Multiple Reactions Chapter 6 Multiple Reactions Seldom is



















- Slides: 58

Multiple Reactions Chapter 6

Multiple Reactions • Seldom is the reaction of interest the only one occurs in the chemical reactor. Typically multiple reaction will occur, some desired and some undesired. • The key factor in economic success of chemical plant is minimizing the undesired side reactions.

Multiple Reactions The objectives of this chapter are 1. To discuss the general mole balance and reactor selection for multiple reaction. 2. Define different types of multiple reactions such as series, parallel, independent and complex reactions. 3. Define the selectivity parameters in terms of minimizing the undesired products. 4. Develop the algorithm to solve process with multiple reactions.





Desired & Undesired Reactions (Selectivity and Yield)

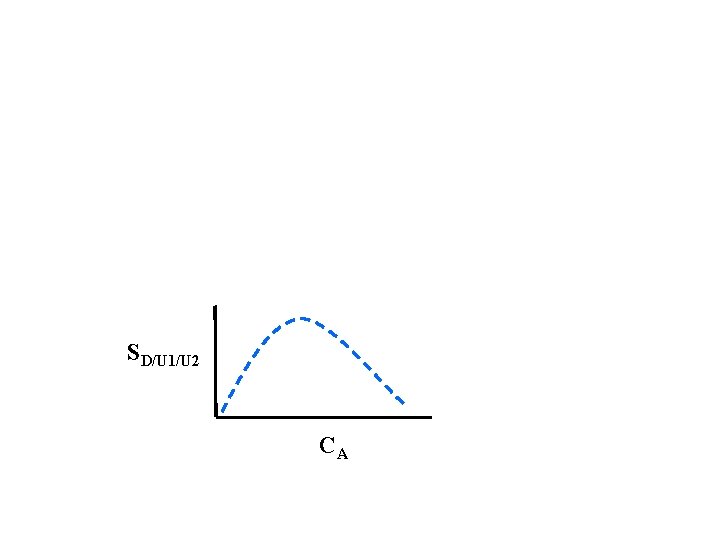
SD/U 1/U 2 CA

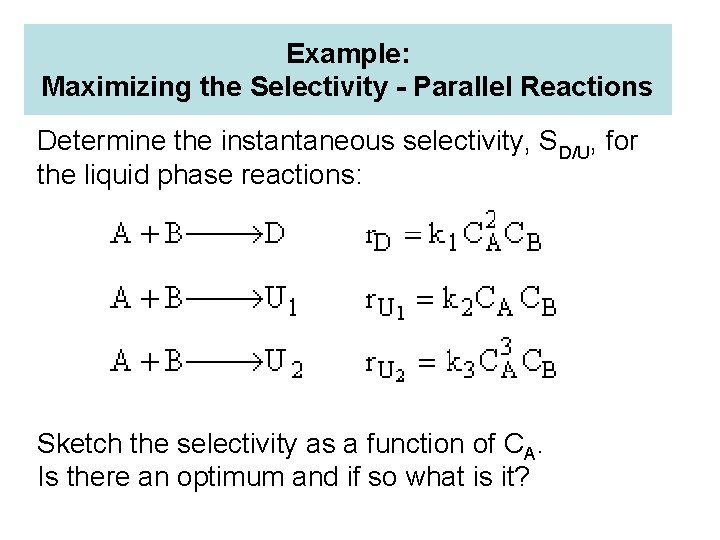
Example: Maximizing the Selectivity - Parallel Reactions Determine the instantaneous selectivity, SD/U, for the liquid phase reactions: Sketch the selectivity as a function of CA. Is there an optimum and if so what is it?

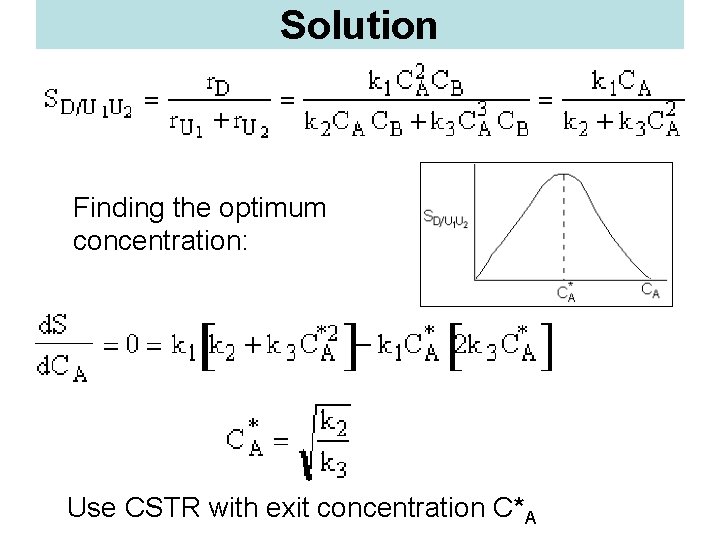
Solution Finding the optimum concentration: Use CSTR with exit concentration C*A

Solution In general if the order of desired product higher, then CA should be maintained higher (batch & PFR) are recommended If the order of undesired reaction is higher then CA should be maintained at minimum (CSTR is recommended)

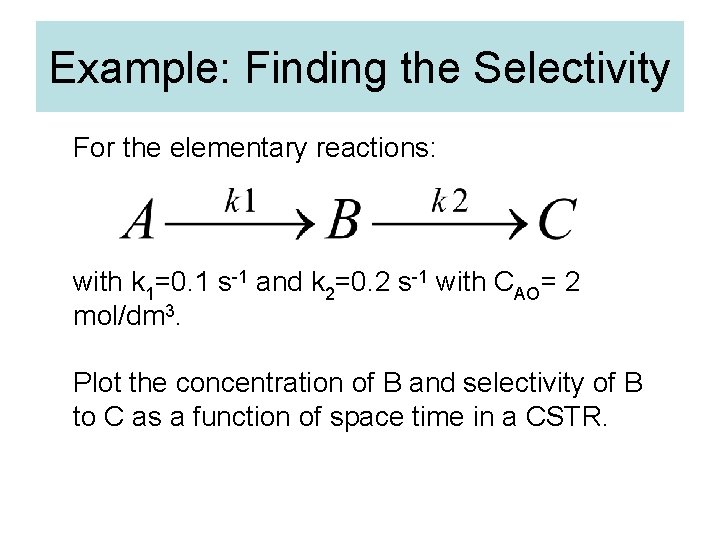
Example: Finding the Selectivity For the elementary reactions: with k 1=0. 1 s-1 and k 2=0. 2 s-1 with CAO= 2 mol/dm 3. Plot the concentration of B and selectivity of B to C as a function of space time in a CSTR.

