Minimally Invasive Approaches in the Treatment of Urothelial















- Slides: 43

Minimally Invasive Approaches in the Treatment of Urothelial Carcinoma “Robotic Radical Cystectomy” Douglas S. Scherr, M. D. Weill Medical College of Cornell University

Robotics Beyond The Prostate • Radical Cystectomy • Can we achieve equal oncological outcome?

Radical Cystectomy • Gold Standard for Invasive Disease • Role in T 1 Disease • Quality of surgery impacts outcome and survival

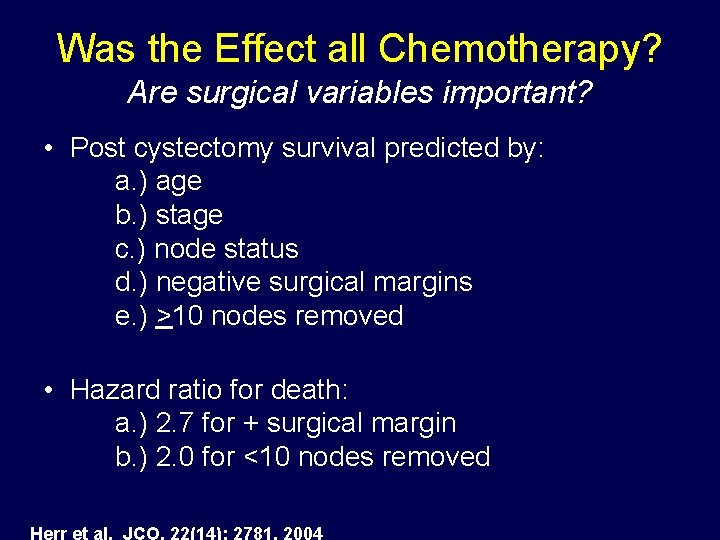
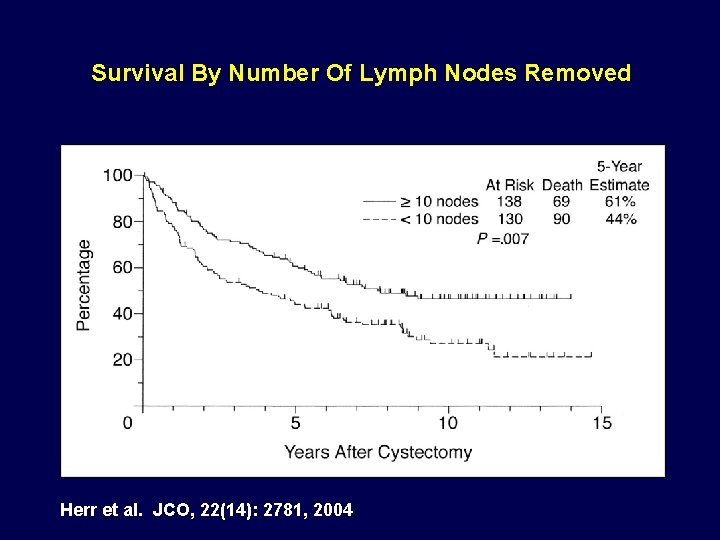
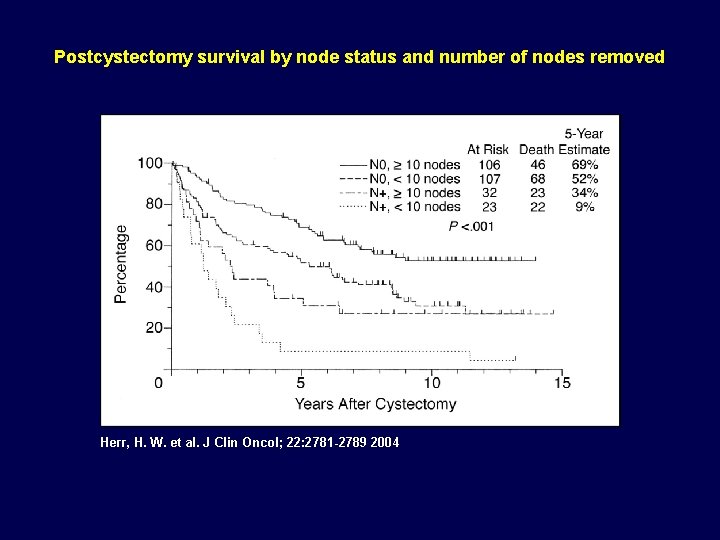
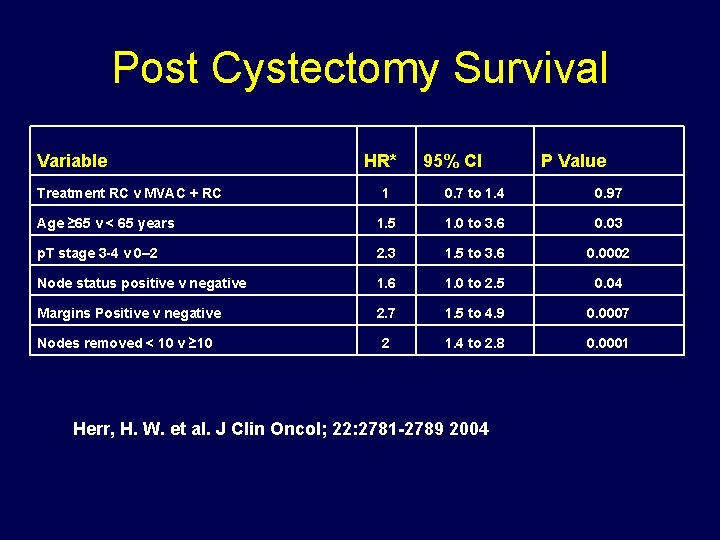
Was the Effect all Chemotherapy? Are surgical variables important? • Post cystectomy survival predicted by: a. ) age b. ) stage c. ) node status d. ) negative surgical margins e. ) >10 nodes removed • Hazard ratio for death: a. ) 2. 7 for + surgical margin b. ) 2. 0 for <10 nodes removed Herr et al. JCO, 22(14): 2781, 2004

