NCEA Chemistry 2 2 Identify Ions AS 91162




![Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-28.jpg)
![Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3 Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-29.jpg)
![Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-30.jpg)
![Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ + Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ +](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-31.jpg)
- Slides: 42

NCEA Chemistry 2. 2 Identify Ions AS 91162

What is this NCEA Achievement Standard? When a student achieves a standard, they gain a number of credits. Students must achieve a certain number of credits to gain an NCEA certificate (80 for Level 2) The standard you will be assessed on is called Chemistry 2. 2 Carry out procedures to identify ions present in solution It will be internally (in Class) assessed as part of a Investigation and will count towards 3 credits for your Level 2 NCEA in Chemistry 2

What are the main steps required in this Internal Assessment? AS 91162 Carry out procedures to identify ions present in solution The method Carry out procedures to identify ions involves collecting primary data and using these observations to identify ions in a solution using a procedure provided. Identification of ions must be supported by experimental observations and identification of all precipitates formed. Ions to be identified will be limited to: Ag+, Al 3+, Ba 2+, Cu 2+, Fe 3+, Mg 2+, Pb 2+, Na+, Zn 2+, Cl–, CO 32–, I–, NO 3–, OH–, SO 42–. (Na+ and NO 3– are identified by a process of elimination. ) Complex ions may include [Fe. SCN]2+ and those formed when OH–(aq) or NH 3(aq) react with cations listed above, such as [Ag(NH 3)2]+, [Al(OH)4]–, [Pb(OH)4]2–, [Zn(NH 3)4]2+, [Cu(NH 3)4]2+. 3

Aiming for Merit and Excellence Interpretation of evidence for Merit Carry out procedures to justify the identification of ions also includes writing balanced equations for all the reactions where precipitates are formed. Interpretation of evidence for Excellence Carry out procedures to comprehensively justify the identification of ions also includes interpreting observations by recognising the formation of complex ions and writing balanced equations for these reactions.

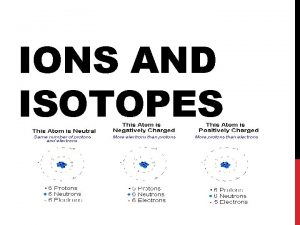
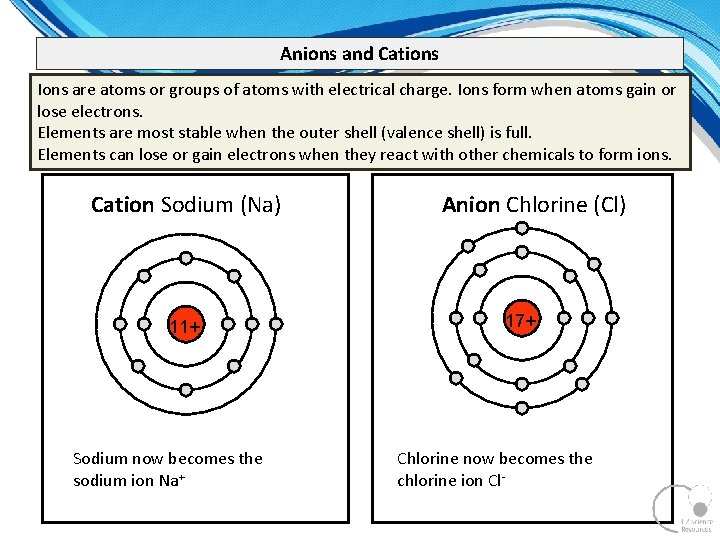
Anions and Cations Ions are atoms or groups of atoms with electrical charge. Ions form when atoms gain or lose electrons. Elements are most stable when the outer shell (valence shell) is full. Elements can lose or gain electrons when they react with other chemicals to form ions. Cation Sodium (Na) 11+ Sodium now becomes the sodium ion Na+ Anion Chlorine (Cl) 17+ Chlorine now becomes the chlorine ion Cl

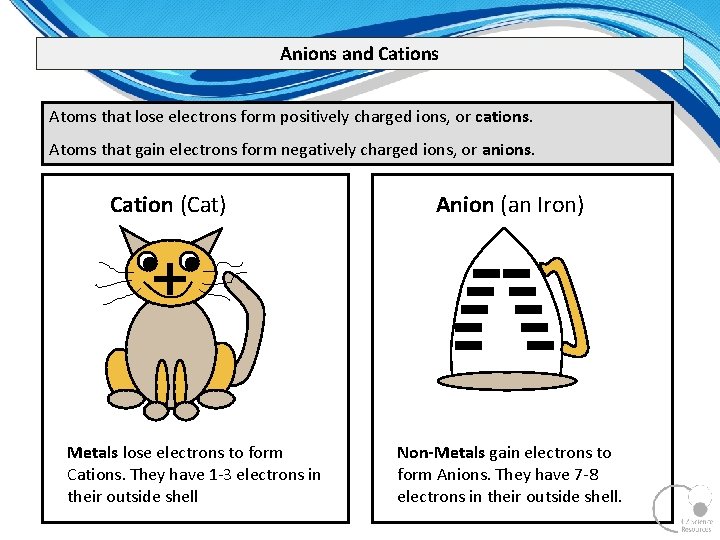
Anions and Cations Atoms that lose electrons form positively charged ions, or cations. Atoms that gain electrons form negatively charged ions, or anions. Cation (Cat) Anion (an Iron) + Metals lose electrons to form Cations. They have 1 3 electrons in their outside shell Non-Metals gain electrons to form Anions. They have 7 8 electrons in their outside shell.

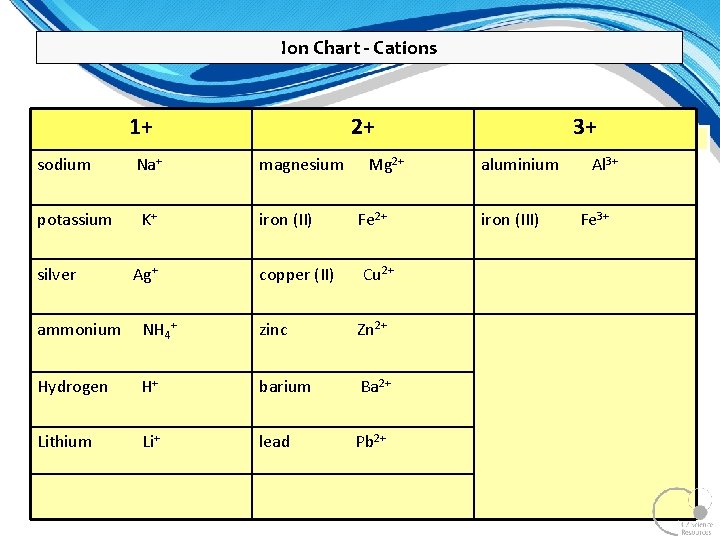
Ion Chart - Cations 1+ sodium potassium silver Na+ K+ Ag+ 2+ magnesium iron (II) Mg 2+ Fe 2+ copper (II) Cu 2+ ammonium NH 4+ zinc Zn 2+ Hydrogen H+ barium Ba 2+ Lithium Li+ lead Pb 2+ 7 3+ aluminium iron (III) Al 3+ Fe 3+

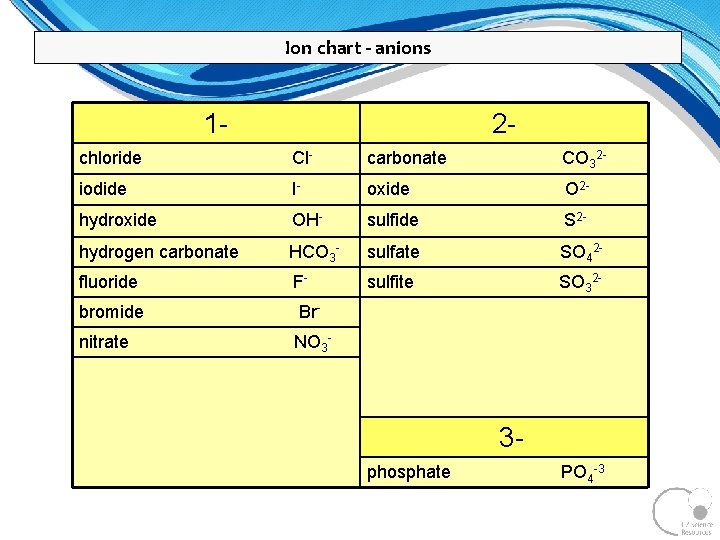
Ion chart - anions 1 - 2 - chloride Cl- carbonate CO 32 - iodide I- oxide O 2 - hydroxide OH- sulfide S 2 - hydrogen carbonate HCO 3 - sulfate SO 42 - fluoride F- sulfite SO 32 - bromide Br- nitrate NO 3 - 3 phosphate PO 4 -3

