NATS 101 Lecture 2 TR Atmospheric Composition Vertical




















- Slides: 43

NATS 101 Lecture 2 TR Atmospheric Composition Vertical Structure Weather & Climate

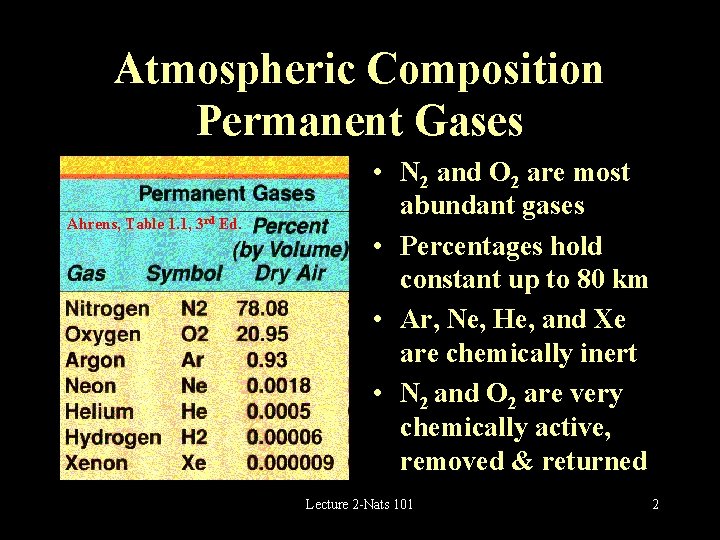
Atmospheric Composition Permanent Gases Ahrens, Table 1. 1, 3 rd Ed. • N 2 and O 2 are most abundant gases • Percentages hold constant up to 80 km • Ar, Ne, He, and Xe are chemically inert • N 2 and O 2 are very chemically active, removed & returned Lecture 2 -Nats 101 2

N 2 and O 2 N 2 Boiling point: 77 °K or -196°C or – 320 °F O 2 Boiling point: 90 °K or -183 °C or -297 °F Balance between input (production) and output (destruction): Input: plant/animal decaying Input: plant photosynthesis Sink: soil bacteria; Sink: organic matter decay oceanic plankton-->nutrients Lecture 2 -Nats 101 chemical bonding (oxidation) breathing 3

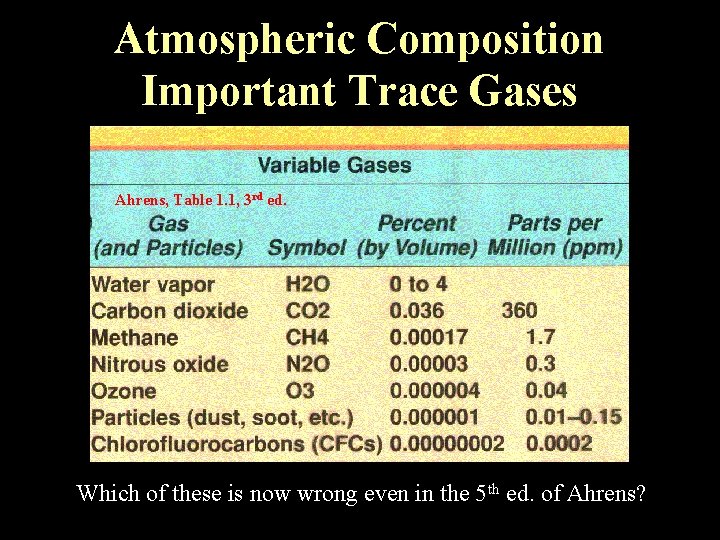
Atmospheric Composition Important Trace Gases Ahrens, Table 1. 1, 3 rd ed. th ed. of Ahrens? Which of these is now wrong even in the 5 Lecture 2 -Nats 101 4

Carbon Dioxide CO 2 Sources vegetative decay volcanic eruptions animal exhalation combustion of fossil fuels (CH 4 + 2 O 2 > 2 H 2 O + CO 2) Sinks photosynthesis (oxygen production) dissolves in water phytoplankton absorption (limestone formation) Lecture 2 -Nats 101 5

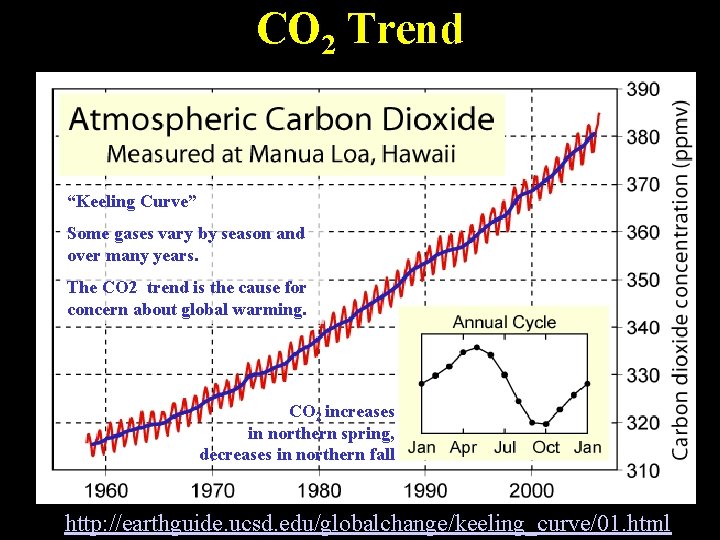
CO 2 Trend “Keeling Curve” Some gases vary by season and over many years. The CO 2 trend is the cause for concern about global warming. CO 2 increases in northern spring, decreases in northern fall Lecture 2 -Nats 101 6 http: //earthguide. ucsd. edu/globalchange/keeling_curve/01. html