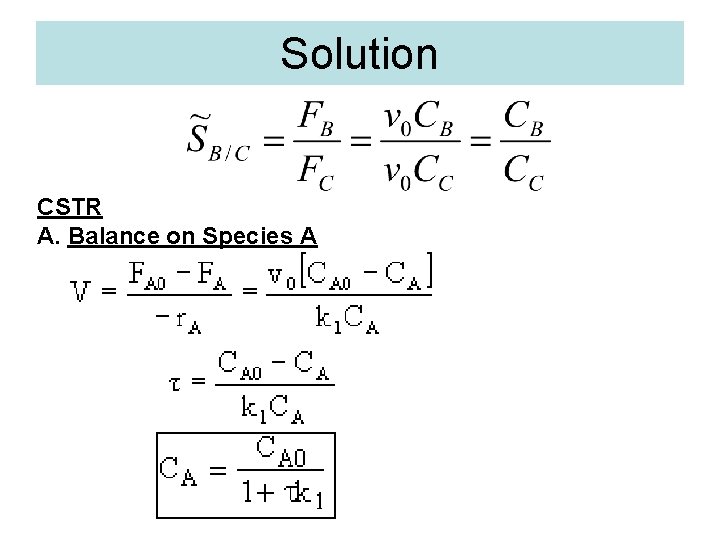
Solution CSTR A. Balance on Species A

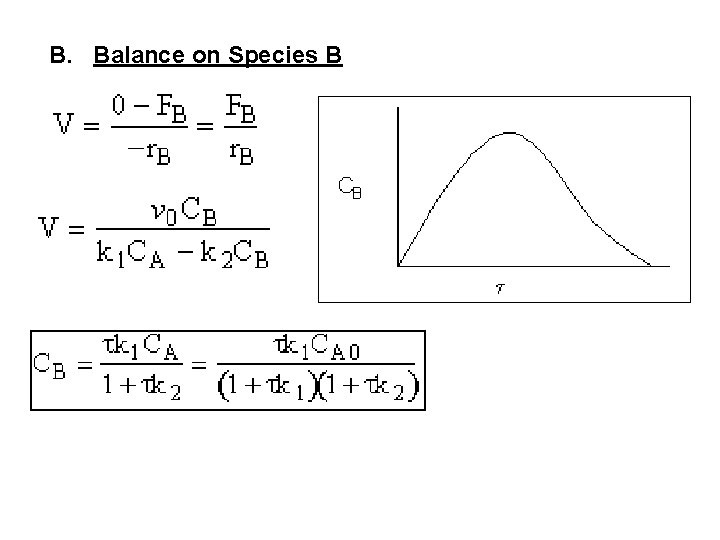
B. Balance on Species B

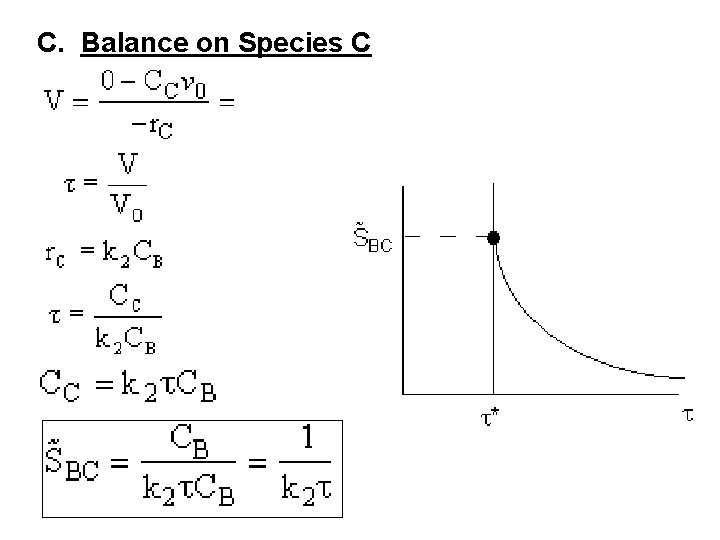
C. Balance on Species C

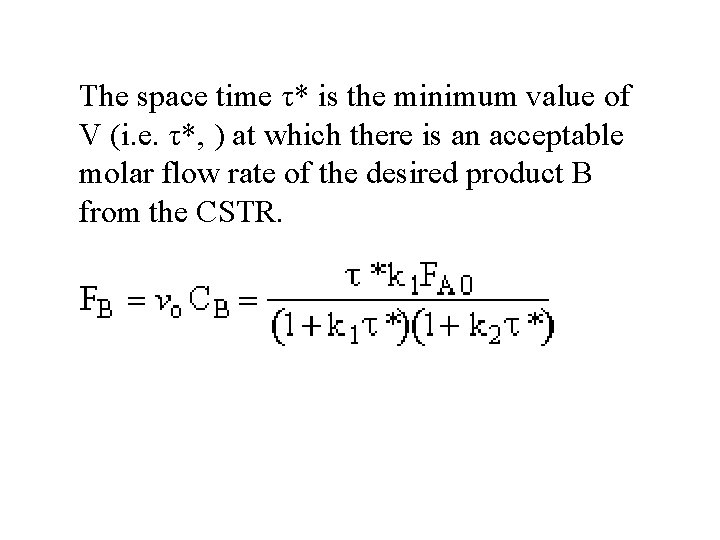
The space time τ* is the minimum value of V (i. e. τ*, ) at which there is an acceptable molar flow rate of the desired product B from the CSTR.