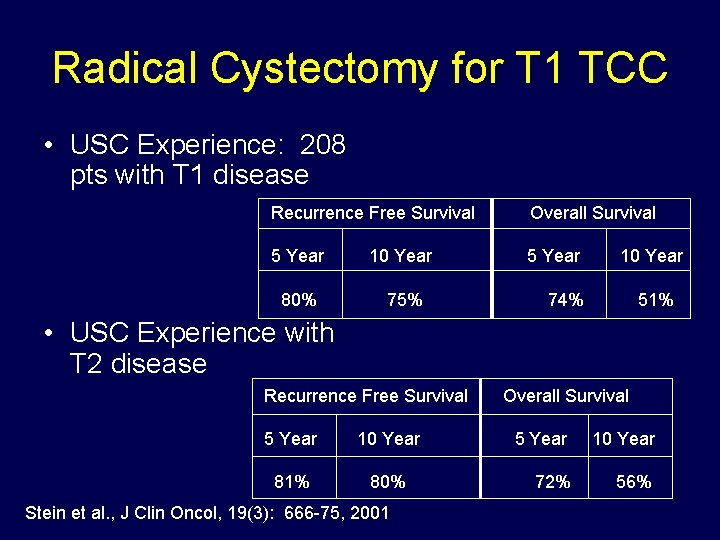
Radical Cystectomy for T 1 TCC • USC Experience: 208 pts with T 1 disease Recurrence Free Survival Overall Survival 5 Year 10 Year 5 Year 80% 75% 10 Year 74% 51% • USC Experience with T 2 disease Recurrence Free Survival 5 Year 10 Year 81% 80% Stein et al. , J Clin Oncol, 19(3): 666 -75, 2001 Overall Survival 5 Year 10 Year 72% 56%

Early Vs. Late Cystectomy • 90 pts who had TUR + BCG ultimately underwent cystectomy • 41/90 had T 1 disease • Median Follow up of 96 mos Early cystectomy (<2 years): 92% survival Late cystectomy (>2 years): 56% survival Herr and Sogani, J Urol, 166: 1296 -9, 2001

Extent of Lymphadenectomy • Is there more to the node dissection than staging? • 1936 Colston and Leadbetter performed studies on 98 cadavers “limited metastatic disease was restricted to the pelvic nodes” • 1946 – Dr. Jewett “cardinal site of metastasis” Colston and Leadbetter, J Urol, 36: 669, 1936 Jewett et al. J Urol, 55: 366, 1946

Extent of Lymphadenectomy • Node positive patients can enjoy long term survival • 24% of grossly node positive disease survived 10 years without adjuvant therapy • More nodes removed correlates with improved survival Sanderson et al. Urol Oncol. , 22: 205, 2004