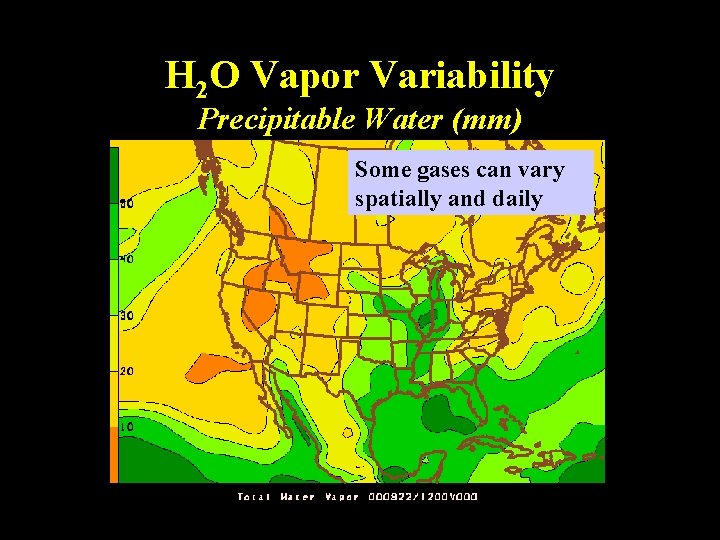
H 2 O Vapor Variability Precipitable Water (mm) Some gases can vary spatially and daily Lecture 2 -Nats 101 7

Aerosols 1 cm 3 of air can contain as many as 200, 000 non-gaseous particles. – – – – dust dirt (soil) salt from ocean spray volcanic ash water pollen pollutants Lecture 2 -Nats 101 8

Aerosols - Volcanic Ash Lecture 2 -Nats 101 9 Fig. 1 -4, p. 6

Aerosols - Dust Particles Dust Storm on Interstate 10, between Phoenix and Tucson, AZ. Lecture 2 -Nats 101 10

Aerosols • Provide surfaces upon which water vapor can condense. • Provide a surface area or catalyst needed for much atmospheric chemistry. • Aerosols can deplete stratospheric ozone. They can also cool the planet by reflecting sunlight back to space. Lecture 2 -Nats 101 11

Two Important Concepts Let’s introduce two new concepts. . . Density Pressure Lecture 2 -Nats 101 12

What is Density? Density (ρ) = Mass (M) per unit Volume (V) ρ = M/V ρ = Greek letter “rho” Typical Units: kg/m 3, gm/cm 3 Mass = # molecules x molecular weight (gm/mole) Avogadro number (6. 023 x 1023 molecules/mole) Lecture 2 -Nats 101 13

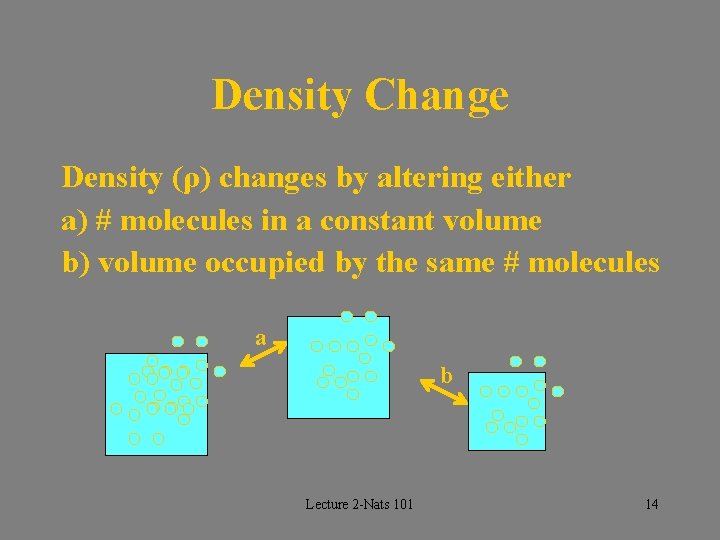
Density Change Density (ρ) changes by altering either a) # molecules in a constant volume b) volume occupied by the same # molecules a b Lecture 2 -Nats 101 14