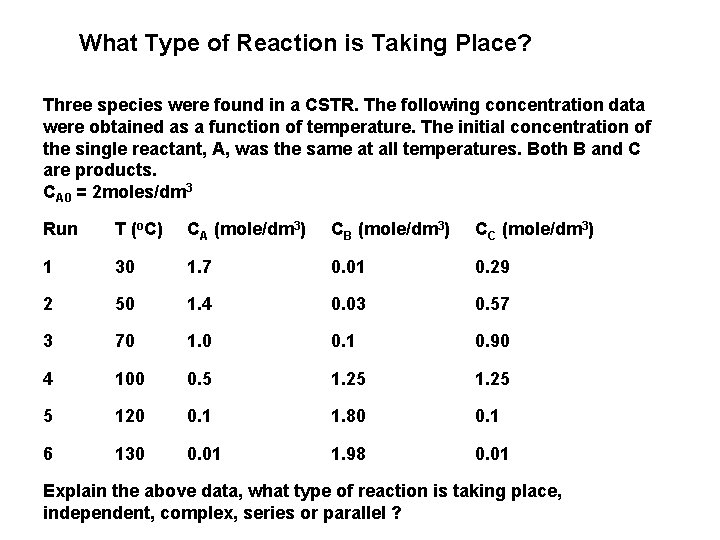
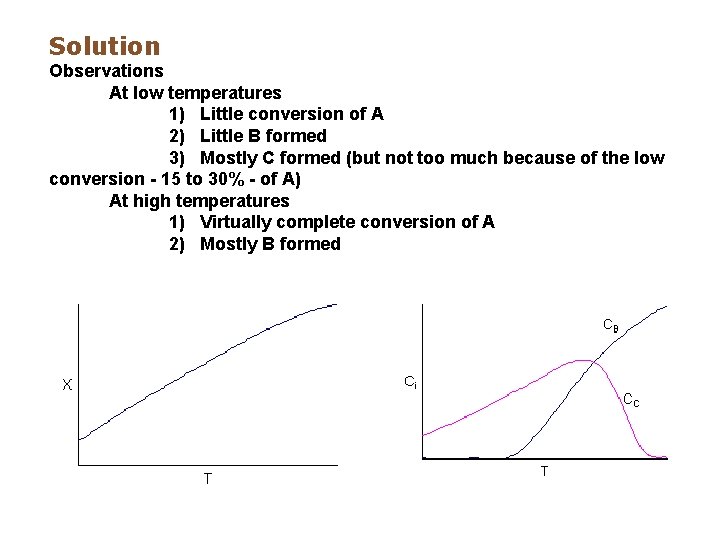
What Type of Reaction is Taking Place? Three species were found in a CSTR. The following concentration data were obtained as a function of temperature. The initial concentration of the single reactant, A, was the same at all temperatures. Both B and C are products. CA 0 = 2 moles/dm 3 Run T (o. C) CA (mole/dm 3) CB (mole/dm 3) CC (mole/dm 3) 1 30 1. 7 0. 01 0. 29 2 50 1. 4 0. 03 0. 57 3 70 1. 0 0. 1 0. 90 4 100 0. 5 1. 25 5 120 0. 1 1. 80 0. 1 6 130 0. 01 1. 98 0. 01 Explain the above data, what type of reaction is taking place, independent, complex, series or parallel ?

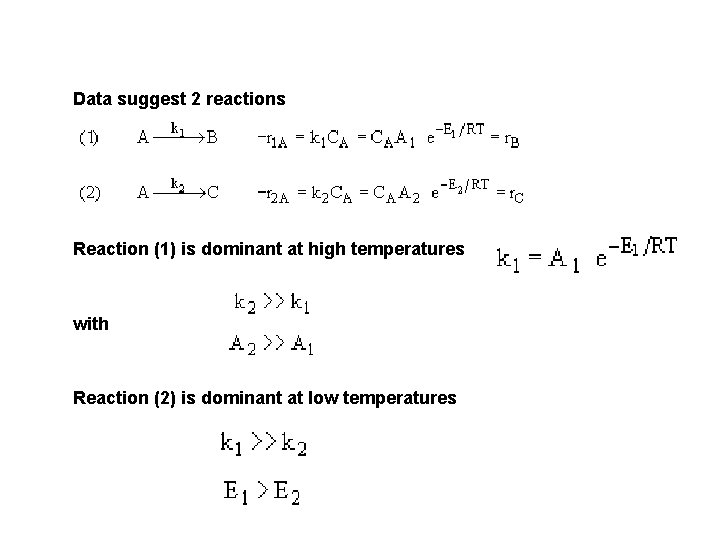
Solution Observations At low temperatures 1) Little conversion of A 2) Little B formed 3) Mostly C formed (but not too much because of the low conversion - 15 to 30% - of A) At high temperatures 1) Virtually complete conversion of A 2) Mostly B formed

Data suggest 2 reactions Reaction (1) is dominant at high temperatures with Reaction (2) is dominant at low temperatures


Reactor selection and operation • Types of reactors – Batch reactor – PFR (with and without recycle) – CSTR (with and without recycle) – Semibatch reactors – Membrane reactor

