop laxatives when initiating therapy § Additional review Ø Poulsen JL, et al. Clin Exp Gastroenterol 2014; 7: 345 -58.

Eluxadoline (Viberzi™) § Indication Ø Treatment of irritable bowel syndrome with diarrhea (IBS-D) § Pharmacology Ø Mu-opioid receptor agonist Ø Delta-opioid receptor antagonist Viberzi PI 2015

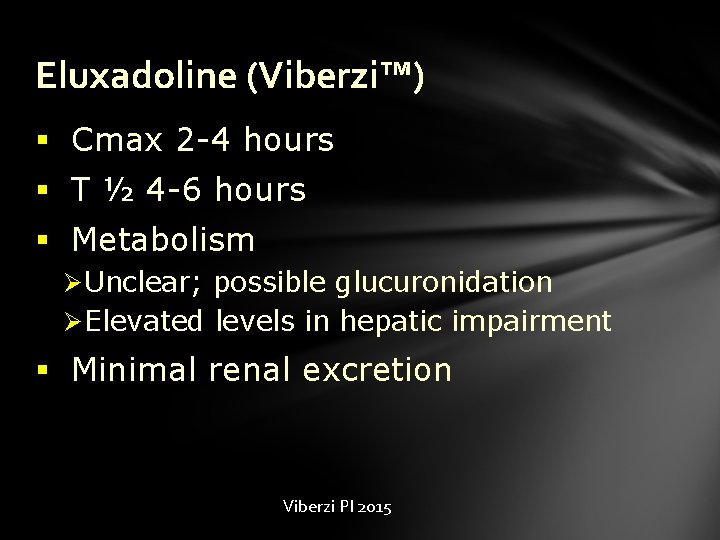
Eluxadoline (Viberzi™) § Cmax 2 -4 hours § T ½ 4 -6 hours § Metabolism Ø Unclear; possible glucuronidation Ø Elevated levels in hepatic impairment § Minimal renal excretion Viberzi PI 2015

Eluxadoline (Viberzi™) § Contraindications Ø Biliary obstruction, sphincter of Oddi dysfunction, Et. OH use, pancreatitis, severe liver impairment, history of chronic or severe constipation, GI obstruction § Warnings Ø Risk of pancreatitis & sphincter of Oddi spasm Viberzi PI 2015

Eluxadoline (Viberzi™) § Drug interactions Ø OATP 1 B 1 Inhibitors Ø Increase levels of eluxadoline Ø Cyclosporine, gemfibrozil, antiretrovirals Ø OATP 1 B 1 & BCRP substrates Ø Rosuvastatin levels may increase Ø Drugs causing constipation Ø Anticholinergics, opioids Viberzi PI 2015

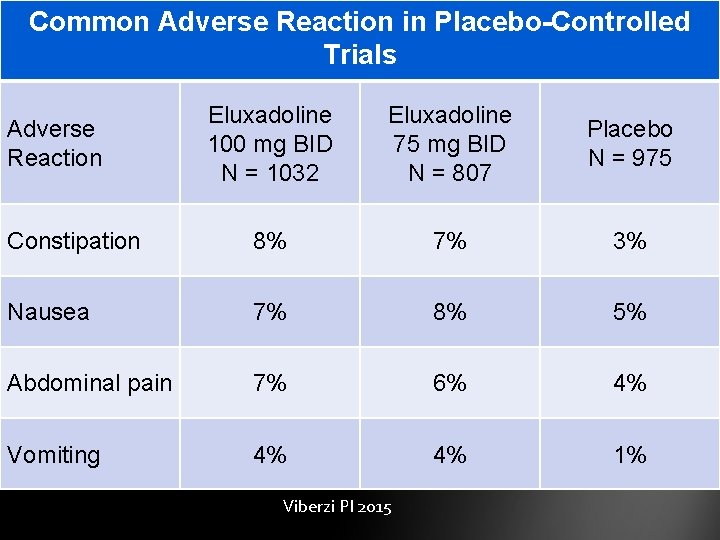
Common Adverse Reaction in Placebo-Controlled Trials Eluxadoline 100 mg BID N = 1032 Eluxadoline 75 mg BID N = 807 Placebo N = 975 Constipation 8% 7% 3% Nausea 7% 8% 5% Abdominal pain 7% 6% 4% Vomiting 4% 4% 1% Adverse Reaction Viberzi PI 2015

