Minimizing Onset to Stroke Treatment Time MOST Stroke









- Slides: 35

Minimizing Onset to Stroke Treatment Time (MOST) Stroke Trial A Randomized, Open-Label, Multi-Arm, Adaptive Phase 3 Clinical Trial Opeolu Adeoye, MD MS Andrew Barreto, MD MS Joseph Broderick, MD James Grotta, MD August 13, 2014

Team � Co-PIs ◦ ◦ Opeolu Adeoye, University of Cincinnati Andrew Barreto, University of Texas-Houston Jim Grotta, Memorial Hermann Hospital, Houston Joe Broderick, University of Cincinnati � Primary Statisticians ◦ Berry Consultants � Data Management/Unblinded Statisticians ◦ Stroke. NET NDMC at MUSC � Enrollment ◦ Stroke. NET, NETT, US/International 2

Background � Stroke. NET Primary Objective: ◦ “…an adaptive trial to identify which of the currently available intravenous thrombolytic drugs, either alone or in combination with other strategies, are superior for the treatment of acute ischemic stroke within the current time window. ” � IV rt-PA alone opens ~50% of occluded arteries; 14 -34% of these reocclude within 2 hours leading to worse outcomes Alexandrov Neurology 2002; Alexandrov NEJM 2004; Rha Stroke 2007 3

Adjunctive Treatments to rt-PA � Medical ◦ Thrombin inhibition – Argatroban ◦ Platelet inhibition – Eptifibatide ◦ Both previously combined with rt-PA as SPOTRIAS projects. � Three � Two Phase 2 trials completed ongoing � The best available evidence for adjunctive medications that combined with rt-PA may: ◦ Augment thrombolysis ◦ Prevent re-occlusion ◦ Result in improved outcomes over standard IV rt-PA. 4

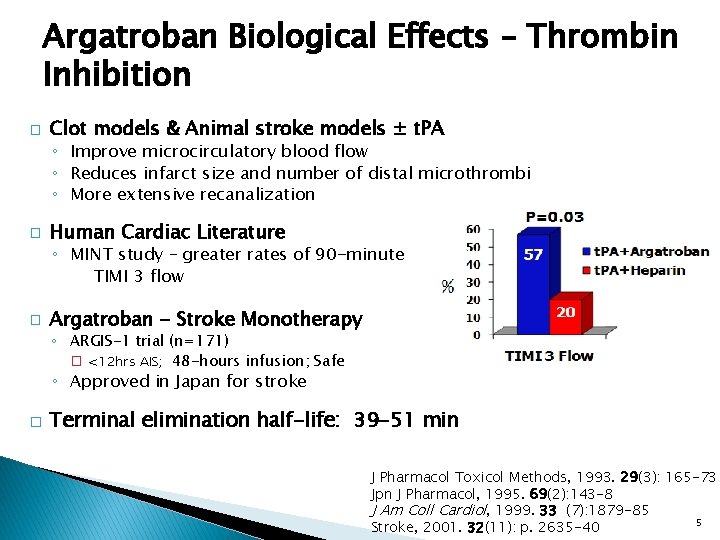
Argatroban Biological Effects – Thrombin Inhibition � Clot models & Animal stroke models ± t. PA ◦ Improve microcirculatory blood flow ◦ Reduces infarct size and number of distal microthrombi ◦ More extensive recanalization � Human Cardiac Literature ◦ MINT study – greater rates of 90 -minute TIMI 3 flow � Argatroban - Stroke Monotherapy ◦ ARGIS-1 trial (n=171) � <12 hrs AIS; 48 -hours infusion; Safe ◦ Approved in Japan for stroke � Terminal elimination half-life: 39 -51 min J Pharmacol Toxicol Methods, 1993. 29(3): 165 -73 Jpn J Pharmacol, 1995. 69(2): 143 -8 J Am Coll Cardiol, 1999. 33 (7): 1879 -85 5 Stroke, 2001. 32(11): p. 2635 -40