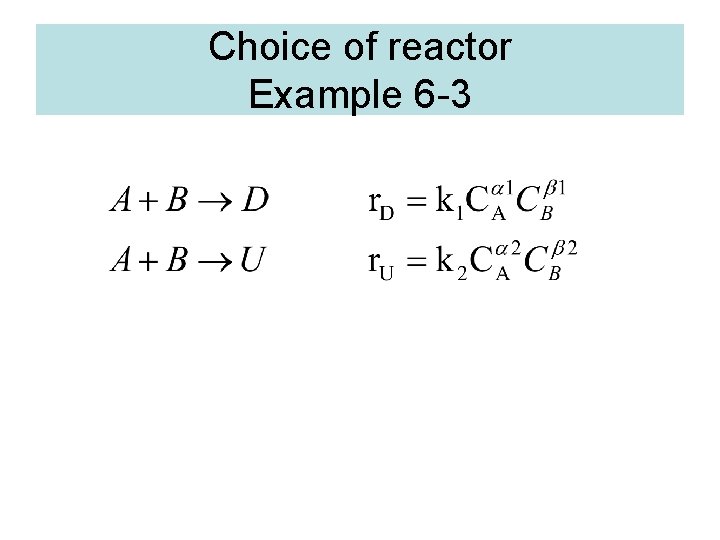
Choice of reactor Example 6 -3





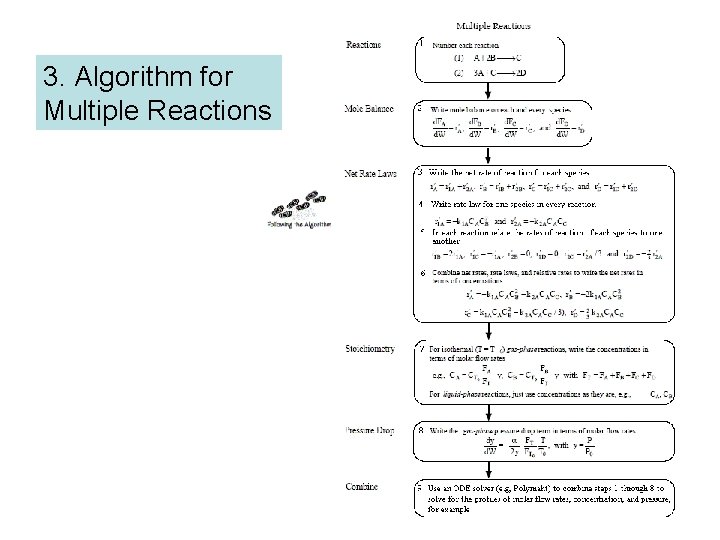
3. Algorithm for Multiple Reactions

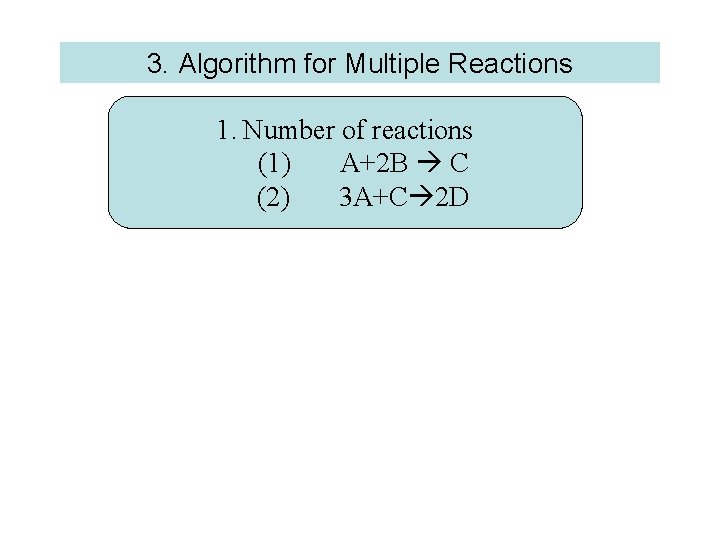
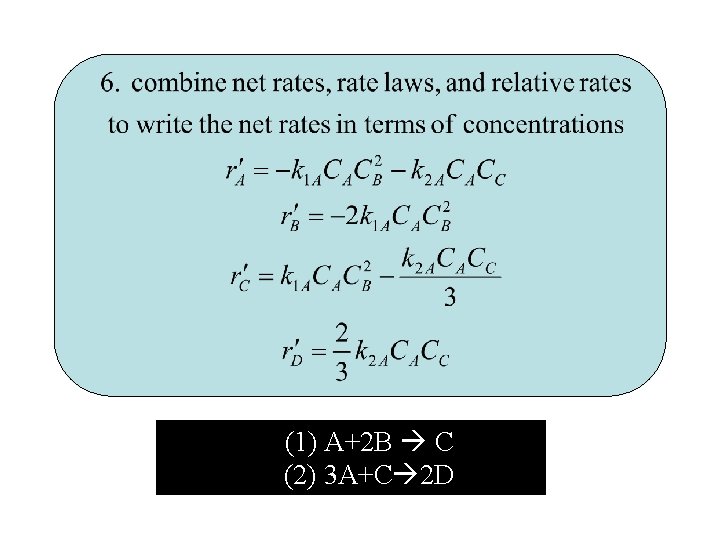
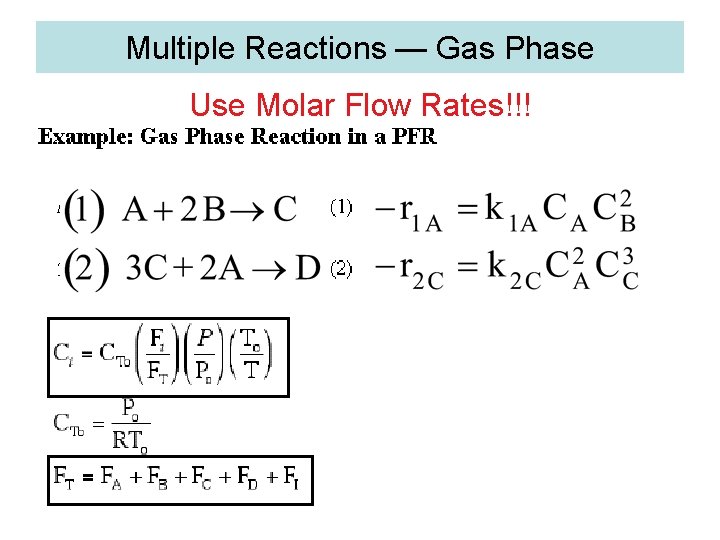
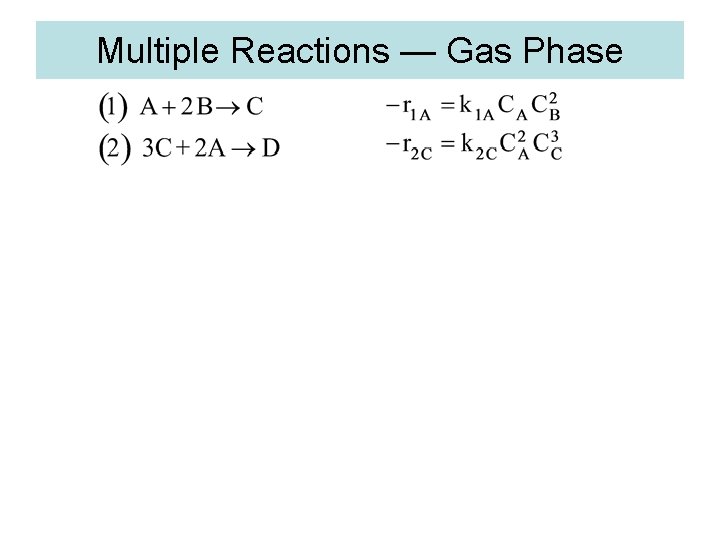
3. Algorithm for Multiple Reactions 1. Number of reactions (1) A+2 B C (2) 3 A+C 2 D