What is Pressure? Pressure (p) = Force (F) per unit Area (A) Typical Units: pounds per square inch (psi), millibars (mb), inches Hg Average pressure at sea-level: 14. 7 psi 1013 mb 29. 92 in. Hg Lecture 2 -Nats 101 15

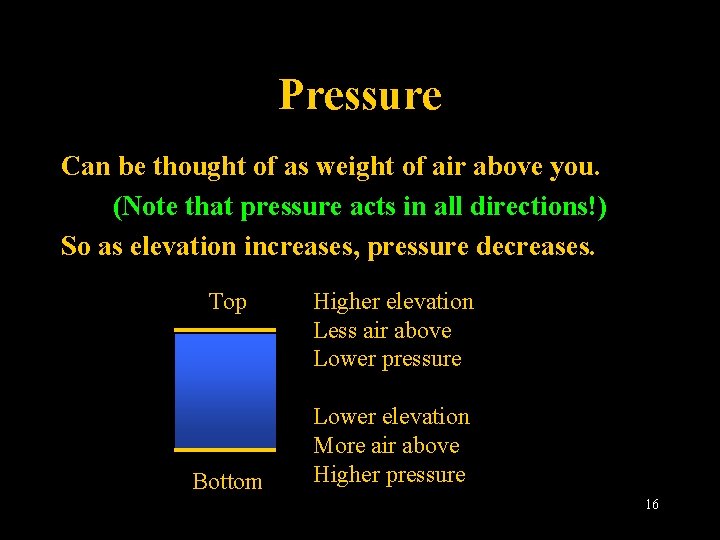
Pressure Can be thought of as weight of air above you. (Note that pressure acts in all directions!) So as elevation increases, pressure decreases. Top Bottom Higher elevation Less air above Lower pressure Lower elevation More air above Higher pressure Lecture 2 -Nats 101 16

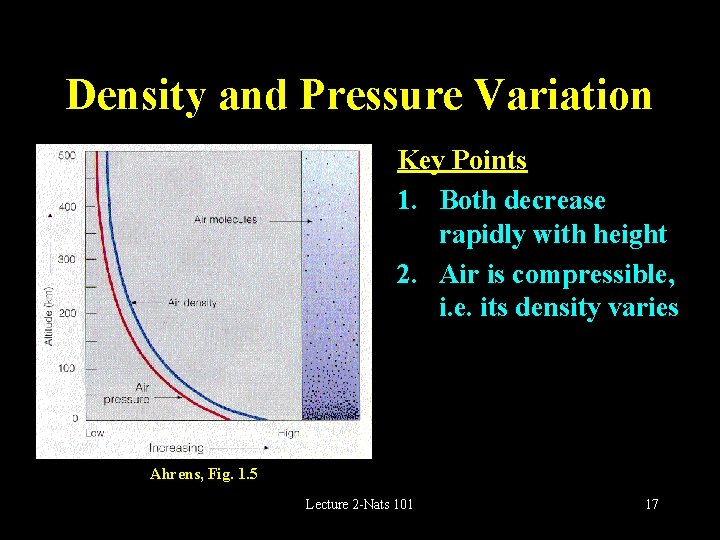
Density and Pressure Variation Key Points 1. Both decrease rapidly with height 2. Air is compressible, i. e. its density varies Ahrens, Fig. 1. 5 Lecture 2 -Nats 101 17

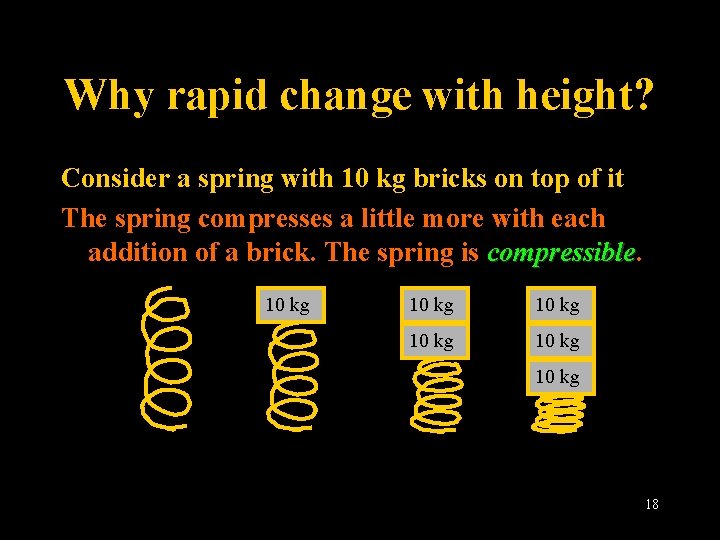
Why rapid change with height? Consider a spring with 10 kg bricks on top of it The spring compresses a little more with each addition of a brick. The spring is compressible 10 kg 10 kg Lecture 2 -Nats 101 18