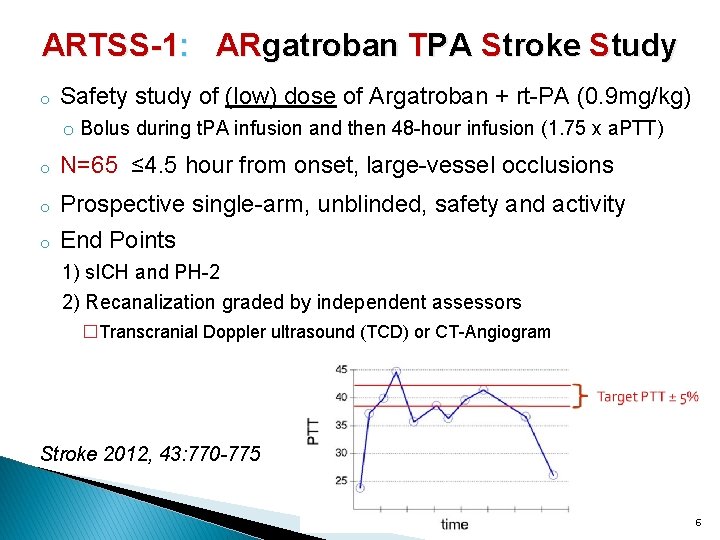
ARTSS-1: ARgatroban TPA Stroke Study o Safety study of (low) dose of Argatroban + rt-PA (0. 9 mg/kg) o Bolus during t. PA infusion and then 48 -hour infusion (1. 75 x a. PTT) o N=65 ≤ 4. 5 hour from onset, large-vessel occlusions o Prospective single-arm, unblinded, safety and activity End Points o 1) s. ICH and PH-2 2) Recanalization graded by independent assessors �Transcranial Doppler ultrasound (TCD) or CT-Angiogram Stroke 2012, 43: 770 -775 6

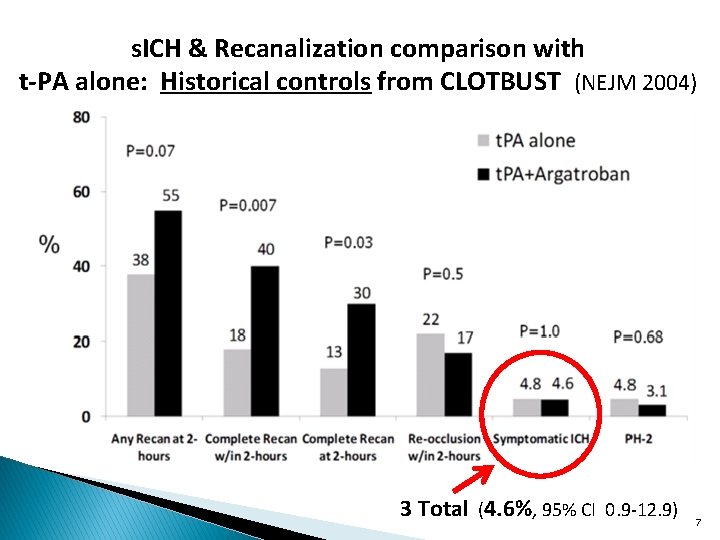
s. ICH & Recanalization comparison with t-PA alone: Historical controls from CLOTBUST (NEJM 2004) 3 Total (4. 6%, 95% CI 0. 9 -12. 9) 7