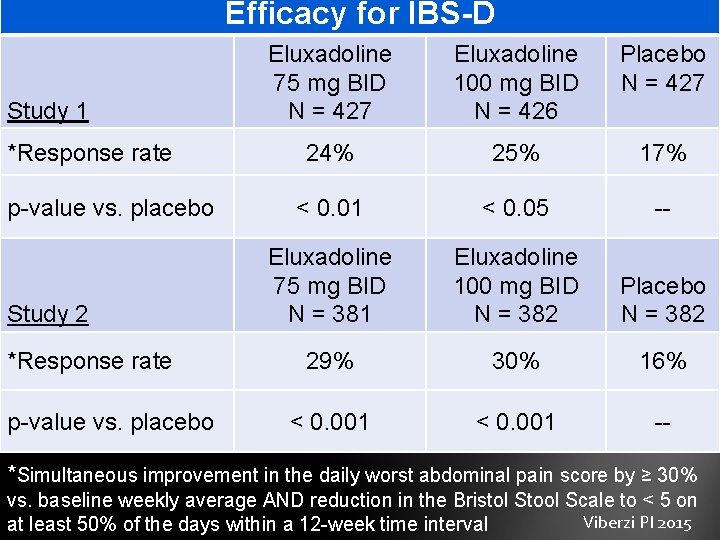
Efficacy for IBS-D Study 1 *Response rate p-value vs. placebo Study 2 *Response rate p-value vs. placebo Eluxadoline 75 mg BID N = 427 Eluxadoline 100 mg BID N = 426 Placebo N = 427 24% 25% 17% < 0. 01 < 0. 05 -- Eluxadoline 75 mg BID N = 381 Eluxadoline 100 mg BID N = 382 Placebo N = 382 29% 30% 16% < 0. 001 -- *Simultaneous improvement in the daily worst abdominal pain score by ≥ 30% vs. baseline weekly average AND reduction in the Bristol Stool Scale to < 5 on Viberzi PI 2015 at least 50% of the days within a 12 -week time interval

Eluxadoline (Viberzi™) § Dosing Ø 100 mg BID with food Ø 75 mg BID if: Ø No gallbladder Ø Mild-moderate hepatic impairment Ø Does not tolerate 100 mg BID Ø Taking a OATP 1 B 1 inhibitor Ø Discontinue if constipation develops and lasts for > 4 days Viberzi PI 2015

Eluxadoline (Viberzi™) § Bottom line Ø Another option for IBS-D patients Ø No active comparator trials Ø Opioid-agonist; Schedule IV controlled substance § Additional review Ø Garnock-Jones. Drugs; 75: 1305 -10. Viberzi PI 2015

Elbasvir/Grazoprevir (Zepatier®) § Indication Ø Genotype 1 or 4 Hepatitis C Ø With or without Ribavirin § Mechanism of action: Ø Elbasvir Ø HCV NS 5 A inhibitor Ø Grazoprevir Ø HCV NS 3/4 A protease inhibitor Zepatier [PI] 2016.