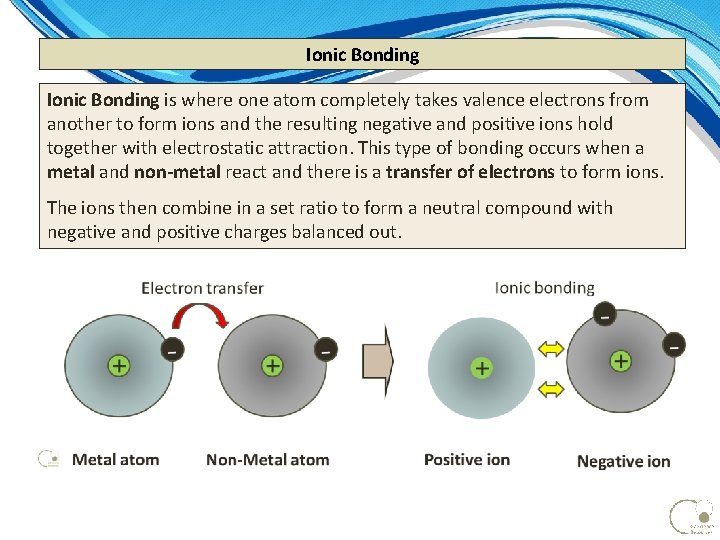
Ionic Bonding is where one atom completely takes valence electrons from another to form ions and the resulting negative and positive ions hold together with electrostatic attraction. This type of bonding occurs when a metal and non-metal react and there is a transfer of electrons to form ions. The ions then combine in a set ratio to form a neutral compound with negative and positive charges balanced out.

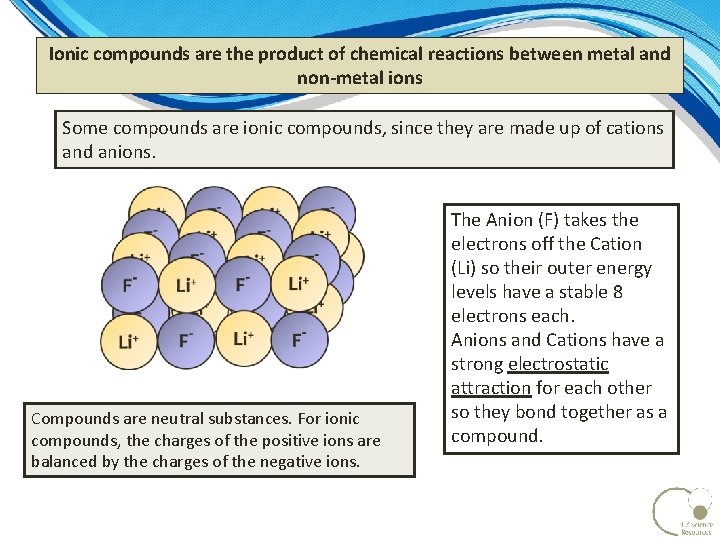
Ionic compounds are the product of chemical reactions between metal and non-metal ions Some compounds are ionic compounds, since they are made up of cations and anions. Compounds are neutral substances. For ionic compounds, the charges of the positive ions are balanced by the charges of the negative ions. The Anion (F) takes the electrons off the Cation (Li) so their outer energy levels have a stable 8 electrons each. Anions and Cations have a strong electrostatic attraction for each other so they bond together as a compound.

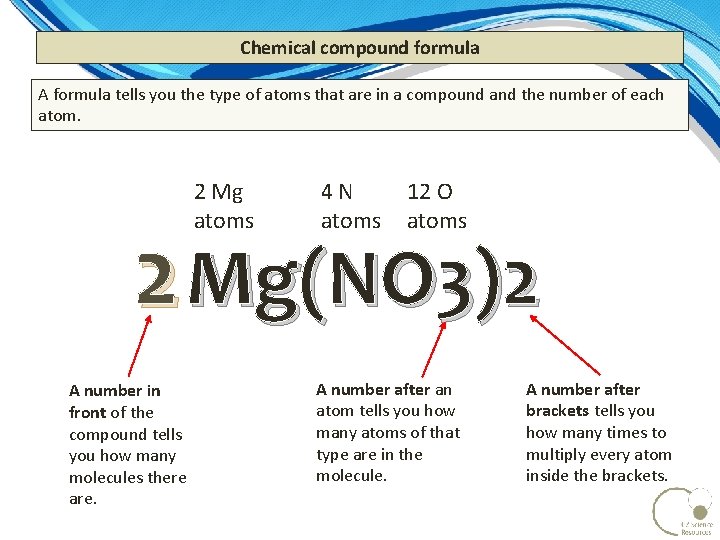
Chemical compound formula A formula tells you the type of atoms that are in a compound and the number of each atom. 2 Mg atoms 4 N atoms 12 O atoms 2 Mg(NO 3)2 A number in front of the compound tells you how many molecules there are. A number after an atom tells you how many atoms of that type are in the molecule. A number after brackets tells you how many times to multiply every atom inside the brackets.

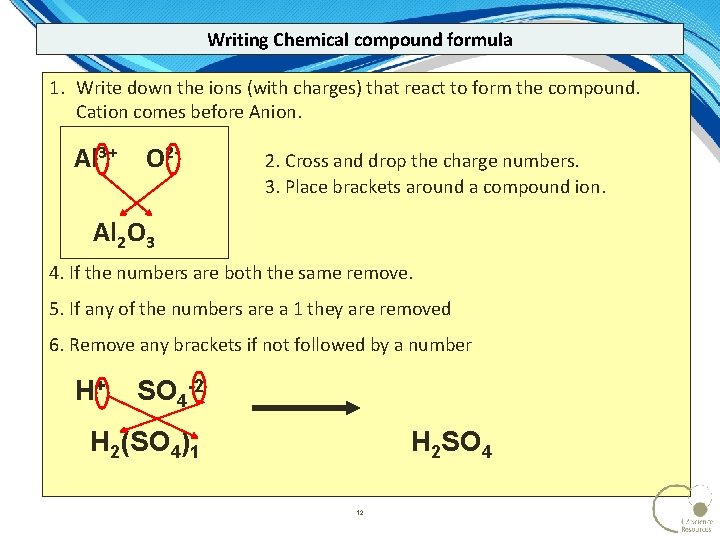
Writing Chemical compound formula 1. Write down the ions (with charges) that react to form the compound. Cation comes before Anion. Al 3+ O 2 - 2. Cross and drop the charge numbers. 3. Place brackets around a compound ion. Al 2 O 3 4. If the numbers are both the same remove. 5. If any of the numbers are a 1 they are removed 6. Remove any brackets if not followed by a number H+ SO 4 -2 H 2(SO 4)1 H 2 SO 4 12

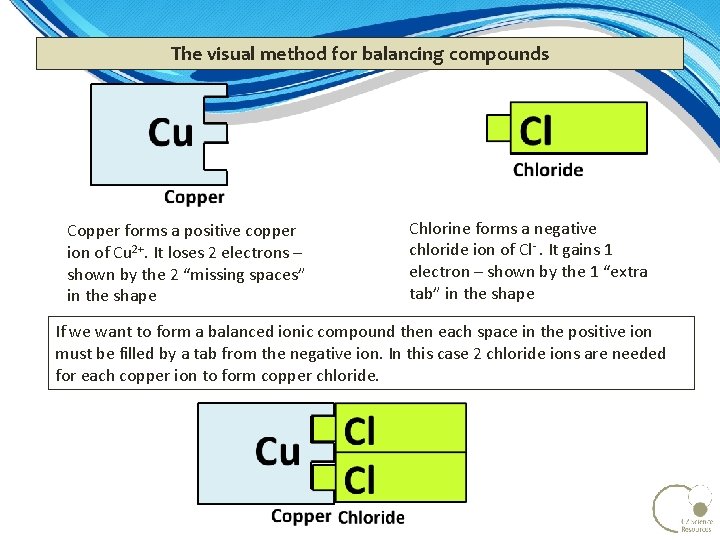
The visual method for balancing compounds Copper forms a positive copper ion of Cu 2+. It loses 2 electrons – shown by the 2 “missing spaces” in the shape Chlorine forms a negative chloride ion of Cl . It gains 1 electron – shown by the 1 “extra tab” in the shape If we want to form a balanced ionic compound then each space in the positive ion must be filled by a tab from the negative ion. In this case 2 chloride ions are needed for each copper ion to form copper chloride.