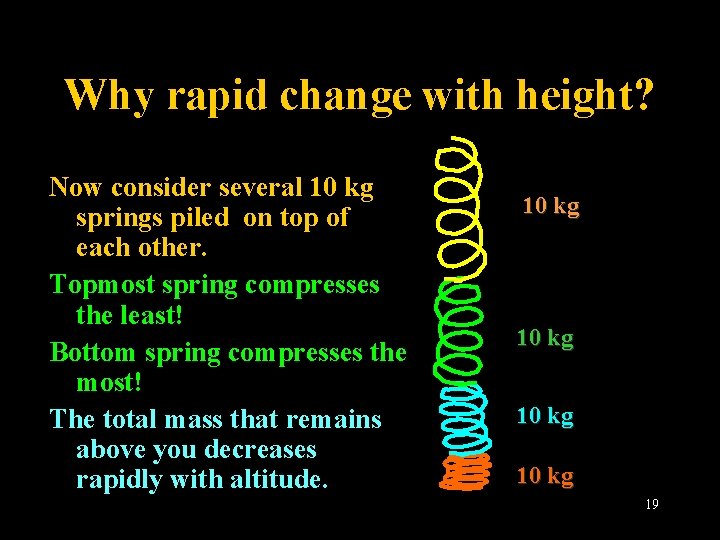
Why rapid change with height? Now consider several 10 kg springs piled on top of each other. Topmost spring compresses the least! Bottom spring compresses the most! The total mass that remains above you decreases rapidly with altitude. Lecture 2 -Nats 101 10 kg 19

Why rapid change with height? Finally, consider piled-up parcels of air, each with the same # molecules. The bottom parcel is squished the most. Its density is the highest. Density more slowly as elevation increases. Lecture 2 -Nats 101 20

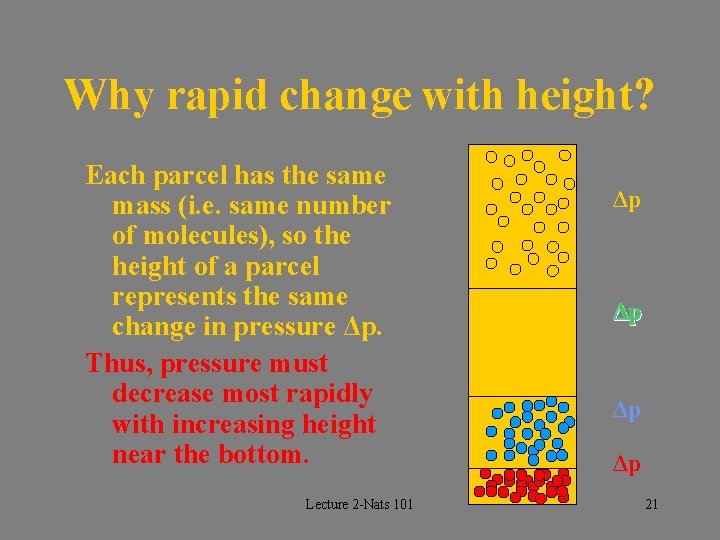
Why rapid change with height? Each parcel has the same mass (i. e. same number of molecules), so the height of a parcel represents the same change in pressure Δp. Thus, pressure must decrease most rapidly with increasing height near the bottom. Lecture 2 -Nats 101 Δp Δp 21

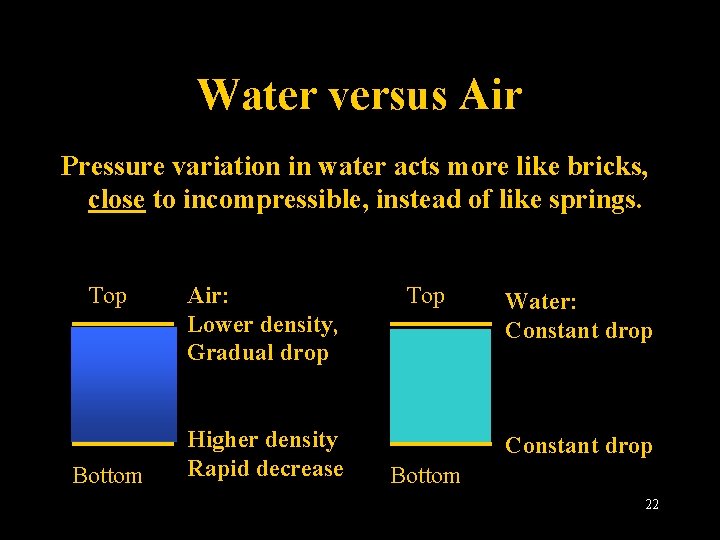
Water versus Air Pressure variation in water acts more like bricks, close to incompressible, instead of like springs. Top Bottom Air: Lower density, Gradual drop Higher density Rapid decrease Top Water: Constant drop Bottom Lecture 2 -Nats 101 22