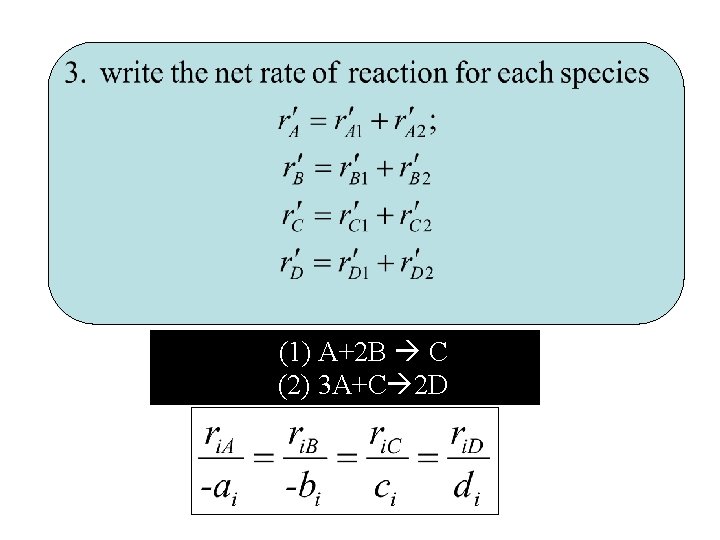
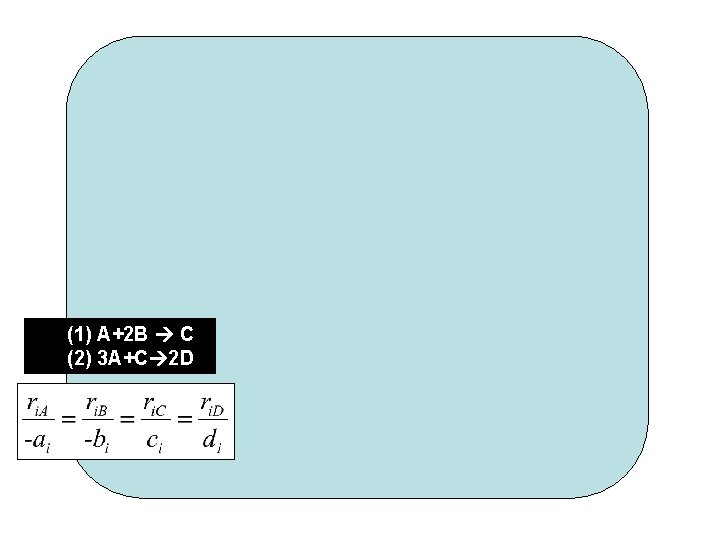
(1) A+2 B C (2) 3 A+C 2 D

(1) A+2 B C (2) 3 A+C 2 D

(1) A+2 B C (2) 3 A+C 2 D

(1) A+2 B C (2) 3 A+C 2 D




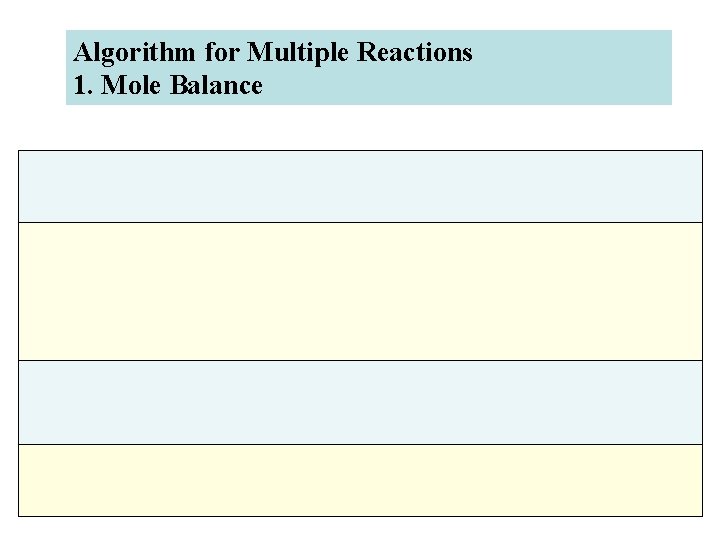
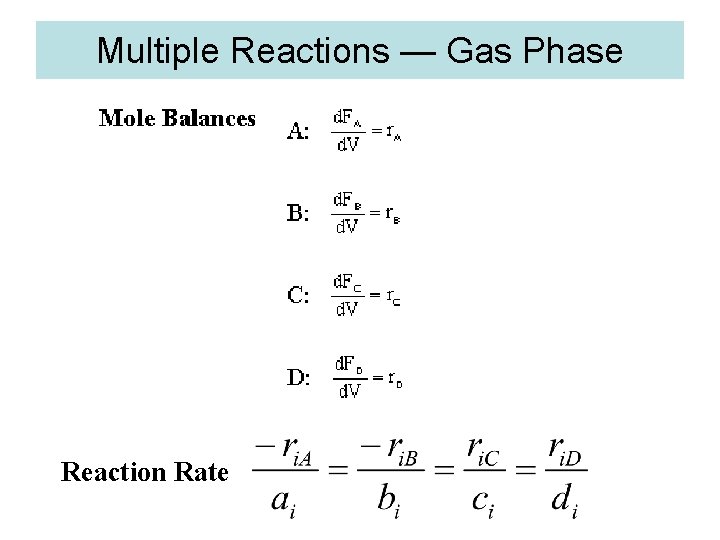
Algorithm for Multiple Reactions 1. Mole Balance