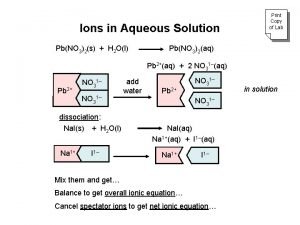
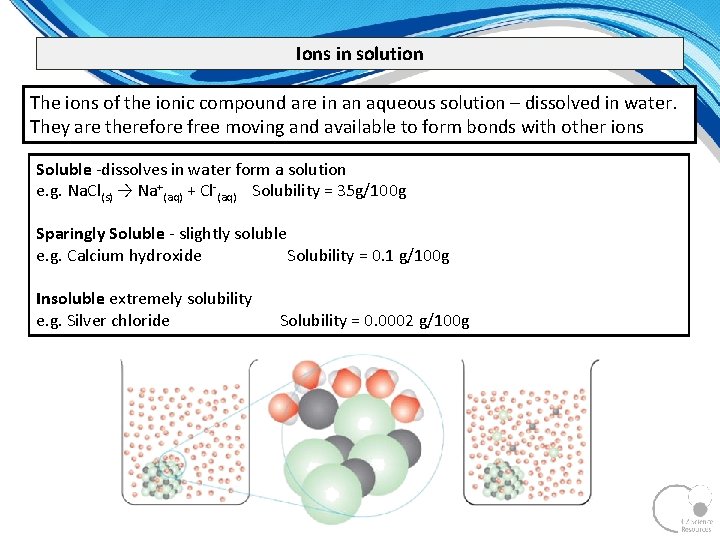
Ions in solution The ions of the ionic compound are in an aqueous solution – dissolved in water. They are therefore free moving and available to form bonds with other ions Soluble dissolves in water form a solution e. g. Na. Cl(s) → Na+(aq) + Cl (aq) Solubility = 35 g/100 g Sparingly Soluble slightly soluble e. g. Calcium hydroxide Solubility = 0. 1 g/100 g Insoluble extremely solubility e. g. Silver chloride Solubility = 0. 0002 g/100 g

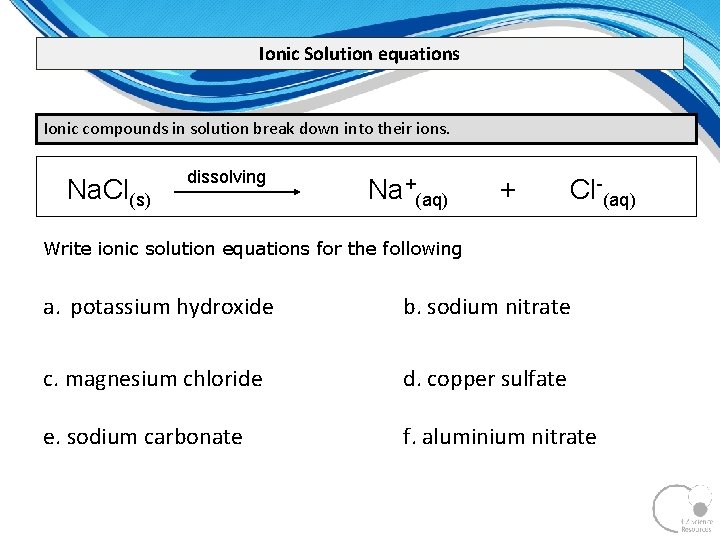
Ionic Solution equations Ionic compounds in solution break down into their ions. Na. Cl(s) dissolving Na+(aq) + Cl-(aq) Write ionic solution equations for the following a. potassium hydroxide b. sodium nitrate c. magnesium chloride d. copper sulfate e. sodium carbonate f. aluminium nitrate

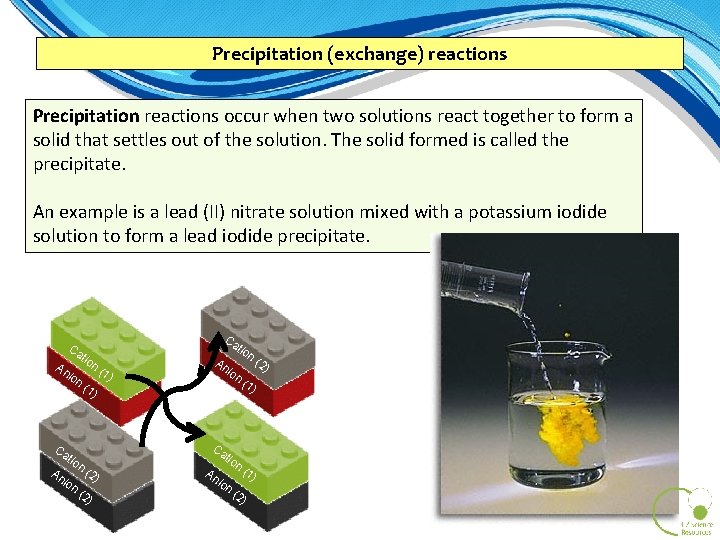
Precipitation (exchange) reactions Precipitation reactions occur when two solutions react together to form a solid that settles out of the solution. The solid formed is called the precipitate. An example is a lead (II) nitrate solution mixed with a potassium iodide solution to form a lead iodide precipitate. Ca An ion Ca An tio ion tio Ca n( (1 n( (2 1) tio n An ion Ca 2) ) (1 ) ) An tio ion n( (2 1) ) (2 )

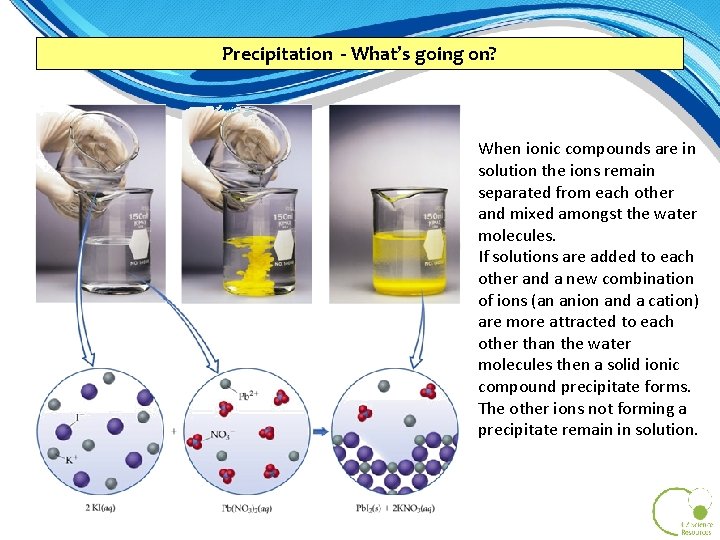
Precipitation - What’s going on? When ionic compounds are in solution the ions remain separated from each other and mixed amongst the water molecules. If solutions are added to each other and a new combination of ions (an anion and a cation) are more attracted to each other than the water molecules then a solid ionic compound precipitate forms. The other ions not forming a precipitate remain in solution.