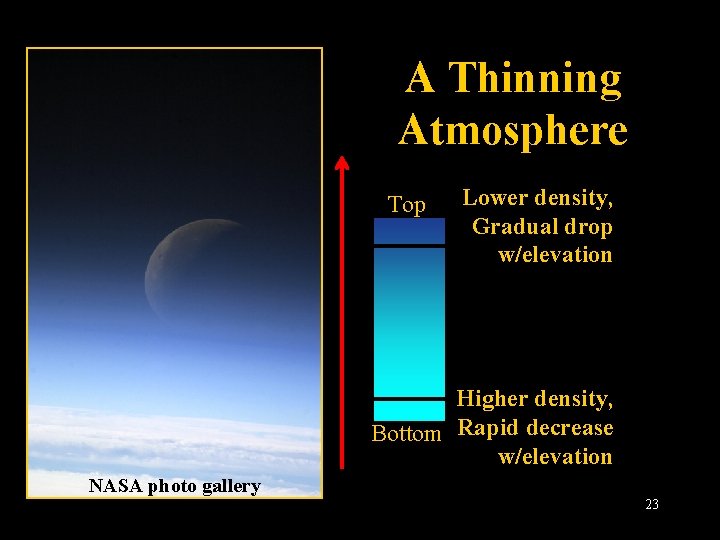
A Thinning Atmosphere Top Lower density, Gradual drop w/elevation Higher density, Bottom Rapid decrease w/elevation NASA photo gallery Lecture 2 -Nats 101 23

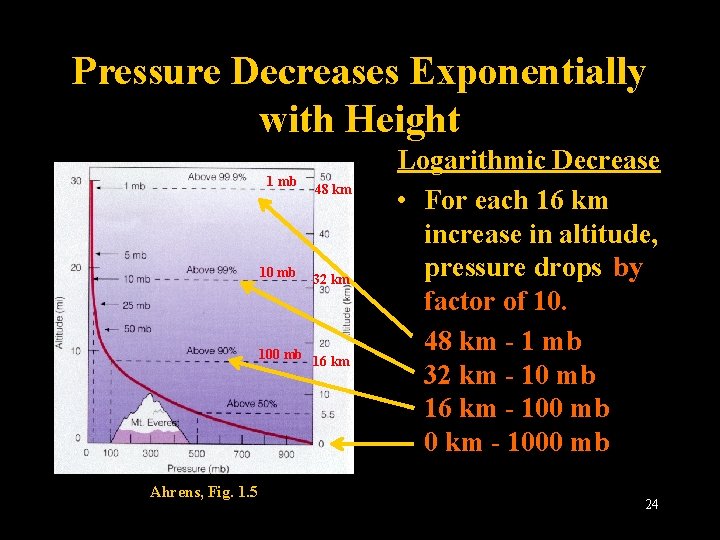
Pressure Decreases Exponentially with Height 1 mb 10 mb 48 km 32 km 100 mb 16 km Ahrens, Fig. 1. 5 Logarithmic Decrease • For each 16 km increase in altitude, pressure drops by factor of 10. 48 km - 1 mb 32 km - 10 mb 16 km - 100 mb 0 km - 1000 mb Lecture 2 -Nats 101 24

Equation for Pressure Variation We can Quantify Pressure Change with Height Lecture 2 -Nats 101 26

What is Pressure at 2. 8 km? (Summit of Mt. Lemmon) Use Equation for Pressure Change Lecture 2 -Nats 101 27

What is Pressure at Tucson? Use Equation for Pressure Change Let’s get cocky… How about Denver? Z=1, 600 m How about Mt. Everest? Z=8, 700 m You try these examples at home for practice Lecture 2 -Nats 101 28

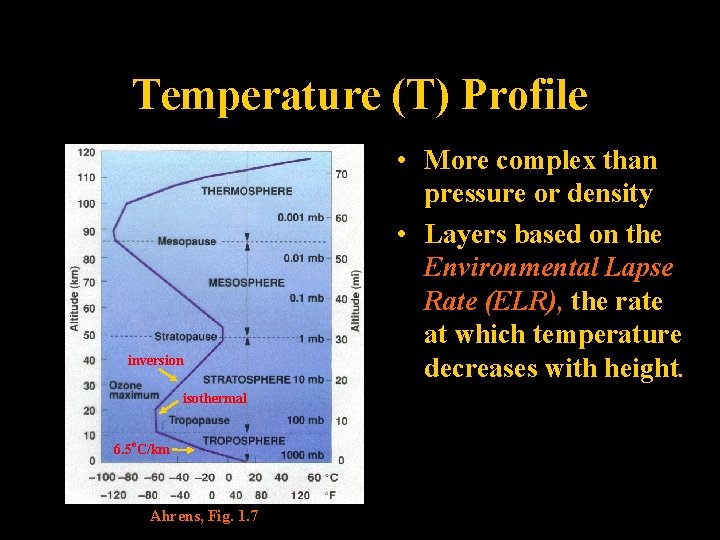
Temperature (T) Profile inversion • More complex than pressure or density • Layers based on the Environmental Lapse Rate (ELR), the rate at which temperature decreases with height. isothermal 6. 5 o. C/km Ahrens, Fig. 1. 7 Lecture 2 -Nats 101 29