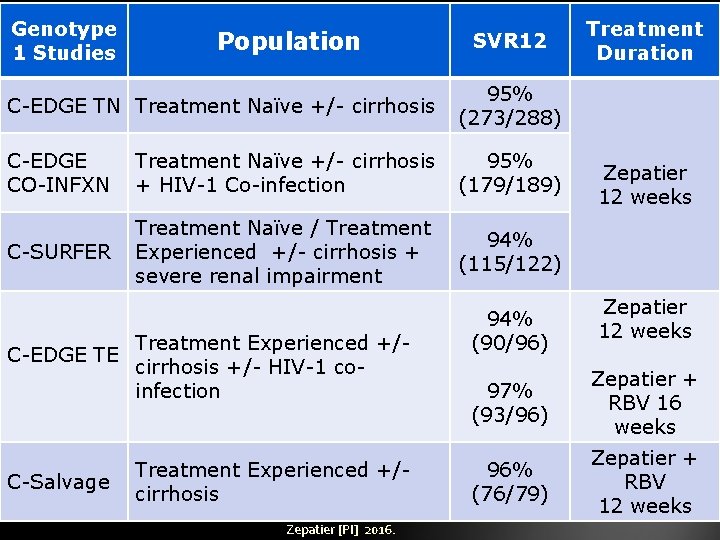
Genotype 1 Studies Population SVR 12 C-EDGE TN Treatment Naïve +/- cirrhosis 95% (273/288) C-EDGE CO-INFXN Treatment Naïve +/- cirrhosis + HIV-1 Co-infection 95% (179/189) C-SURFER Treatment Naïve / Treatment Experienced +/- cirrhosis + severe renal impairment 94% (115/122) C-EDGE TE C-Salvage Treatment Experienced +/cirrhosis +/- HIV-1 coinfection Treatment Experienced +/cirrhosis Zepatier [PI] 2016. 94% (90/96) Treatment Duration Zepatier 12 weeks 97% (93/96) Zepatier + RBV 16 weeks 96% (76/79) Zepatier + RBV 12 weeks

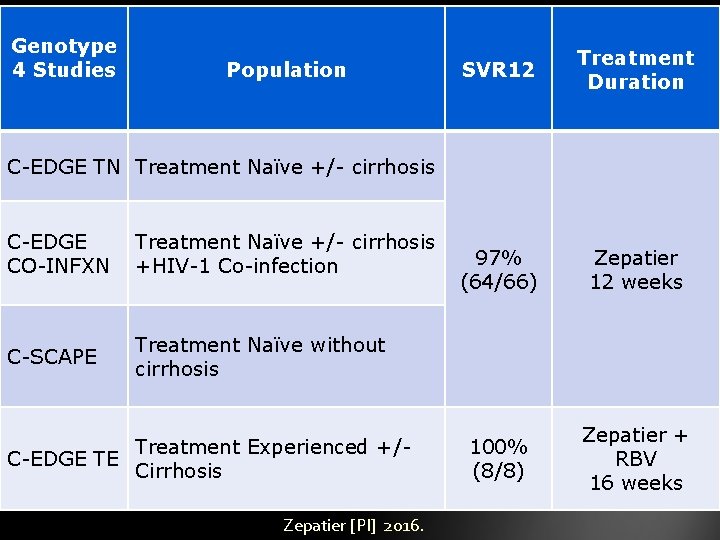
Genotype 4 Studies Population SVR 12 Treatment Duration 97% (64/66) Zepatier 12 weeks 100% (8/8) Zepatier + RBV 16 weeks C-EDGE TN Treatment Naïve +/- cirrhosis C-EDGE CO-INFXN Treatment Naïve +/- cirrhosis +HIV-1 Co-infection C-SCAPE Treatment Naïve without cirrhosis Treatment Experienced +/C-EDGE TE Cirrhosis Zepatier [PI] 2016.

Elbasvir and Grazoprevir (Zepatier®) § Adverse effects Ø Headache, nausea, fatigue § Dosage and Administration Ø One tablet once daily with or without food Ø 50 mg elbasvir /100 mg grazeprevir § No adjustments in renal dysfunction or dialysis Zepatier [PI] 2016.

Elbasvir and Grazoprevir (Zepatier®) § Bottom line Ø One tablet, once daily treatment for HCV genotypes 1 or 4 infections Ø Cure rates of >94% Ø Duration of 12 weeks for most patient groups Ø NS 5 A polymorphism testing recommended Ø Approved in renal failure § Additional review Ø Bell, AM, et al. International Journal of Hepatology, 2016; 3852126. Zepatier [PI] 2016.