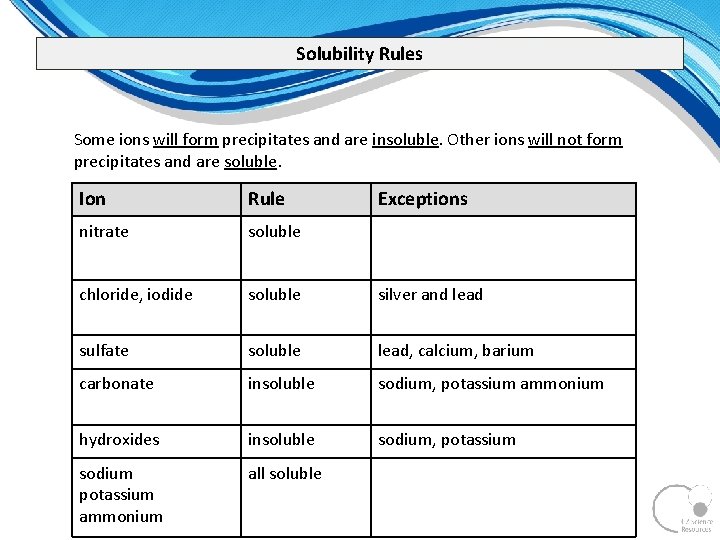
Solubility Rules Some ions will form precipitates and are insoluble. Other ions will not form precipitates and are soluble. Ion Rule Exceptions nitrate soluble chloride, iodide soluble silver and lead sulfate soluble lead, calcium, barium carbonate insoluble sodium, potassium ammonium hydroxides insoluble sodium, potassium sodium potassium ammonium all soluble

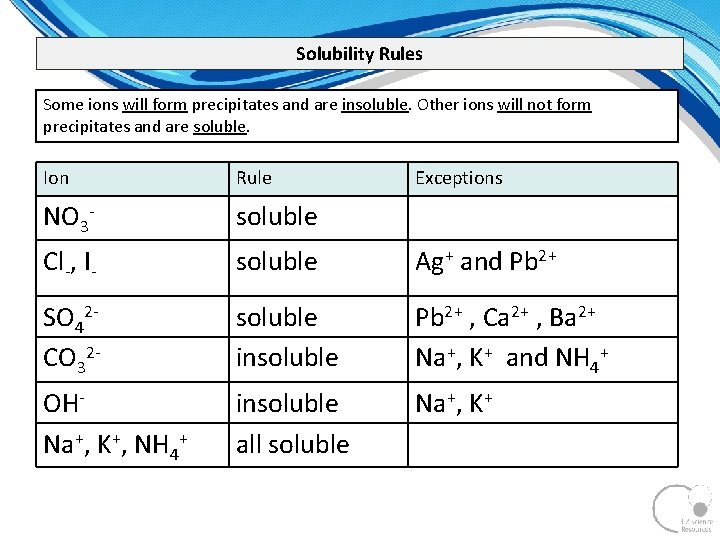
Solubility Rules Some ions will form precipitates and are insoluble. Other ions will not form precipitates and are soluble. Ion Rule Exceptions NO 3 soluble Cl , I soluble Ag+ and Pb 2+ SO 42 CO 32 soluble insoluble Pb 2+ , Ca 2+ , Ba 2+ Na+, K+ and NH 4+ OH Na+, K+, NH 4+ insoluble all soluble Na+, K+

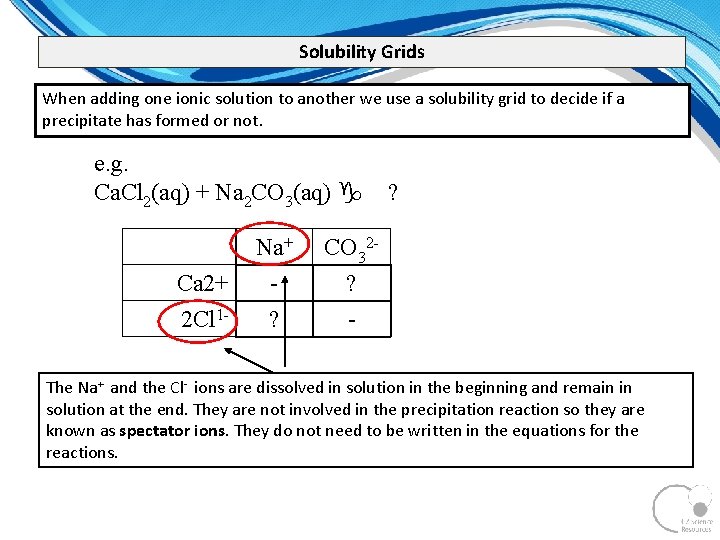
Solubility Grids When adding one ionic solution to another we use a solubility grid to decide if a precipitate has formed or not. e. g. Ca. Cl 2(aq) + Na 2 CO 3(aq) g Ca 2+ 2 Cl 1 - Na+ ? ? CO 32? - The Na+ and the Cl ions are dissolved in solution in the beginning and remain in solution at the end. They are not involved in the precipitation reaction so they are known as spectator ions. They do not need to be written in the equations for the reactions.

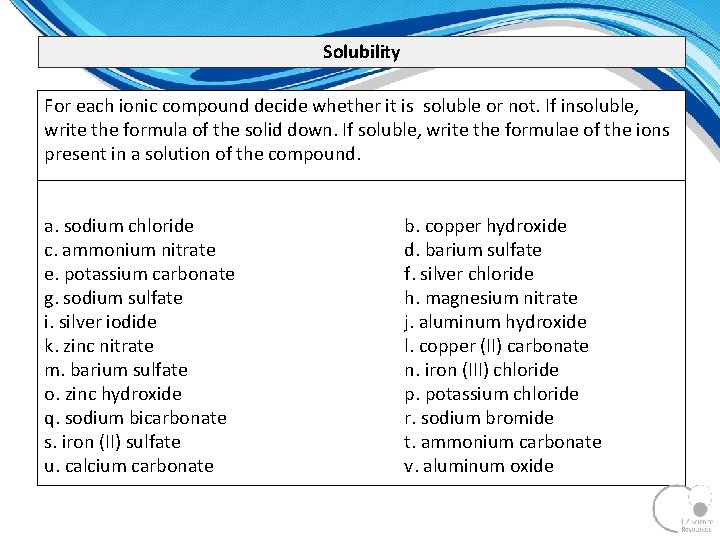
Solubility For each ionic compound decide whether it is soluble or not. If insoluble, write the formula of the solid down. If soluble, write the formulae of the ions present in a solution of the compound. a. sodium chloride c. ammonium nitrate e. potassium carbonate g. sodium sulfate i. silver iodide k. zinc nitrate m. barium sulfate o. zinc hydroxide q. sodium bicarbonate s. iron (II) sulfate u. calcium carbonate b. copper hydroxide d. barium sulfate f. silver chloride h. magnesium nitrate j. aluminum hydroxide l. copper (II) carbonate n. iron (III) chloride p. potassium chloride r. sodium bromide t. ammonium carbonate v. aluminum oxide

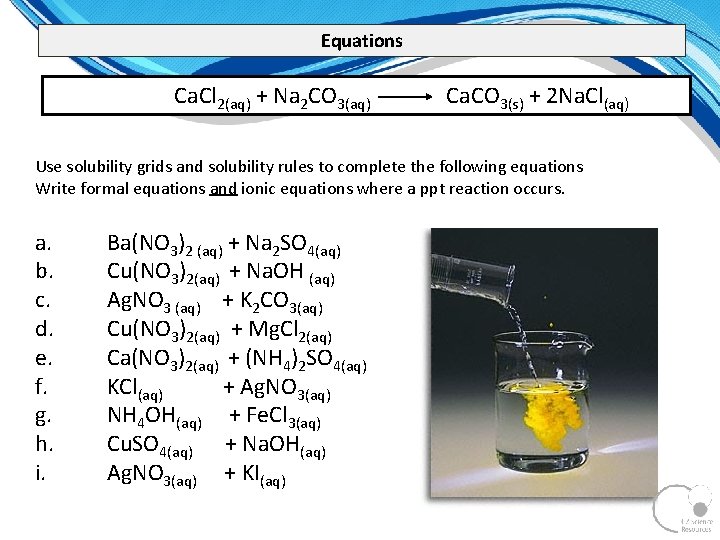
Equations Ca. Cl 2(aq) + Na 2 CO 3(aq) Ca. CO 3(s) + 2 Na. Cl(aq) Use solubility grids and solubility rules to complete the following equations Write formal equations and ionic equations where a ppt reaction occurs. a. b. c. d. e. f. g. h. i. Ba(NO 3)2 (aq) + Na 2 SO 4(aq) Cu(NO 3)2(aq) + Na. OH (aq) Ag. NO 3 (aq) + K 2 CO 3(aq) Cu(NO 3)2(aq) + Mg. Cl 2(aq) Ca(NO 3)2(aq) + (NH 4)2 SO 4(aq) KCl(aq) + Ag. NO 3(aq) NH 4 OH(aq) + Fe. Cl 3(aq) Cu. SO 4(aq) + Na. OH(aq) Ag. NO 3(aq) + KI(aq)