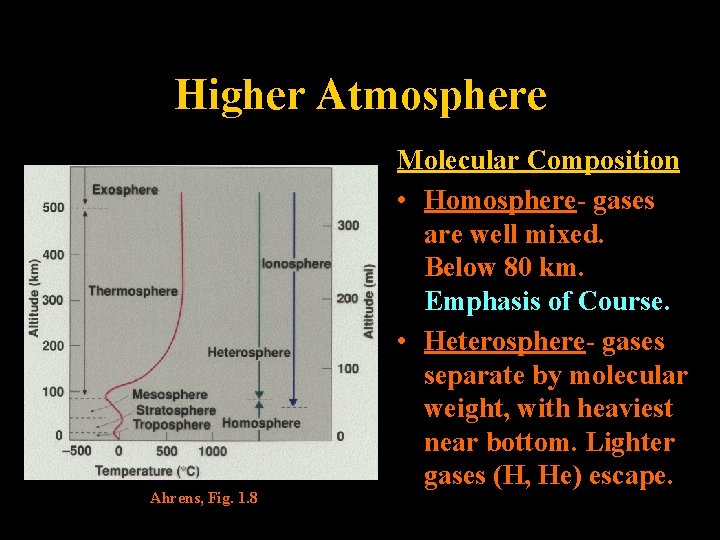
Higher Atmosphere Ahrens, Fig. 1. 8 Molecular Composition • Homosphere- gases are well mixed. Below 80 km. Emphasis of Course. • Heterosphere- gases separate by molecular weight, with heaviest near bottom. Lighter gases (H, He) escape. Lecture 2 -Nats 101 30

Atmospheric Layers Essentials • Thermosphere-above 85 km Temps warm w/height Gases settle by molecular weight (Heterosphere) • Mesosphere-50 to 85 km Temps cool w/height • Stratosphere-10 to 50 km Temps warm w/height, very dry • Troposphere-0 to 10 km (to the nearest 5 km) Temps cool with height Contains “all” H 2 O vapor, weather of public interest Lecture 2 -Nats 101 31

Take Home Points • Many gases make up air N 2 and O 2 account for ~99% Trace gases: CO 2, H 2 O, O 3, etc. Some are very important…more later • Pressure and Density Decrease rapidly with height • Temperature Complex vertical structure Lecture 2 -Nats 101 32

Climate and Weather “Climate is what you expect. Weather is what you get. ” -Robert A. Heinlein Lecture 2 -Nats 101 33

Weather – The state of the atmosphere: for a specific place at a particular time Weather Elements 1) Temperature 2) Pressure 3) Humidity 4) Wind 5) Visibility 6) Clouds 7) Significant Weather Lecture 2 -Nats 101 34

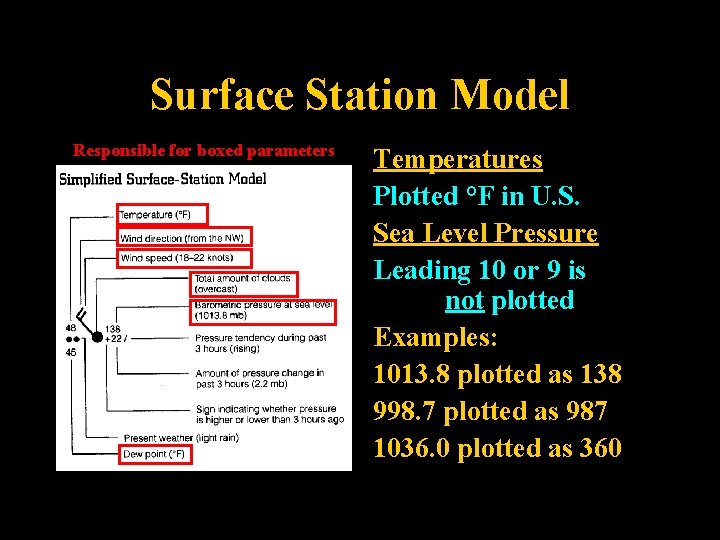
Surface Station Model Responsible for boxed parameters Ahrens, p 431 Temperatures Plotted °F in U. S. Sea Level Pressure Leading 10 or 9 is not plotted Examples: 1013. 8 plotted as 138 998. 7 plotted as 987 1036. 0 plotted as 360 Lecture 2 -Nats 101 35