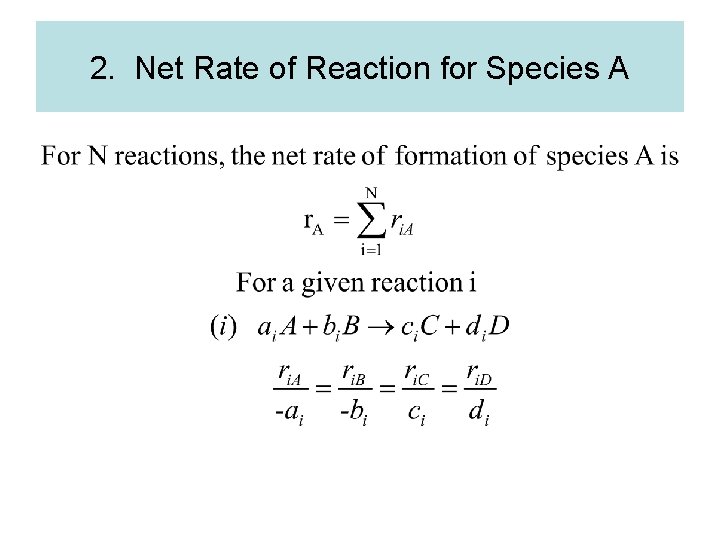
2. Net Rate of Reaction for Species A

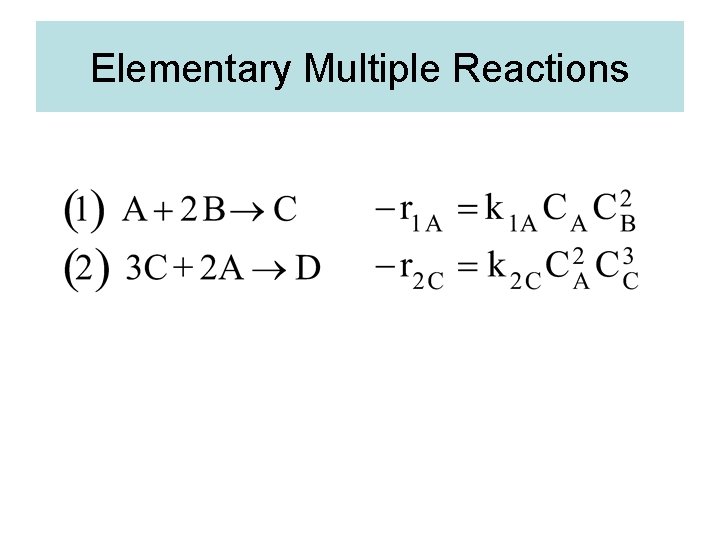
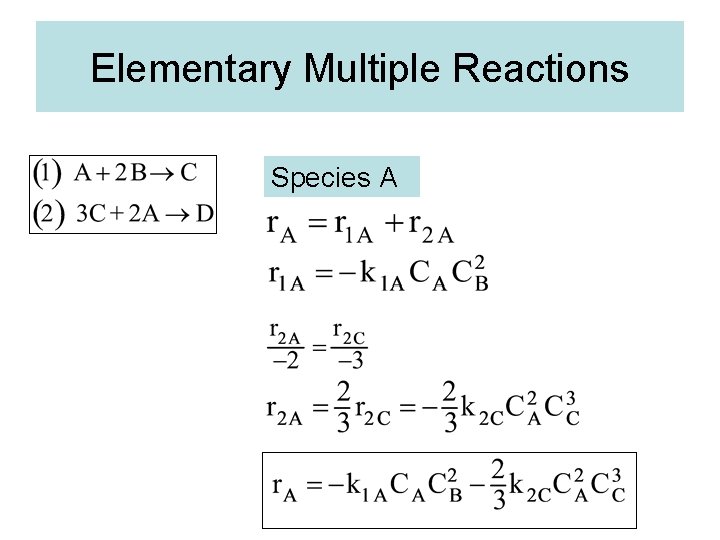
Elementary Multiple Reactions