Precipitate observations

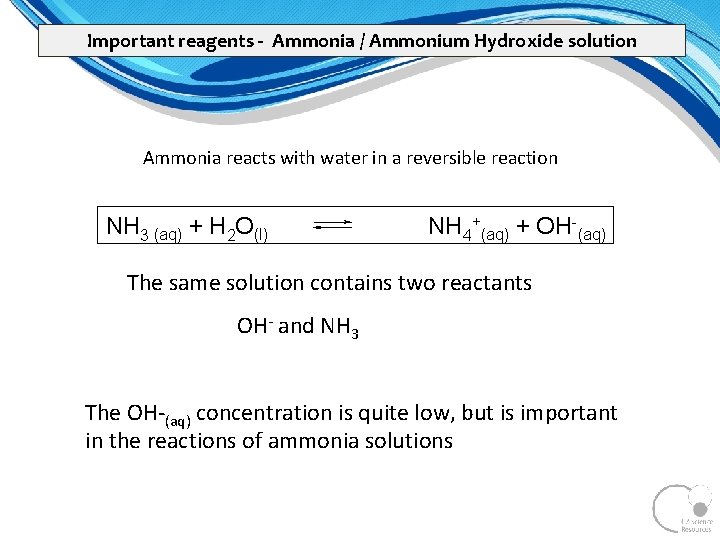
Important reagents - Ammonia / Ammonium Hydroxide solution Ammonia reacts with water in a reversible reaction NH 3 (aq) + H 2 O(l) NH 4+(aq) + OH-(aq) The same solution contains two reactants OH and NH 3 The OH (aq) concentration is quite low, but is important in the reactions of ammonia solutions

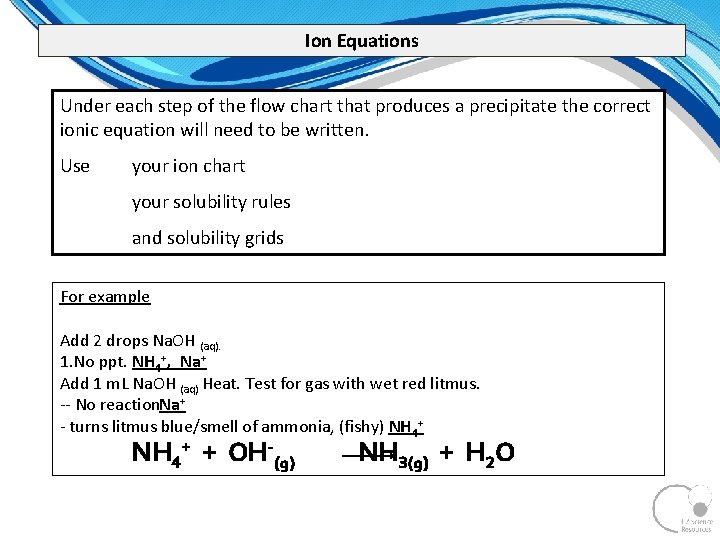
Ion Equations Under each step of the flow chart that produces a precipitate the correct ionic equation will need to be written. Use your ion chart your solubility rules and solubility grids For example Add 2 drops Na. OH (aq). 1. No ppt. NH 4+, Na+ Add 1 m. L Na. OH (aq) Heat. Test for gas with wet red litmus. No reaction. Na+ turns litmus blue/smell of ammonia, (fishy) NH 4+ + OH-(g) NH 3(g) + H 2 O

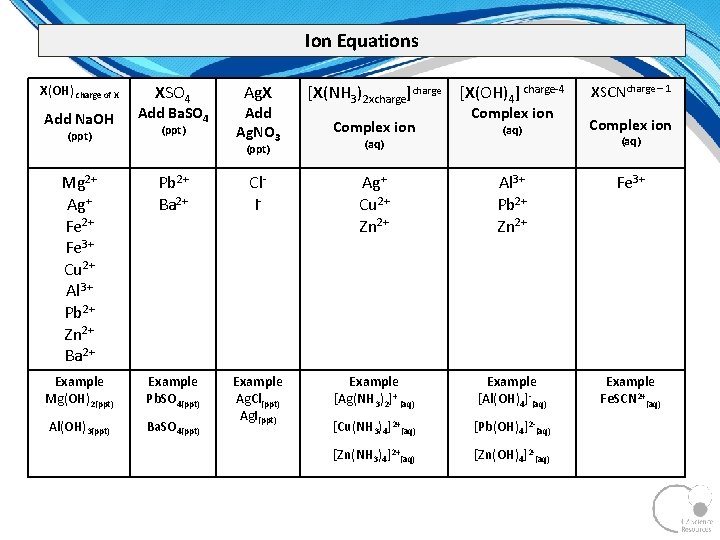
Ion Equations X(OH) charge of X Add Na. OH (ppt) XSO 4 Add Ba. SO 4 (ppt) Ag. X Add Ag. NO 3 [X(NH 3)2 xcharge]charge [X(OH)4] charge 4 XSCNcharge – 1 Complex ion (aq) Complex ion (ppt) (aq) Complex ion (aq) Mg 2+ Ag+ Fe 2+ Fe 3+ Cu 2+ Al 3+ Pb 2+ Zn 2+ Ba 2+ Pb 2+ Ba 2+ Cl I Ag+ Cu 2+ Zn 2+ Al 3+ Pb 2+ Zn 2+ Fe 3+ Example Mg(OH)2(ppt) Example Pb. SO 4(ppt) Example [Ag(NH 3)2]+ (aq) Example [Al(OH)4] (aq) Example Fe. SCN 2+(aq) Al(OH)3(ppt) Ba. SO 4(ppt) Example Ag. Cl(ppt) Ag. I(ppt) [Cu(NH 3)4]2+(aq) [Pb(OH)4]2 (aq) [Zn(NH 3)4]2+(aq) [Zn(OH)4]2 (aq)