Extent of Lymphadenectomy • Likely no staging advantage to extending the node dissection above the aortic bifurcation • 33% of unsuspected nodes found at common iliacs • Practice patterns vary widely: a. ) 40% of cystectomies have no LND b. ) 12. 7% of LND had <4 nodes removed Lymph node density (# pos nodes/total # nodes) Konety et al. J Urol, 170: 1765, 2003

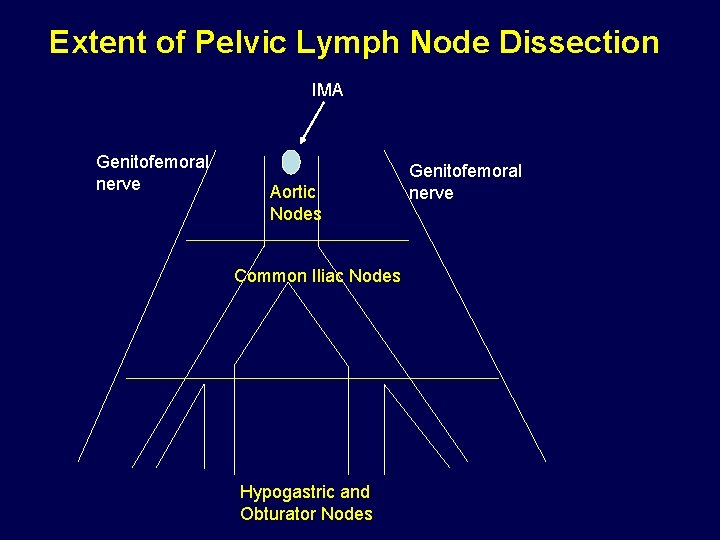
Extent of Pelvic Lymph Node Dissection IMA Genitofemoral nerve Aortic Nodes Common Iliac Nodes Hypogastric and Obturator Nodes Genitofemoral nerve

Survival By Number Of Lymph Nodes Removed Herr et al. JCO, 22(14): 2781, 2004

Postcystectomy survival by node status and number of nodes removed Herr, H. W. et al. J Clin Oncol; 22: 2781 -2789 2004

Post Cystectomy Survival Variable Treatment RC v MVAC + RC HR* 95% CI P Value 1 0. 7 to 1. 4 0. 97 Age ≥ 65 v < 65 years 1. 5 1. 0 to 3. 6 0. 03 p. T stage 3 -4 v 0– 2 2. 3 1. 5 to 3. 6 0. 0002 Node status positive v negative 1. 6 1. 0 to 2. 5 0. 04 Margins Positive v negative 2. 7 1. 5 to 4. 9 0. 0007 Nodes removed < 10 v ≥ 10 2 1. 4 to 2. 8 0. 0001 Herr, H. W. et al. J Clin Oncol; 22: 2781 -2789 2004

Gold Standard • Open radical cystectomy (RC) is the gold standard for treatment of muscle-invasive bladder cancer.

Minimally Invasive Bladder Cancer Surgery • Efforts to reduce the operative morbidity of RC have fostered interest in minimally invasive approaches. • Laparoscopic RC • Robot-assisted laparoscopic RC

Concerns of Robotic Cystectomy? • Concerns regarding minimally invasive RC – Absence of long term oncologic outcomes – Absence of long term functional outcomes – Limited pelvic lymphadenectomy – Longer operative time – Increased cost Miller NL et al: World J Urol (2006) 24: 180

Outcome Measures of Minimally Invasive Bladder Surgery • Previous reports comparing open versus minimally invasive RC have focused on perioperative outcomes. – – – Blood loss Operative time Analgesic requirement Time to regular diet Length of hospital stay Hemal AK et al: Urol Clin N Am (2004) 31: 719 Basillote JB et al: J Urol (2004) 172: 489 Taylor GD et al: J Urol (2004) 172: 1291 Galich A et al: JSLS (2006) 10: 145 Rhee JJ et al: BJU Int (2006) 98: 1059

Comparison of Surgical Techniques • However, direct comparison between open and minimally invasive RC of early oncologic parameters is lacking. • Lymph node yield • Margin status

Study Comparison • Comparison of perioperative and early pathologic outcomes in a consecutive series of open and robotic RCs at our institution.