Sofosbuvir and Velpatasvir (Epclusa®) § Indication Ø Genotype 1, 2, 3, 4, 5, or 6 infection Ø Without cirrhosis or with compensated cirrhosis Ø With decompensated cirrhosis in combination with ribavirin HCV § Mechanism of Action Ø Sofosbuvir: NS 5 B polymerase inhibitor Ø Velpatasvir: NS 5 A inhibitor Epclusa [PI] 2016.

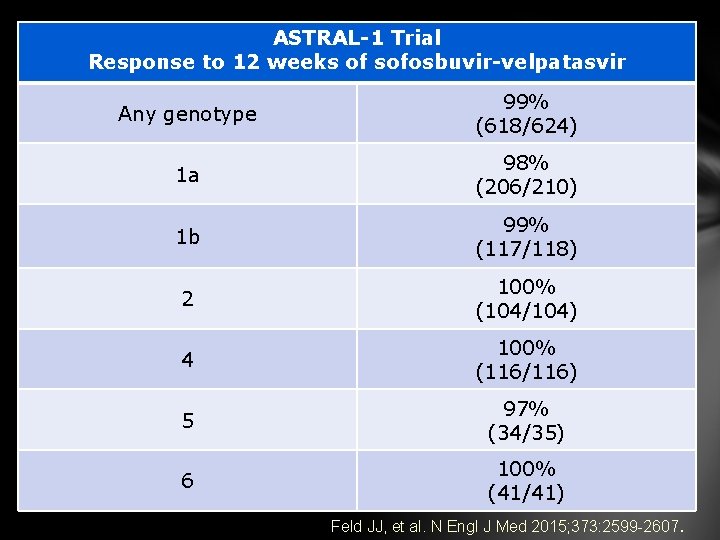
ASTRAL-1 Trial Response to 12 weeks of sofosbuvir-velpatasvir Any genotype 99% (618/624) 1 a 98% (206/210) 1 b 99% (117/118) 2 100% (104/104) 4 100% (116/116) 5 97% (34/35) 6 100% (41/41) Feld JJ, et al. N Engl J Med 2015; 373: 2599 -2607.

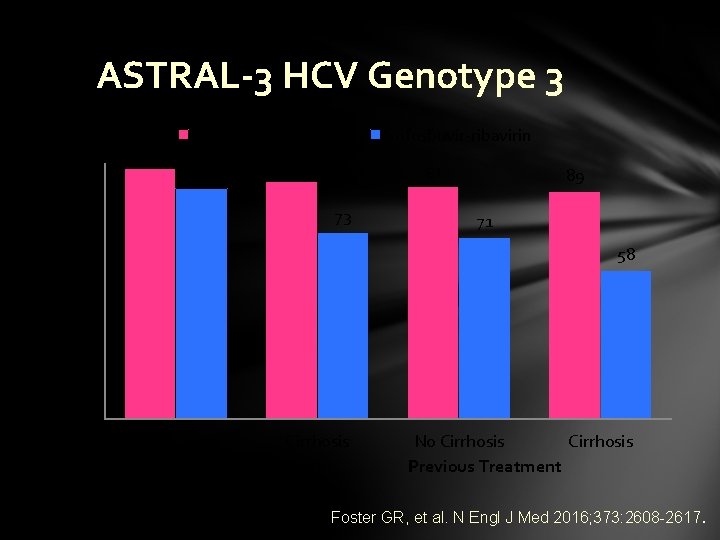
Sustained Virologic Response % ASTRAL-3 HCV Genotype 3 100 90 80 70 60 50 40 30 20 10 0 98 Sofosbuvir-velpatasvir 90 93 Sofosbuvir-ribavirin 91 73 89 71 58 No Cirrhosis No Previous Treatment No Cirrhosis Previous Treatment Foster GR, et al. N Engl J Med 2016; 373: 2608 -2617.