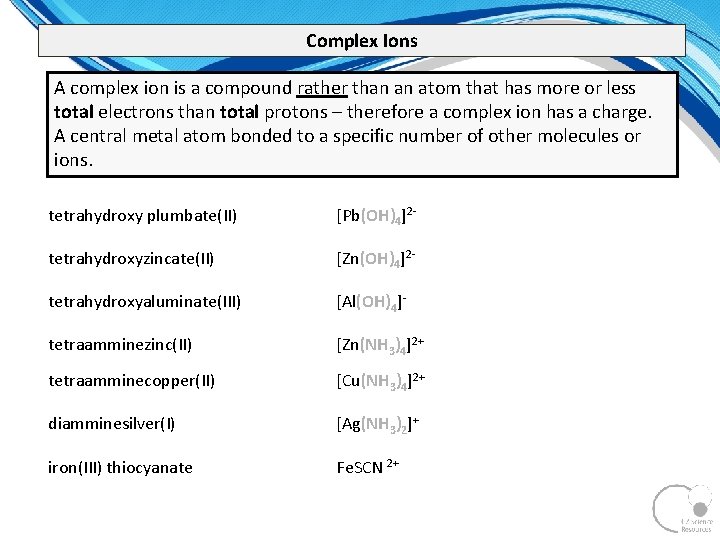
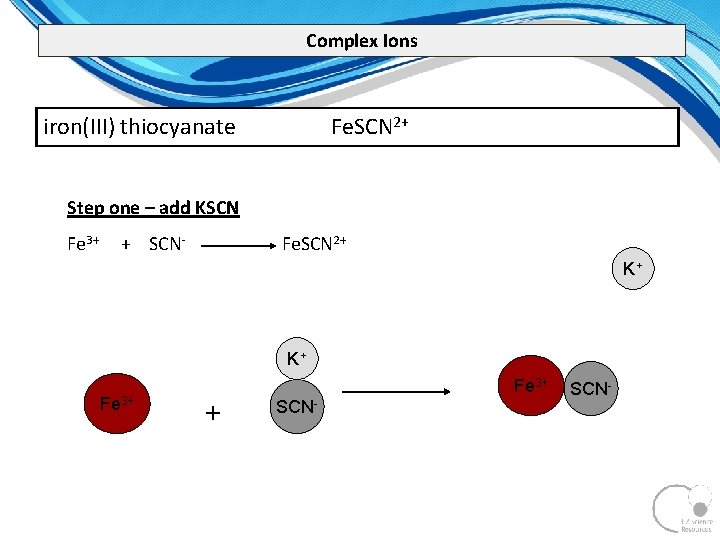
Complex Ions A complex ion is a compound rather than an atom that has more or less total electrons than total protons – therefore a complex ion has a charge. A central metal atom bonded to a specific number of other molecules or ions. tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 tetrahydroxyaluminate(III) [Al(OH)4] tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ diamminesilver(I) [Ag(NH 3)2]+ iron(III) thiocyanate Fe. SCN 2+
![Complex Ions tetrahydroxy plumbateII PbOH42 tetrahydroxyzincateII ZnOH42 Step one add Na OH Pb Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-28.jpg)
Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb 2+ + 2 OH Pb(OH)2 Step two – add excess Na. OH Pb(OH)2 + 2 OH [Pb(OH)4]2 OH- OHOH- Pb 2+ OHOH-
![Complex Ions tetrahydroxyaluminateIII AlOH4 Step one add Na OH Al 3 3 Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-29.jpg)
Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3 OH Al(OH)3 Step two – add excess Na. OH Al(OH)3 + OH [Al(OH)4] OH- OHOH- Al 3+ OH-
![Complex Ions tetraamminezincII ZnNH 342 tetraamminecopperII CuNH 342 NH 3 H 2 O Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-30.jpg)
Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O NH 4+ + OH- Step one – add 2 drops NH 3 Zn 2+ + 2 OH Zn(OH)2 Step two – add excess NH 3 Zn(OH)2 OH- Zn 2+ + 4 NH 3 OH- [Zn(NH 3)4]2+ + + NH 3 OH- NH 3 OH- 2 OH - Zn 2+ NH 3
![Complex Ions diamminesilverI AgNH 32 NH 3 H 2 O NH 4 Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ +](https://slidetodoc.com/presentation_image_h2/f82cacfb7f92a89bbe564a21a0b1c2f7/image-31.jpg)
Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ + OH- Step one – add 2 drops NH 3 Ag+ + OH Ag. OH Step two – add excess NH 3 Ag. OH + 2 NH 3 [Ag(NH 3)2]+ + OH OH- Ag+ OH- + NH 3 Ag+ NH 3

Complex Ions iron(III) thiocyanate Fe. SCN 2+ Step one – add KSCN Fe 3+ + SCN Fe. SCN 2+ K+ K+ Fe 3+ + SCN-

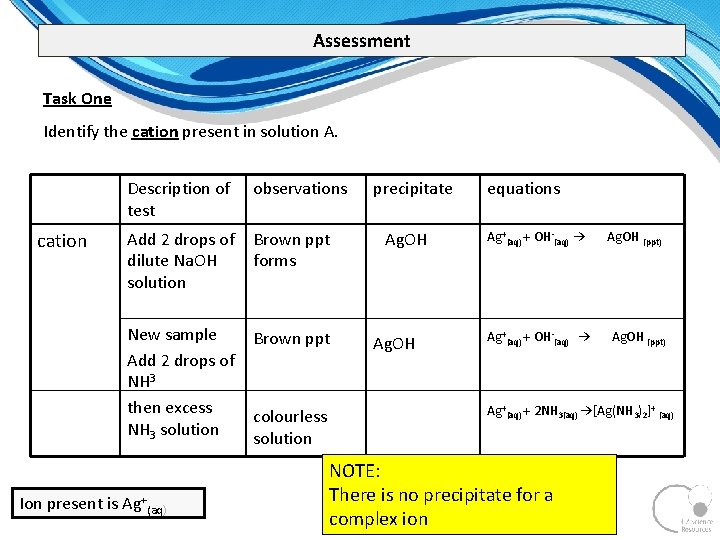
Assessment Task One Identify the cation present in solution A. cation Description of test observations Add 2 drops of dilute Na. OH solution Brown ppt forms New sample Add 2 drops of NH 3 then excess NH 3 solution Brown ppt Ion present is Ag+(aq) colourless solution precipitate Ag. OH equations Ag+(aq) + OH (aq) → Ag. OH (ppt) Ag+(aq) + 2 NH 3(aq) →[Ag(NH 3)2]+ (aq) NOTE: There is no precipitate for a complex ion

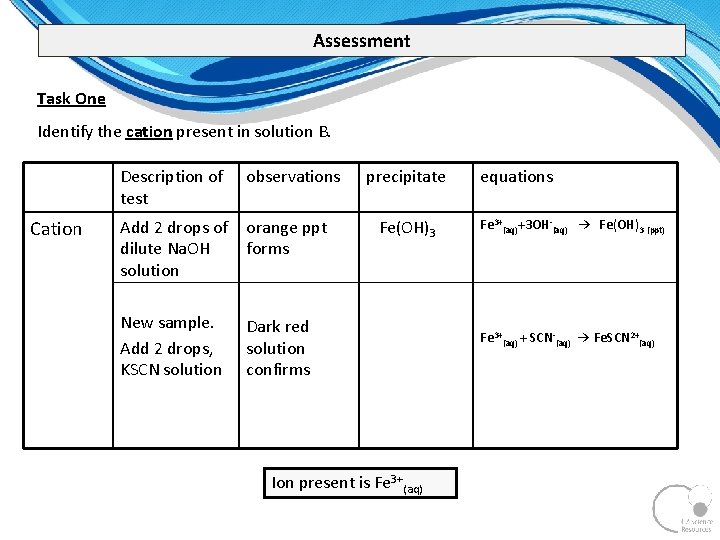
Assessment Task One Identify the cation present in solution B. Cation Description of test observations Add 2 drops of dilute Na. OH solution orange ppt forms New sample. Add 2 drops, KSCN solution Dark red solution confirms precipitate Fe(OH)3 Ion present is Fe 3+(aq) equations Fe 3+(aq)+3 OH (aq) → Fe(OH)3 (ppt) Fe 3+(aq) + SCN (aq) → Fe. SCN 2+(aq)

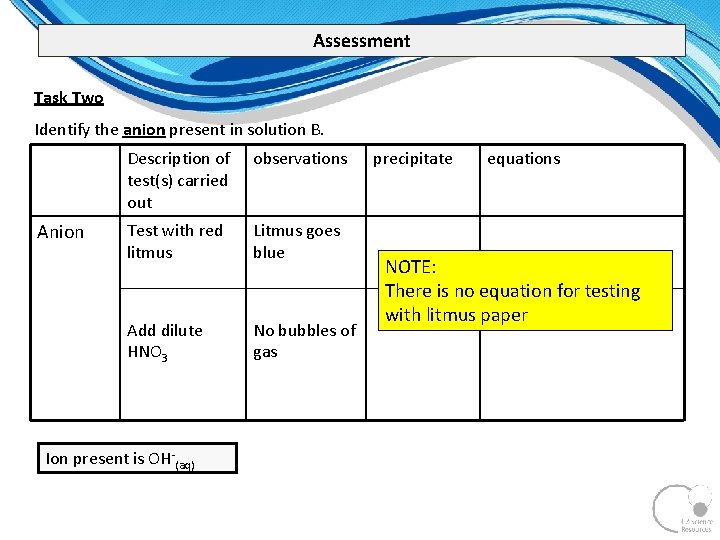
Assessment Task Two Identify the anion present in solution B. Anion Description of test(s) carried out observations Test with red litmus Litmus goes blue Add dilute HNO 3 No bubbles of gas Ion present is OH (aq) precipitate equations NOTE: There is no equation for testing with litmus paper