Sky Cover and Weather Symbols Ahrens, p 431 Lecture 2 -Nats 101 36

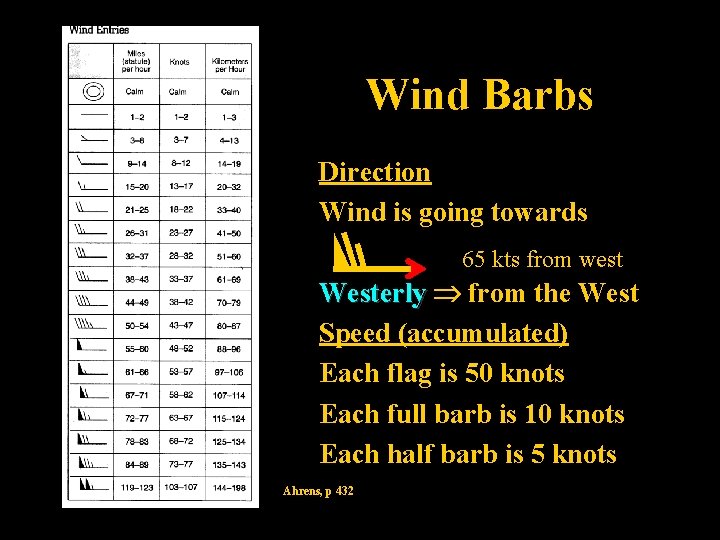
Wind Barbs Direction Wind is going towards 65 kts from west Westerly from the West Speed (accumulated) Each flag is 50 knots Each full barb is 10 knots Each half barb is 5 knots Ahrens, p 432 Lecture 2 -Nats 101 38

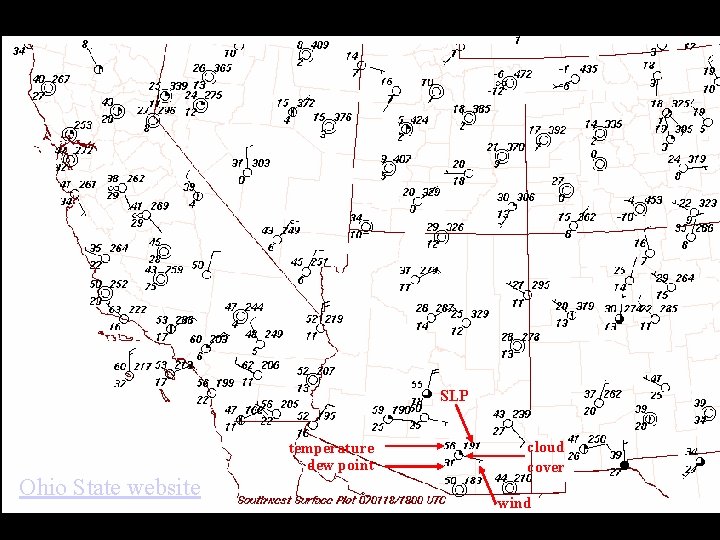
SLP Ohio State website temperature dew point Lecture 2 -Nats 101 cloud cover wind 39

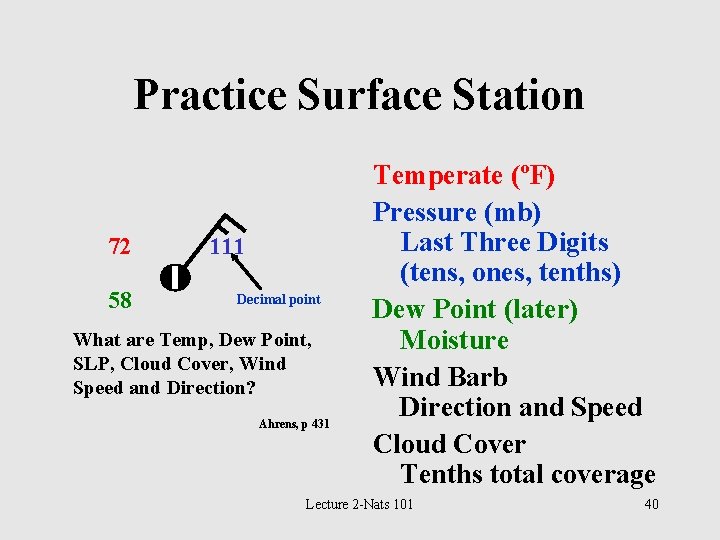
Practice Surface Station 72 58 111 Decimal point What are Temp, Dew Point, SLP, Cloud Cover, Wind Speed and Direction? Ahrens, p 431 Temperate (o. F) Pressure (mb) Last Three Digits (tens, ones, tenths) Dew Point (later) Moisture Wind Barb Direction and Speed Cloud Cover Tenths total coverage Lecture 2 -Nats 101 40