Methods • 100 consecutive patients underwent RC by a single surgeon at our institution 20062007 • 22 open • 78 robotic

Technique • • Posterior dissection Isolation of ureters Lateral dissection Control of bladder pedicles Anterior dissection Control of DVC and division of urethra Control of prostate pedicles and nerve-sparing Pelvic lymph node dissection – External iliac, hypogastric, and obturator lymphadenectomy up to the level of the mid-common iliac vessels • Extracorporeal urinary diversion through a 5 -7 cm midline incision – Orthotopic neobladder: robot re-docked for urethral neovesical anastomosis


Data Collection and Analysis • Data was collected prospectively – Patient characteristics – Perioperative outcomes – Early pathologic outcomes • Data analysis – Chi-square test – Fisher’s exact test – Student’s t-test

Results: Patient Characteristics • There was no difference in the following parameters among the 2 cohorts. • Age • BMI • ASA class • Prior abdominal surgery • Prior abdominal radiation • Neoadjuvant chemotherapy

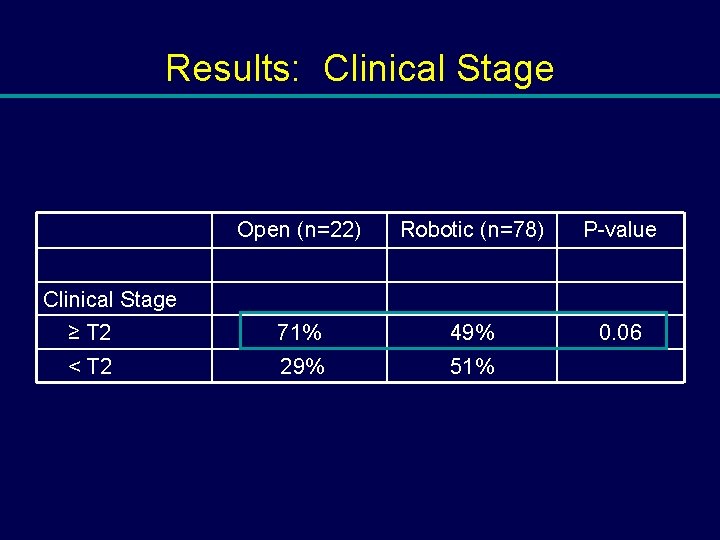
Results: Clinical Stage Open (n=22) Robotic (n=78) P-value ≥ T 2 71% 49% 0. 06 < T 2 29% 51% Clinical Stage

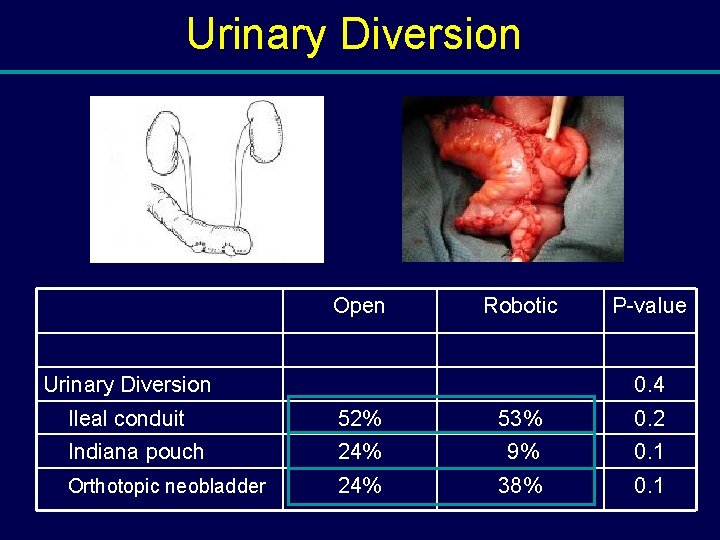
Urinary Diversion Open Robotic Urinary Diversion P-value 0. 4 Ileal conduit 52% 53% 0. 2 Indiana pouch 24% 9% 0. 1 Orthotopic neobladder 24% 38% 0. 1