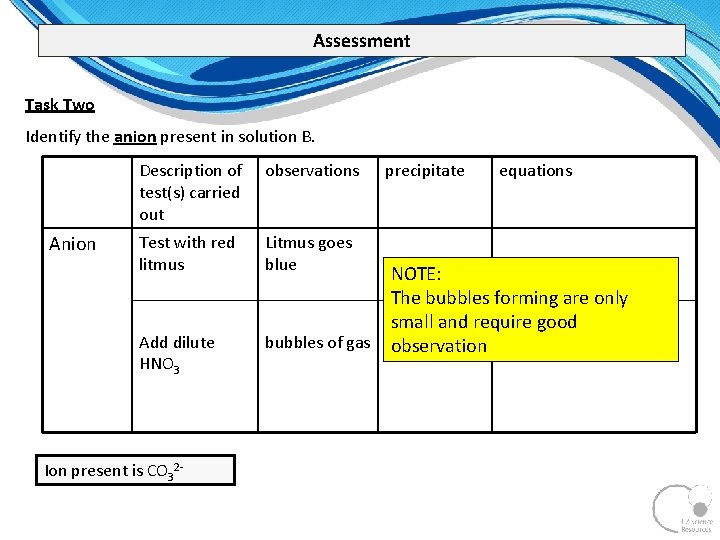
Assessment Task Two Identify the anion present in solution B. Anion Description of test(s) carried out observations Test with red litmus Litmus goes blue Add dilute HNO 3 bubbles of gas Ion present is CO 32 precipitate equations NOTE: The bubbles forming are only small and require good observation

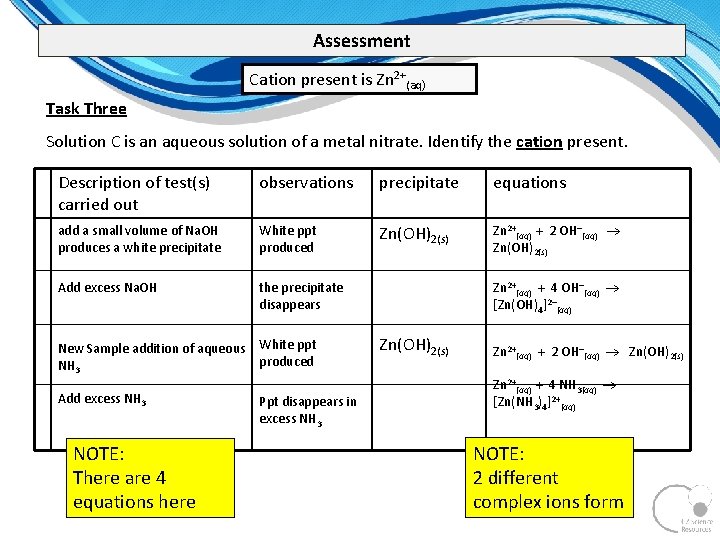
Assessment Cation present is Zn 2+(aq) Task Three Solution C is an aqueous solution of a metal nitrate. Identify the cation present. Description of test(s) carried out observations precipitate equations add a small volume of Na. OH produces a white precipitate White ppt produced Zn(OH)2(s) Zn 2+(aq) + 2 OH (aq) Zn(OH)2(s) Add excess Na. OH the precipitate disappears New Sample addition of aqueous White ppt produced NH 3 Add excess NH 3 NOTE: There are 4 equations here Ppt disappears in excess NH 3 Zn 2+(aq) + 4 OH (aq) [Zn(OH)4]2 (aq) Zn(OH)2(s) Zn 2+(aq) + 2 OH (aq) Zn(OH)2(s) Zn 2+(aq) + 4 NH 3(aq) [Zn(NH 3)4]2+(aq) NOTE: 2 different complex ions form

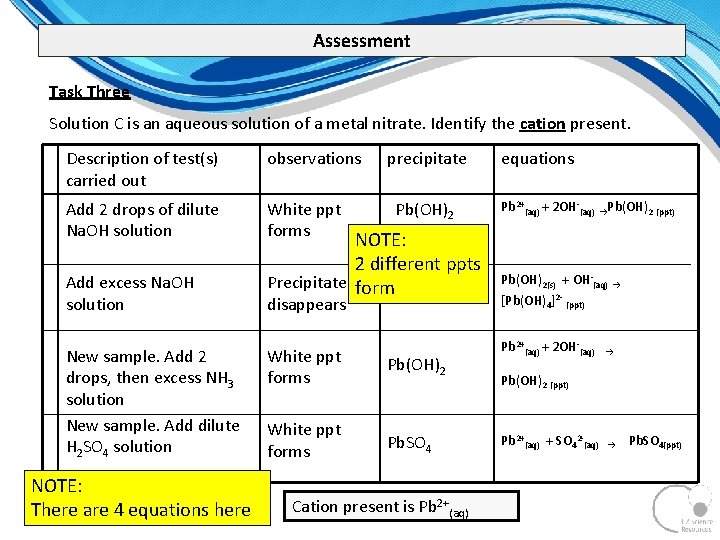
Assessment Task Three Solution C is an aqueous solution of a metal nitrate. Identify the cation present. Description of test(s) carried out observations Add 2 drops of dilute Na. OH solution White ppt forms Add excess Na. OH solution New sample. Add 2 drops, then excess NH 3 solution New sample. Add dilute H 2 SO 4 solution NOTE: There are 4 equations here precipitate Pb(OH)2 NOTE: 2 different ppts Precipitate form disappears White ppt forms Pb(OH)2 White ppt forms Pb. SO 4 Cation present is Pb 2+(aq) equations Pb 2+(aq) + 2 OH (aq) →Pb(OH)2 (ppt) Pb(OH)2(s) + OH (aq) → [Pb(OH)4]2 (ppt) Pb 2+(aq) + 2 OH (aq) → Pb(OH)2 (ppt) Pb 2+(aq) + SO 42 (aq) → Pb. SO 4(ppt)

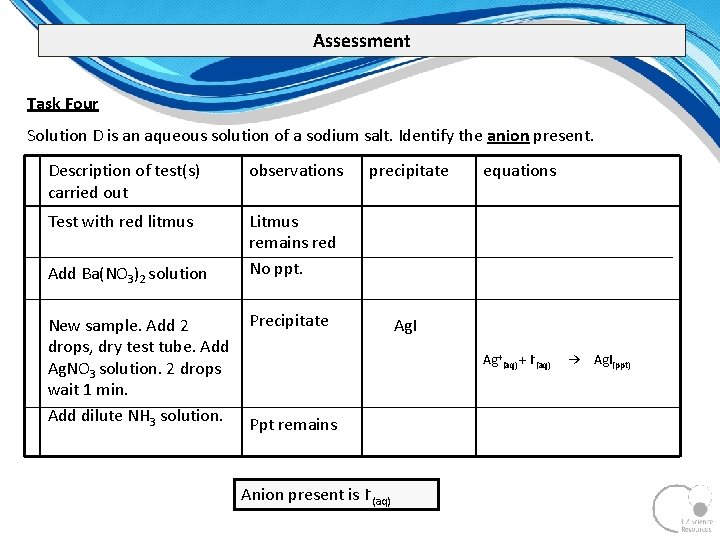
Assessment Task Four Solution D is an aqueous solution of a sodium salt. Identify the anion present. Description of test(s) carried out observations Test with red litmus Litmus remains red No ppt. Add Ba(NO 3)2 solution New sample. Add 2 drops, dry test tube. Add Ag. NO 3 solution. 2 drops wait 1 min. Add dilute NH 3 solution. precipitate Precipitate equations Ag. I Ag+(aq) + I (aq) Ppt remains Anion present is I (aq) → Ag. I(ppt)