Elementary Multiple Reactions Species A

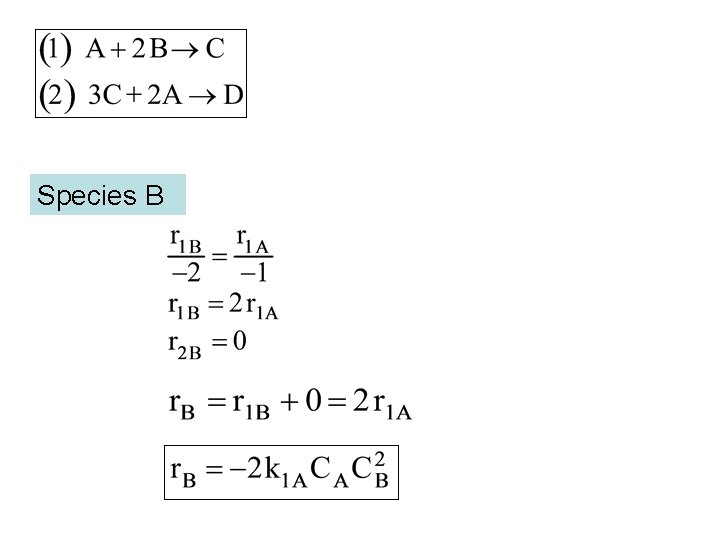
Species B

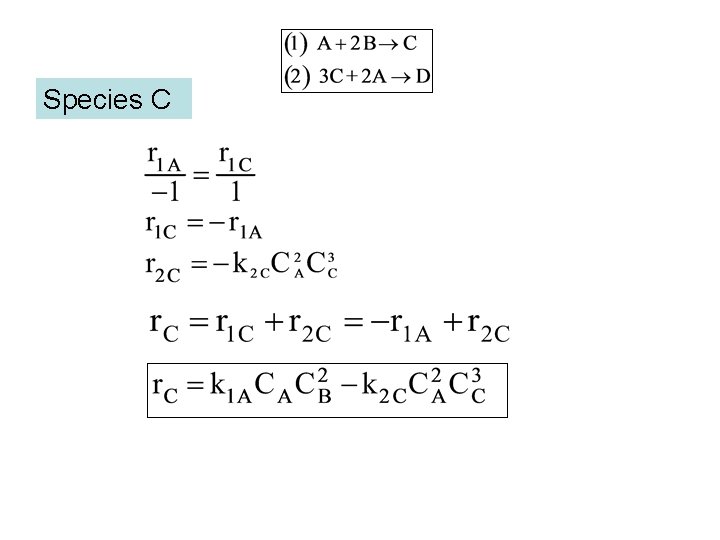
Species C

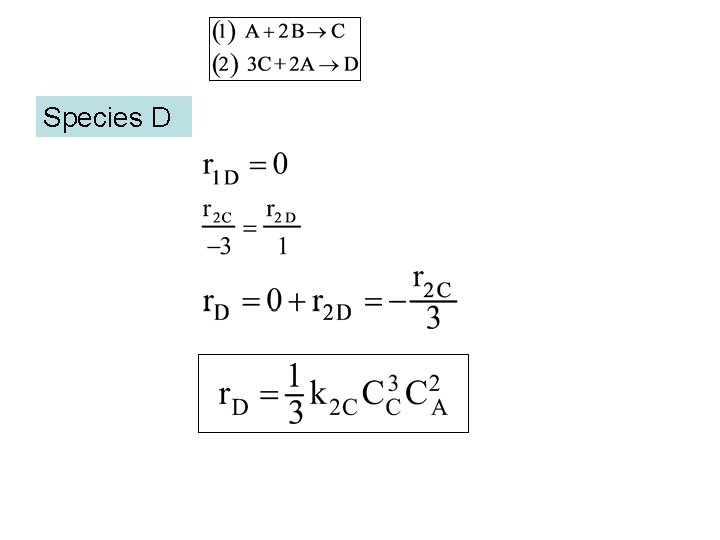
Species D

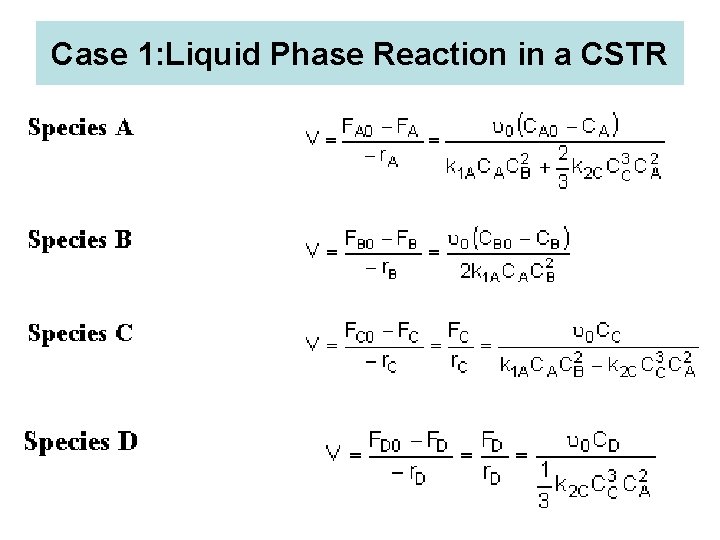
Case 1: Liquid Phase Reaction in a CSTR


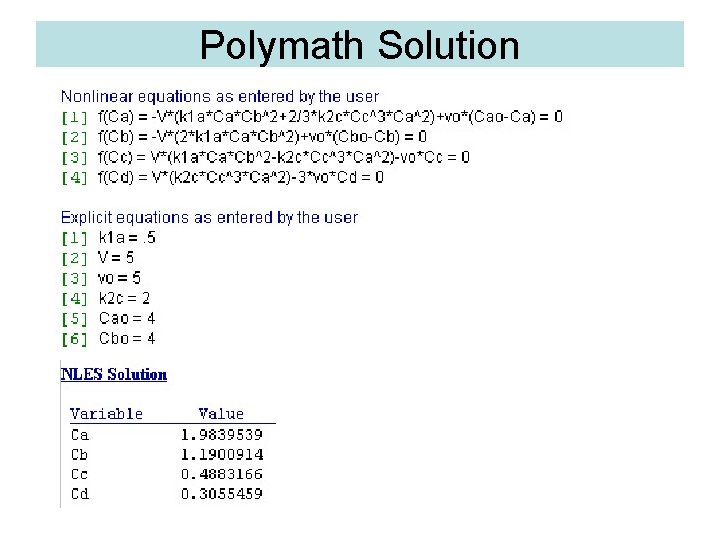
Polymath Solution

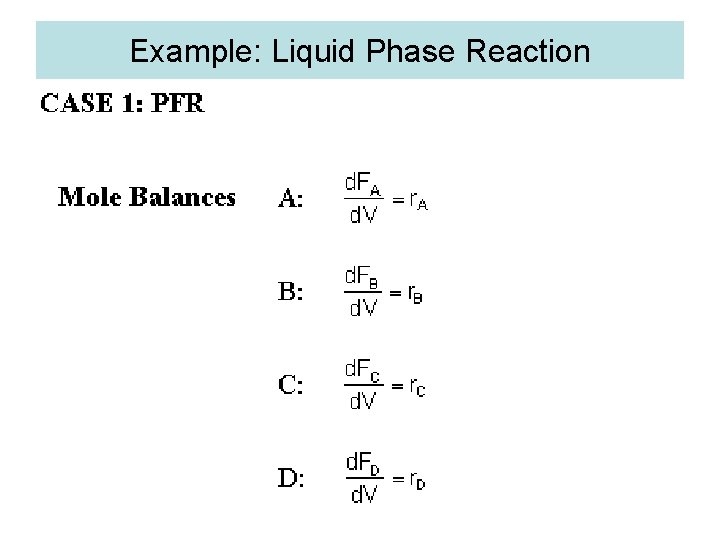
Example: Liquid Phase Reaction

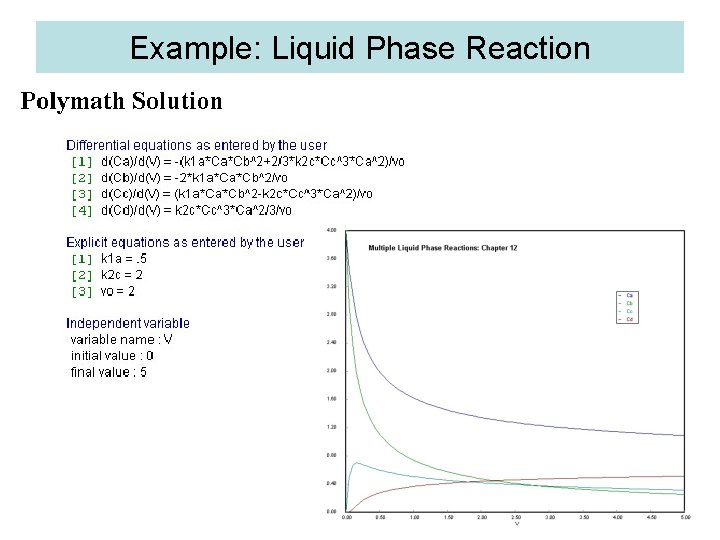
Example: Liquid Phase Reaction