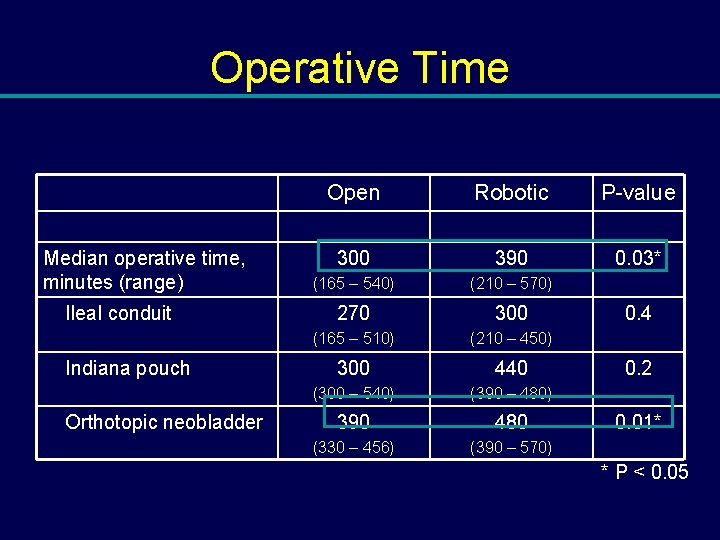
Operative Time Median operative time, minutes (range) Ileal conduit Indiana pouch Orthotopic neobladder Open Robotic P-value 300 390 0. 03* (165 – 540) (210 – 570) 270 300 (165 – 510) (210 – 450) 300 440 (300 – 540) (390 – 480) 390 480 (330 – 456) (390 – 570) 0. 4 0. 2 0. 01* * P < 0. 05

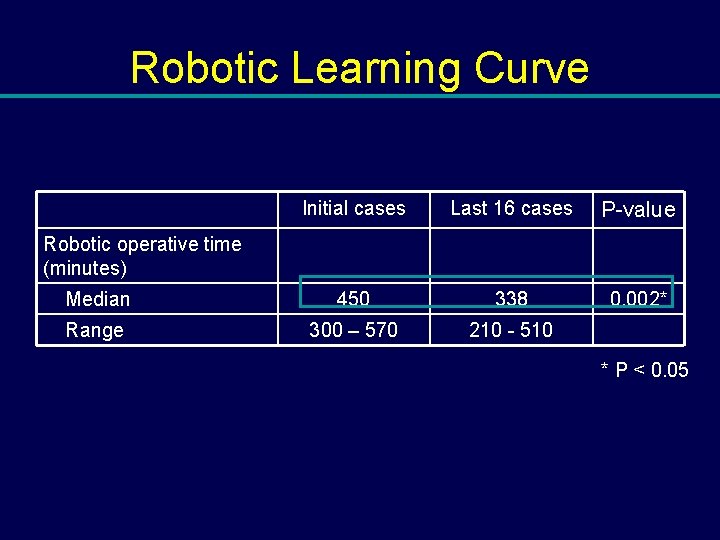
Robotic Learning Curve Initial cases Last 16 cases P-value Median 450 338 0. 002* Range 300 – 570 210 - 510 Robotic operative time (minutes) * P < 0. 05

Blood Loss & Postoperative Parameters Open Robotic P-value 750 400 0. 002* (250 – 2500) (100 – 1200) Median blood transfusions, units PRBCs (range) 2 (0 – 7) 0. 5 (0 – 3) 0. 007* Median time to regular diet, days (range) 5 (4 – 8) 4 (3 – 6) 0. 002* Median length of stay, days (range) 8 (5 – 28) 5 (4 – 18) 0. 007* Median estimated blood loss, m. L (range) * P < 0. 05

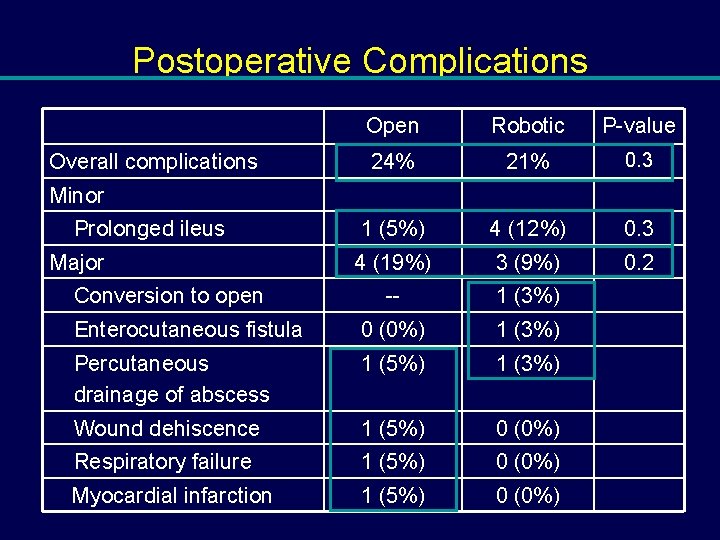
Postoperative Complications Open Robotic P-value 24% 21% 0. 3 1 (5%) 4 (12%) 0. 3 4 (19%) 3 (9%) 0. 2 -- 1 (3%) Enterocutaneous fistula 0 (0%) 1 (3%) Percutaneous drainage of abscess 1 (5%) 1 (3%) Wound dehiscence 1 (5%) 0 (0%) Respiratory failure 1 (5%) 0 (0%) Myocardial infarction 1 (5%) 0 (0%) Overall complications Minor Prolonged ileus Major Conversion to open