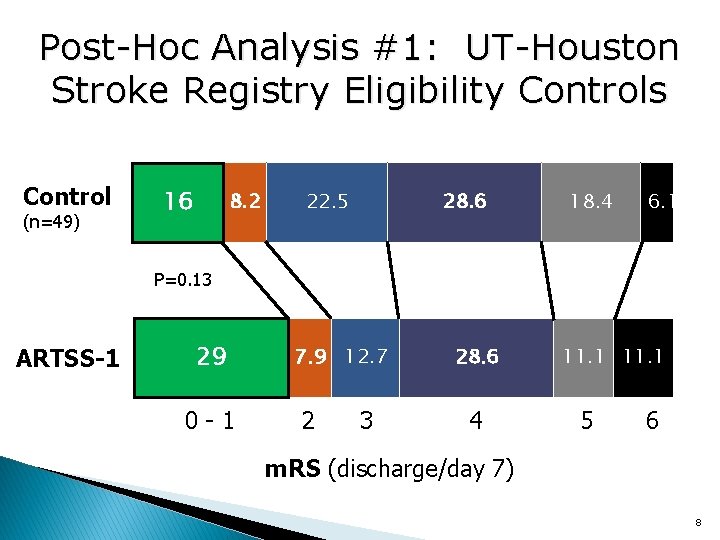
Post-Hoc Analysis #1: UT-Houston Stroke Registry Eligibility Controls Control (n=49) 10. 2 16 6. 18. 2 22. 5 28. 6 18. 4 6. 1 P=0. 13 ARTSS-1 11. 1 2917. 5 0 -1 7. 9 12. 7 2 3 28. 6 4 11. 1 5 6 m. RS (discharge/day 7) 8

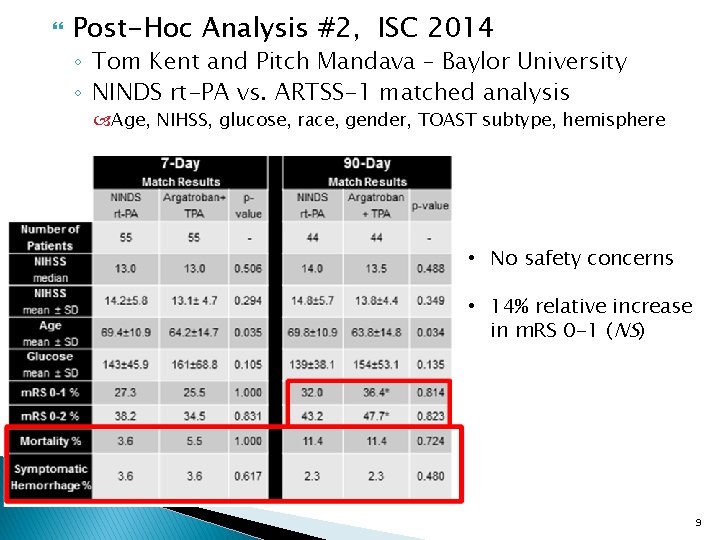
Post-Hoc Analysis #2, ISC 2014 ◦ Tom Kent and Pitch Mandava – Baylor University ◦ NINDS rt-PA vs. ARTSS-1 matched analysis Age, NIHSS, glucose, race, gender, TOAST subtype, hemisphere • No safety concerns • 14% relative increase in m. RS 0 -1 (NS) 9

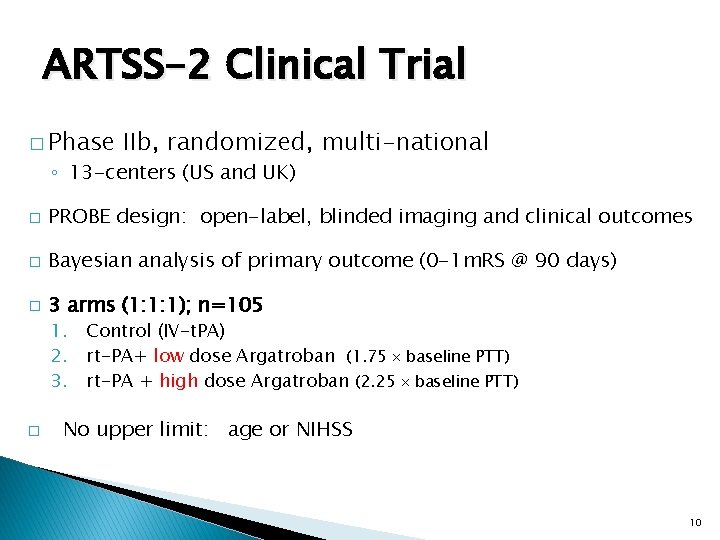
ARTSS-2 Clinical Trial � Phase IIb, randomized, multi-national ◦ 13 -centers (US and UK) � PROBE design: open-label, blinded imaging and clinical outcomes � Bayesian analysis of primary outcome (0 -1 m. RS @ 90 days) � 3 arms (1: 1: 1); n=105 1. Control (IV-t. PA) 2. rt-PA+ low dose Argatroban (1. 75 baseline PTT) 3. rt-PA + high dose Argatroban (2. 25 baseline PTT) � No upper limit: age or NIHSS 10