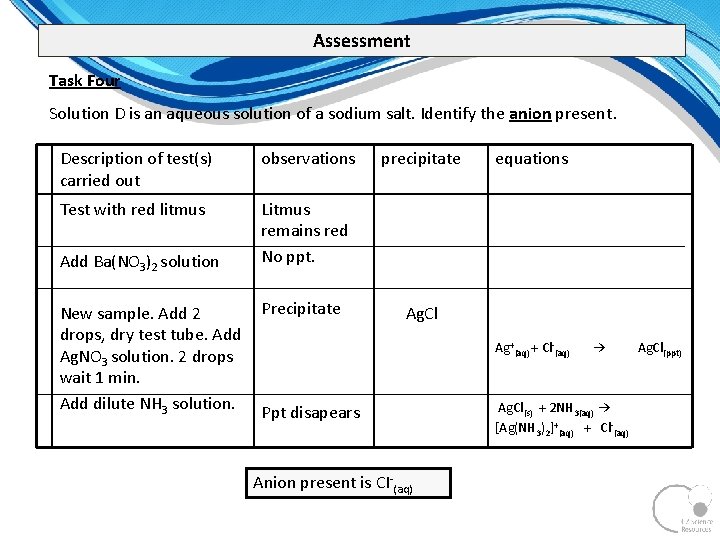
Assessment Task Four Solution D is an aqueous solution of a sodium salt. Identify the anion present. Description of test(s) carried out observations Test with red litmus Litmus remains red No ppt. Add Ba(NO 3)2 solution New sample. Add 2 drops, dry test tube. Add Ag. NO 3 solution. 2 drops wait 1 min. Add dilute NH 3 solution. Precipitate precipitate equations Ag. Cl Ag+(aq) + Cl (aq) Ppt disapears Anion present is CI (aq) → Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl (aq) Ag. Cl(ppt)

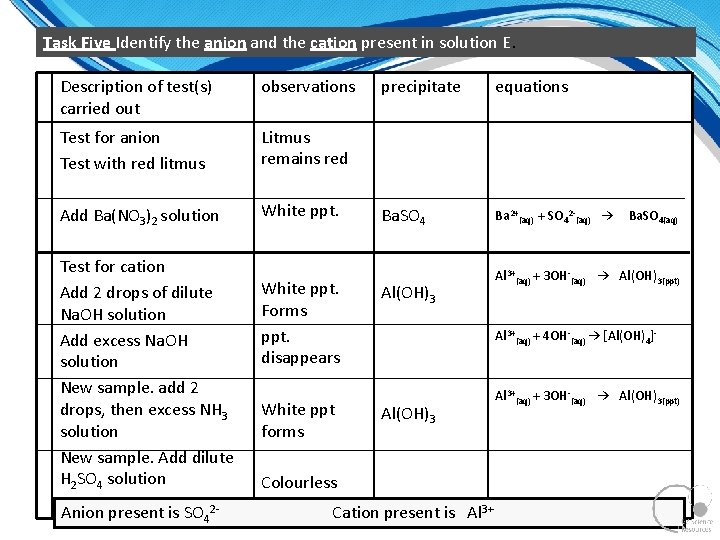
Task Five Identify the anion and the cation present in solution E. Description of test(s) carried out observations Test for anion Test with red litmus Litmus remains red Add Ba(NO 3)2 solution White ppt. Test for cation Add 2 drops of dilute Na. OH solution Add excess Na. OH solution New sample. add 2 drops, then excess NH 3 solution New sample. Add dilute H 2 SO 4 solution Anion present is SO 42 White ppt. Forms ppt. disappears White ppt forms precipitate equations Ba. SO 4 Ba 2+(aq) + SO 42 (aq) → Al(OH)3 Ba. SO 4(aq) Al 3+(aq) + 3 OH (aq) → Al(OH)3(ppt) Al 3+(aq) + 4 OH (aq) → [Al(OH)4] Al(OH)3 Colourless solution Cation present is Al 3+(aq) + 3 OH (aq) → Al(OH)3(ppt)

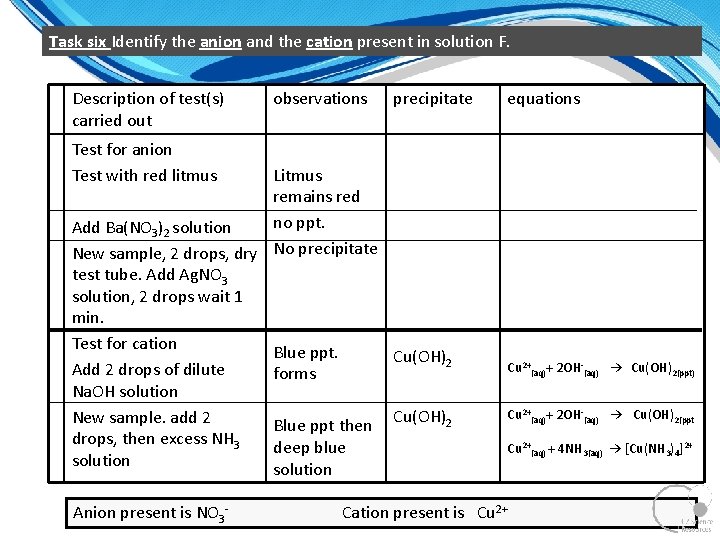
Task six Identify the anion and the cation present in solution F. Description of test(s) carried out observations precipitate equations Test for anion Test with red litmus Litmus remains red no ppt. Add Ba(NO 3)2 solution New sample, 2 drops, dry No precipitate test tube. Add Ag. NO 3 solution, 2 drops wait 1 min. Test for cation Blue ppt. Cu(OH)2 Add 2 drops of dilute forms Na. OH solution New sample. add 2 Blue ppt then Cu(OH)2 drops, then excess NH 3 deep blue solution Anion present is NO 3 Cu 2+(aq)+ 2 OH (aq) → Cu(OH)2(ppt) Cu 2+(aq)+ 2 OH (aq) → Cu(OH)2(ppt Cu 2+(aq) + 4 NH 3(aq) → [Cu(NH 3)4]2+ Cation present is Cu 2+
 Solubility rules ncea
Solubility rules ncea Ncea scholarship chemistry
Ncea scholarship chemistry Effects of carbon monoxide on humans
Effects of carbon monoxide on humans Positive ions and negative ions table
Positive ions and negative ions table ext{mg}_3 ext n_2mg 3 n 2
ext{mg}_3 ext n_2mg 3 n 2  Spectator ions in chemistry
Spectator ions in chemistry As 91165
As 91165 Ncea
Ncea Physics waves level 2
Physics waves level 2 Ncea
Ncea 2017 level 3 mechanics
2017 level 3 mechanics Solubility rules ncea
Solubility rules ncea Redox reactions ncea level 2
Redox reactions ncea level 2 As90940
As90940 90947
90947 Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Heavy ions
Heavy ions Qulitative analysis
Qulitative analysis What are ions
What are ions Aluminium oxide bond
Aluminium oxide bond Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Common acids
Common acids Enterohepatic circulation
Enterohepatic circulation Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions H ion name
H ion name Mitochondria sem
Mitochondria sem Common ions
Common ions Lewis structure cno-
Lewis structure cno- Qumica
Qumica Why do ions form
Why do ions form H ions
H ions Common polyatomic ions quiz
Common polyatomic ions quiz Sqed
Sqed Monatomic ion definition chemistry
Monatomic ion definition chemistry Srim the stopping and range of ions in matter
Srim the stopping and range of ions in matter Variable charge metal
Variable charge metal Urea ion
Urea ion Polyatomic ions sheet
Polyatomic ions sheet Order of mobility of alkali metal ions
Order of mobility of alkali metal ions Naming monatomic ions
Naming monatomic ions Spectator ions
Spectator ions Noble gas period 6
Noble gas period 6