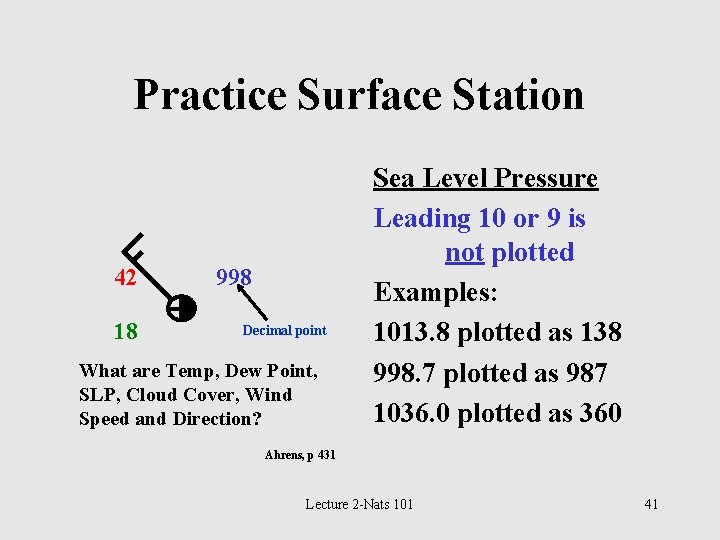
Practice Surface Station 42 18 998 Decimal point What are Temp, Dew Point, SLP, Cloud Cover, Wind Speed and Direction? Sea Level Pressure Leading 10 or 9 is not plotted Examples: 1013. 8 plotted as 138 998. 7 plotted as 987 1036. 0 plotted as 360 Ahrens, p 431 Lecture 2 -Nats 101 41

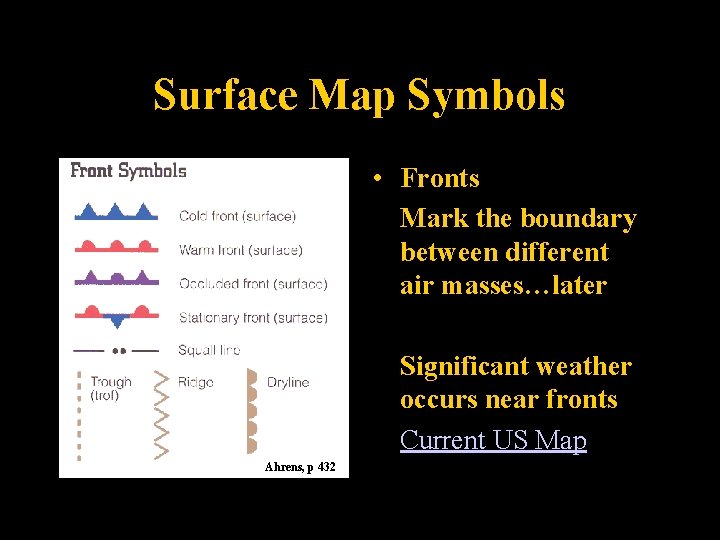
Surface Map Symbols • Fronts Mark the boundary between different air masses…later Significant weather occurs near fronts Current US Map Ahrens, p 432 Lecture 2 -Nats 101 42

Radiosonde Weather balloons, or radiosondes, sample atmospheric to 10 mb. They measure temperature moisture pressure They are tracked by GPS to get winds Lecture 2 -Nats 101 44

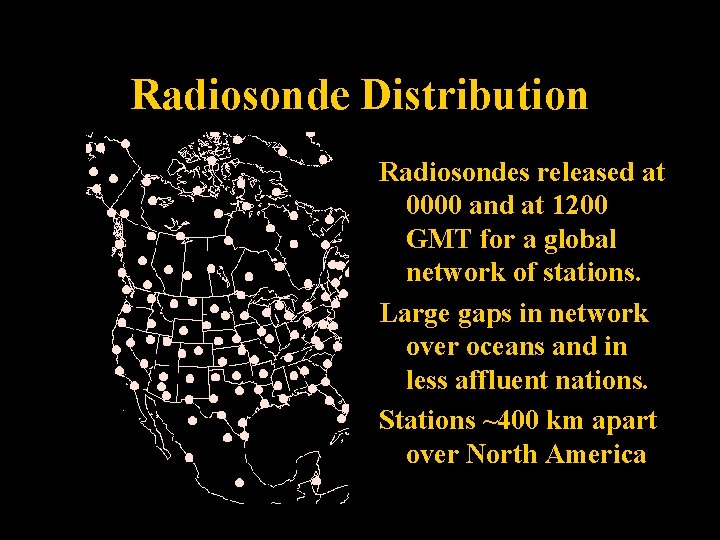
Radiosonde Distribution Radiosondes released at 0000 and at 1200 GMT for a global network of stations. Large gaps in network over oceans and in less affluent nations. Stations ~400 km apart over North America Lecture 2 -Nats 101 45

Next Class Assignment Temperature and Heat • Reading - Ahrens 3 rd: 25 -30 4 th: 25 -30 5 th: 25 -30 • Homework 01 - D 2 L (Due Monday Jan 25 th) 3 rd-Pg 52: 2. 1, 2, 3, 4 4 th-Pg 52: 2. 1, 2, 3, 4 5 th-Pg 52: 2. 1, 2, 3, 4 Lecture 2 -Nats 101 49