Sofosbuvir and Velpatasvir (Epclusa®) § Drug interactions Ø P-gp inducers and CYP inducers decrease concentrations sofosbuvir and velpatasvir Ø Amiodarone may lead to bradycardia when given with sofosbuvir Ø Acid-reducing drugs decrease velpatasvir absorption Ø Avoid proton pump inhibitors Epclusa [PI] 2016.

Sofosbuvir and Velpatasvir (Epclusa®) § Dosing Ø One tablet orally once daily Ø 400 mg sofosbuvir /100 mg velpatasvir Ø With or without food § No dosage recommendations for patients with severe renal impairment (Cr. Cl < 30 ml/min) Epclusa [PI] 2016.

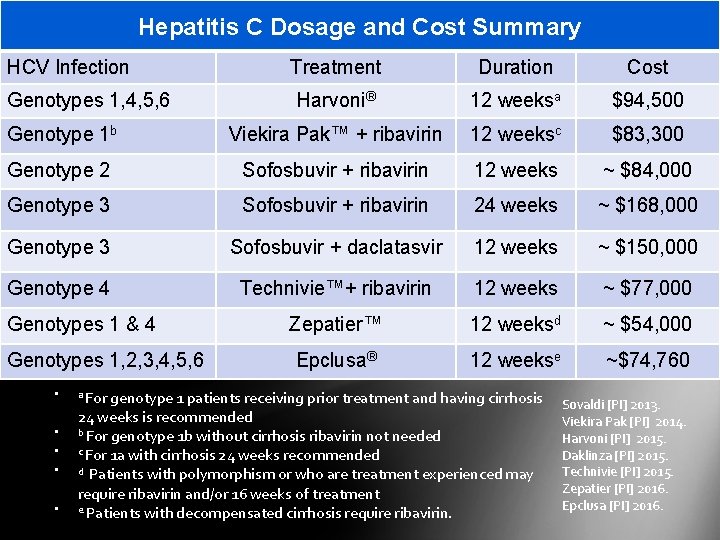
Hepatitis C Dosage and Cost Summary HCV Infection Treatment Duration Cost Harvoni® 12 weeksa $94, 500 Genotype 1 b Viekira Pak™ + ribavirin 12 weeksc $83, 300 Genotype 2 Sofosbuvir + ribavirin 12 weeks ~ $84, 000 Genotype 3 Sofosbuvir + ribavirin 24 weeks ~ $168, 000 Genotype 3 Sofosbuvir + daclatasvir 12 weeks ~ $150, 000 Genotype 4 Technivie™+ ribavirin 12 weeks ~ $77, 000 Zepatier™ 12 weeksd ~ $54, 000 Epclusa® 12 weekse ~$74, 760 Genotypes 1, 4, 5, 6 Genotypes 1 & 4 Genotypes 1, 2, 3, 4, 5, 6 • • • a For genotype 1 patients receiving prior treatment and having cirrhosis 24 weeks is recommended b For genotype 1 b without cirrhosis ribavirin not needed c For 1 a with cirrhosis 24 weeks recommended d Patients with polymorphism or who are treatment experienced may require ribavirin and/or 16 weeks of treatment e Patients with decompensated cirrhosis require ribavirin. Sovaldi [PI] 2013. Viekira Pak [PI] 2014. Harvoni [PI] 2015. Daklinza [PI] 2015. Technivie [PI] 2015. Zepatier [PI] 2016. Epclusa [PI] 2016.

Two New Antibiotics § Both approved for c. IAIs & c. UTIs § Used for gram-negative aerobes § Ceftazidime-Avibactam (Avycaz™) Ø Avibactam enhances in vitro activity in presence of some β-lactamases (KPCs) § Ceftolozane/tazobactam (Zerbaxa™) Ø Niche appears to be for multi-drug resistant Pseudomonas infections c. IAI = complicated intrabdominal infections c. UTIs = complicated urinary tract infections Avycaz PI 2015. Zerbaxa PI 2014