ARTSS-2 � 72 of 105 (69%) enrolled ◦ Pre-planned analysis after 75 patients: �Ultra-Early Recanalization �Early Neurological Improvement � 5 DSMB reviews (1 st 50 patients) – no safety concerns � Estimated trial completion Q 1 -2015 11

Eptifibatide Biological Effects – Platelet Inhibition � IMPACT-AMI Trial: § STEMI patients treated with full dose rt-PA (n=52) vs. rt-PA plus eptifibatide (n=49) § 90 -minute TIMI grade 3 flow, 66% combination vs 39% rt-PA only, P=0. 006 § Incidence and speed of reperfusion enhanced when eptifibatide is combined with rt-PA Circulation. 1997 Feb 18; 95(4): 846 -54. 12

Animal Studies � No animal studies of eptifibatide plus rt-PA in ischemic stroke � Several other GP IIb/IIIa molecules have been combined with rt-PA in murine MCA occlusion models: § prevented microvascular platelet aggregation § increased recanalization rates (50% vs. 20%) § reduced infarct volume by 25% J Clin Invest. 1998; 102: 1301 -1310 J Cereb Blood Flow Metab. 2005 Jan; 25(1): 87 -97. J Cereb Blood Flow Metab. 2002 Feb; 22(2): 215 -22. 13

Why Eptifibatide? � � Rapid inhibition of platelet aggregation (within 15 minutes) Short half-life (~2 hours) Rapid dissociation from platelets with 50% restoration of platelet function within 2 -4 hours of discontinuation Reduces risk of serious bleeding and decreases recovery time after treatment Am Heart J 1999; 138: 1093 -104. 14

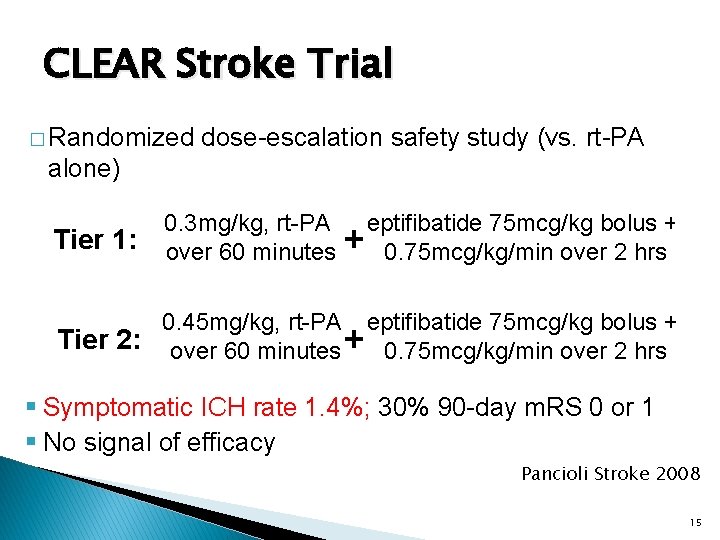
CLEAR Stroke Trial � Randomized dose-escalation safety study (vs. rt-PA alone) Tier 1: 0. 3 mg/kg, rt-PA over 60 minutes 0. 45 mg/kg, rt-PA Tier 2: over 60 minutes eptifibatide 75 mcg/kg bolus + 0. 75 mcg/kg/min over 2 hrs + § Symptomatic ICH rate 1. 4%; 30% 90 -day m. RS 0 or 1 § No signal of efficacy Pancioli Stroke 2008; 39: 3268 -3276 15