Example: Liquid Phase Reaction

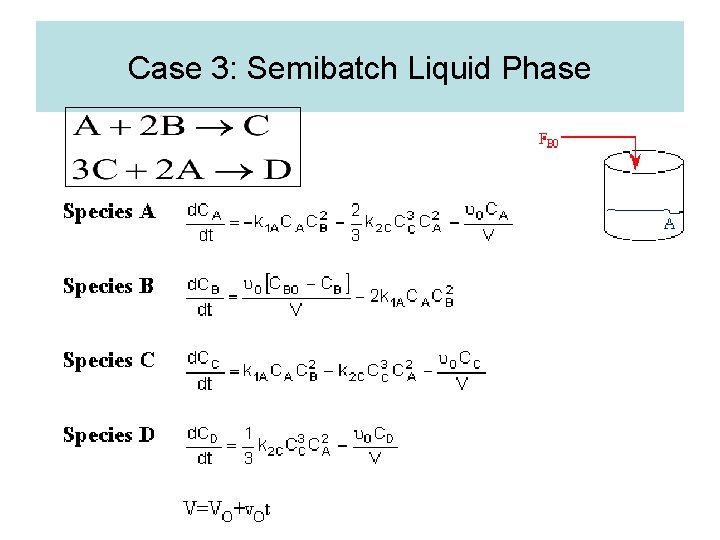
Case 3: Semibatch Liquid Phase

Multiple Reactions — Gas Phase Use Molar Flow Rates!!!

Multiple Reactions — Gas Phase Reaction Rate

Multiple Reactions — Gas Phase

Multiple Reactions — Gas Phase

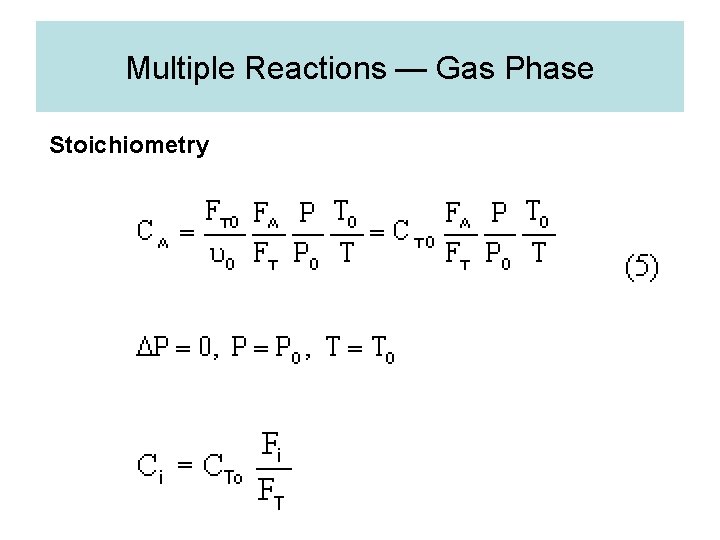
Multiple Reactions — Gas Phase Stoichiometry

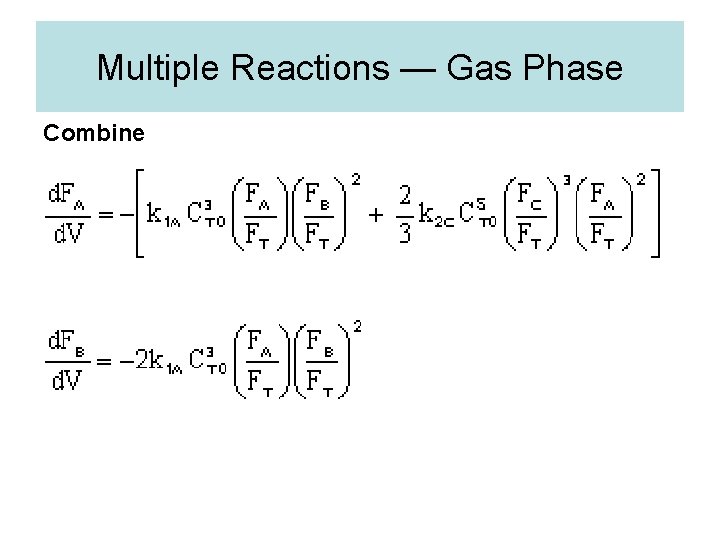
Multiple Reactions — Gas Phase Combine

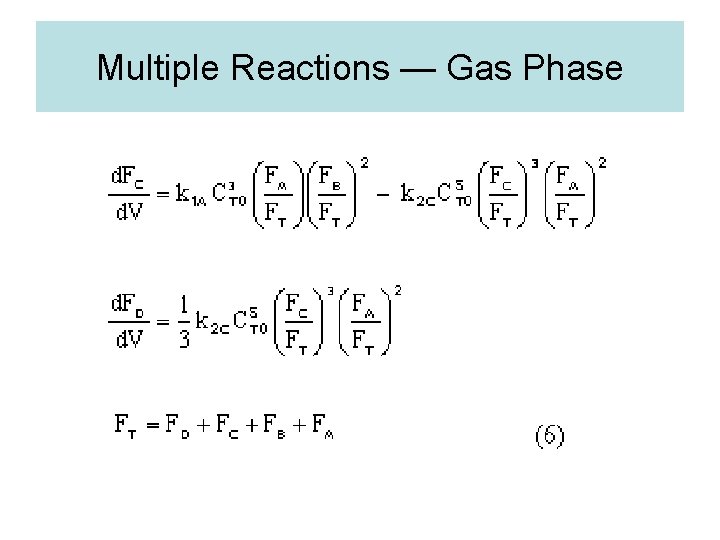
Multiple Reactions — Gas Phase