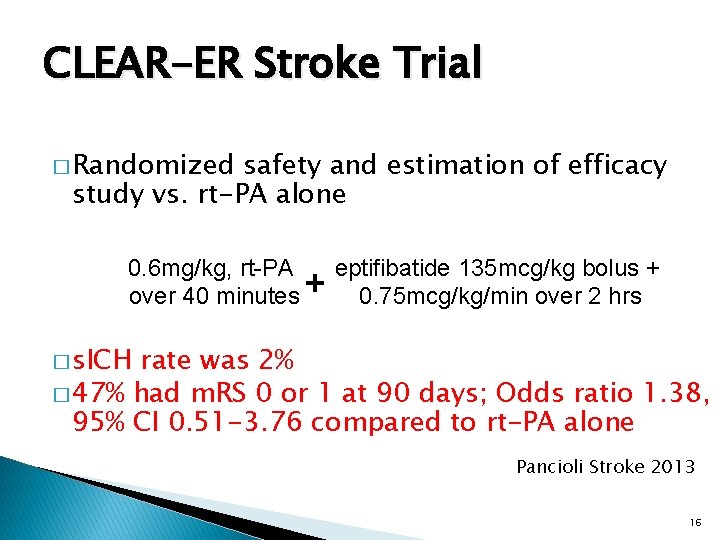
CLEAR-ER Stroke Trial � Randomized safety and estimation of efficacy study vs. rt-PA alone 0. 6 mg/kg, rt-PA over 40 minutes + eptifibatide 135 mcg/kg bolus + 0. 75 mcg/kg/min over 2 hrs � s. ICH rate was 2% � 47% had m. RS 0 or 1 at 90 days; Odds ratio 1. 38, 95% CI 0. 51 -3. 76 compared to rt-PA alone Pancioli Stroke 2013 16

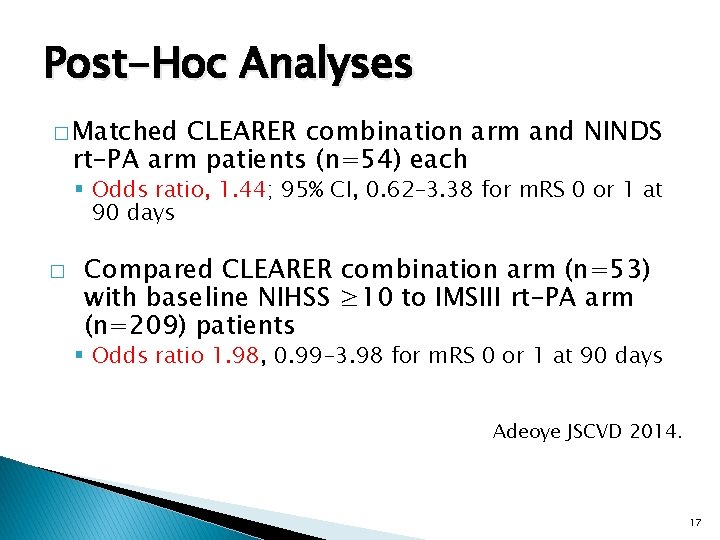
Post-Hoc Analyses � Matched CLEARER combination arm and NINDS rt-PA arm patients (n=54) each § Odds ratio, 1. 44; 95% CI, 0. 62– 3. 38 for m. RS 0 or 1 at 90 days � Compared CLEARER combination arm (n=53) with baseline NIHSS ≥ 10 to IMSIII rt-PA arm (n=209) patients § Odds ratio 1. 98, 0. 99 -3. 98 for m. RS 0 or 1 at 90 days Adeoye JSCVD 2014. 17

CLEAR-FDR Stroke Trial � Single arm safety study 0. 9 mg/kg, rt-PA over 60 minutes + eptifibatide 135 mcg/kg bolus + 0. 75 mcg/kg/min over 2 hrs � Recruitment ongoing, 14 out of 30 patients enrolled to date � Estimated completion Q 1 2015 18

Multi-Arm Adaptive Trial Proposal Phase 3 PROBE • rt-PA-alone • rt-PA + Argatroban 1. Low-Dose 2. High-dose • rt-PA + Eptifibatide 1. 0. 6 mg/kg rt-PA 2. 0. 9 mg/kg rt-PA 19

Approach Rationale � Improved medical reperfusion therapy � Available at any stroke capable hospital or mobile stroke units (CT fitted ambulance) � Great epidemiologic potential for eligible stroke patients – time to treatment is minimized � Like rt-PA alone, does not exclude subsequent endovascular therapy should endovascular therapy be effective in subpopulations of stroke patients 20

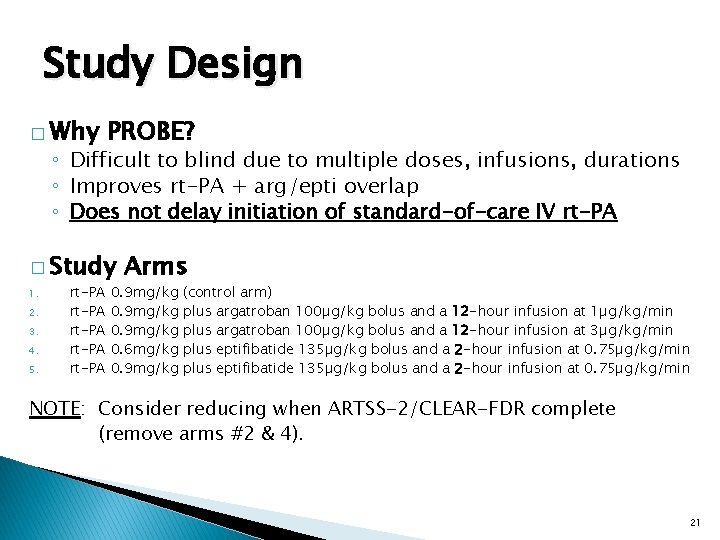
Study Design � Why PROBE? ◦ Difficult to blind due to multiple doses, infusions, durations ◦ Improves rt-PA + arg/epti overlap ◦ Does not delay initiation of standard-of-care IV rt-PA � Study 1. 2. 3. 4. 5. rt-PA rt-PA Arms 0. 9 mg/kg 0. 6 mg/kg 0. 9 mg/kg (control arm) plus argatroban 100µg/kg bolus and a 12 -hour infusion at 1µg/kg/min plus argatroban 100µg/kg bolus and a 12 -hour infusion at 3µg/kg/min plus eptifibatide 135µg/kg bolus and a 2 -hour infusion at 0. 75µg/kg/min NOTE: Consider reducing when ARTSS-2/CLEAR-FDR complete (remove arms #2 & 4). 21

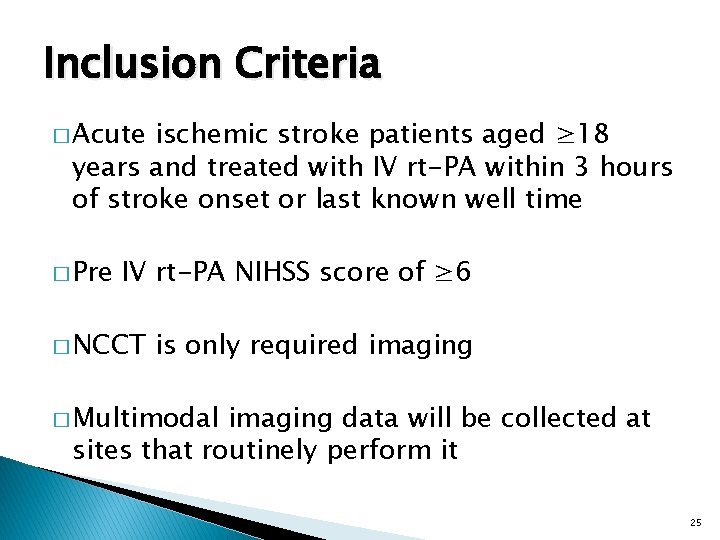
Study Design Fixed 2: 1: 1 Adaptive Randomization Subjects enrolled 0 Number of Study Arms (Including Control) 5 Fixed 1: 1 OR 1. 33: 1: 1 Randomization 500 150 5 1500 Maximum 2 or 3

Response Adaptive Randomization Advantages � Requires lower sample size than multiple two -arm trials � Ethical benefits of treating more subjects with best-performing arms � Novel in acute stroke but well established in cancer clinical trials 23

Aim � Confirm safety and establish efficacy of IV rt. PA plus IV argatroban OR IV rt-PA plus IV eptifibatide over standard IV rt-PA alone for acute ischemic stroke 24

Inclusion Criteria � Acute ischemic stroke patients aged ≥ 18 years and treated with IV rt-PA within 3 hours of stroke onset or last known well time � Pre IV rt-PA NIHSS score of ≥ 6 � NCCT is only required imaging � Multimodal imaging data will be collected at sites that routinely perform it 25

Study Design � � Bayesian approach ◦ Provided no safety concerns, arms with highest posterior probabilities of benefit are carried forward Response Adaptive Randomization ◦ Use 30 -day m. RS for rapid adaptation ◦ Identify patients likely benefiting from combination therapy (e. g. , higher NIHSS/more severe stroke) 26

Primary Endpoints � Efficacy - 90 -day functional outcomes as measured by the m. RS � Safety - s. ICH rates ◦ NINDS stroke trial definition ◦ SITS-MOST definition 27

Recruitment Plan � Stroke. NET centers and NETT sites represent 33 recruiting � Using the network of centers and hospitals in the US, Canada, Europe, Japan, S. Korea and Australia that are participating/participated in ARTSS-2 and IMSIII, we anticipate recruiting a total of 110 centers worldwide � Conservative estimate of 0. 35 patients enrolled/center/month enroll ~462 patients/year with all sites active 28

Sample Size � Maximum – 1500 ◦ Based on initial simulations with both interventions better than rt-PA without a clear difference between interventions � Anticipated years maximum trial duration is five � To avoid premature termination, anticipated minimum enrollment is ~500 29

Establishing Futility � Decisions based on predictive probabilities � Using accruing data from the trial (after minimum enrollment), we will calculate the predictive probability that the trial would be successful if: ◦ The trial proceeded to full enrollment with 50% randomization to rt-PA and one active arm � If all active arms are unlikely to be better than rt-PA (i. e. , <5% probability), the trial will be stopped 30

Early Stopping for Efficacy � Decisions based on predictive probabilities � After minimum enrollment, if one or more active arms is very likely to be better than rt-PA alone (i. e. , >99% probability), the trial may be stopped 31

Discussion – Why 12 Hours of Argatroban? � 48 -hour infusion derived from ARGIS monotherapy trial � Reocclusion after rt-PA occurs within 2 hours of bolus � Early recovery post rt-PA treatment is associated with recanalization only if recanalization achieved within ~5 hrs Neurology 2002; 59: 862 Stroke 2000; 31: 1812 32

Discussion � Why not TNK? � Delays initiation of standard of care IV rt-PA since informed consent needed. Delay in start of rt-PA of 20 minutes or more � TASTE (Australia) and NOR-TEST (Norway) are ongoing phase 3 trials of TNK vs. rt-PA � Augmentation of thrombolysis strategy Saver JAMA 2013 33

Discussion – Endovascular Therapy � For MOST, endovascular therapy within 90 minutes of rt-PA initiation may be considered for confirmed ICAT or basilar artery occlusions with NIHSS≥ 20 � Multiple rt-PA ongoing trials of endovascular therapy vs IV � None provide data about new approaches to medical reperfusion, which is most universally applicable � As data from ongoing endovascular trials become available, timely endovascular therapy may be considered for specific subpopulations of MOST Demchuk Radiology 2014 34

Comments/Questions 35