Pathologic Stage Open Robotic Pathologic stage P-value 0. 3 p. T 0 10% 22% p. Ta 0% 6% p. Tis 19% 28% p. T 1 5% 6% p. T 2 10% 9% p. T 3 24% 22% p. T 4 33% 6% Organ confined, < p. T 3 43% 72% Non-organ confined, p. T 3 -4 57% 28% 0. 03* * P < 0. 05

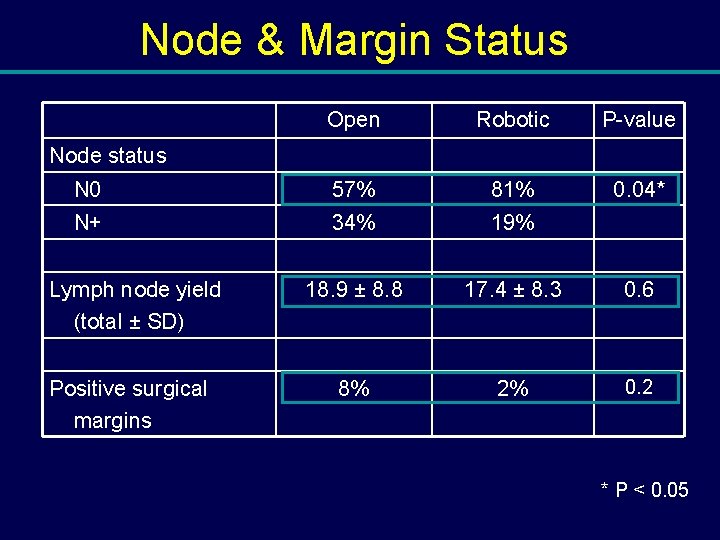
Node & Margin Status Open Robotic P-value N 0 57% 81% 0. 04* N+ 34% 19% 18. 9 ± 8. 8 17. 4 ± 8. 3 0. 6 8% 2% 0. 2 Node status Lymph node yield (total ± SD) Positive surgical margins * P < 0. 05

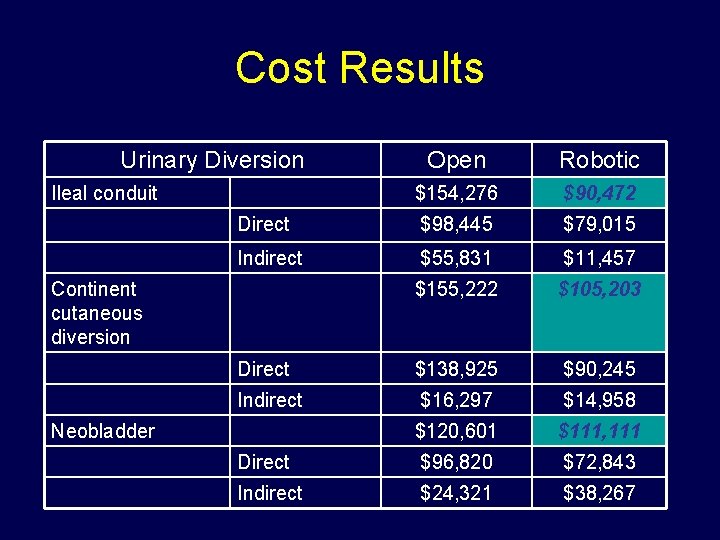
Cost Results Urinary Diversion Open Robotic $154, 276 $90, 472 Direct $98, 445 $79, 015 Indirect $55, 831 $11, 457 $155, 222 $105, 203 Direct $138, 925 $90, 245 Indirect $16, 297 $14, 958 $120, 601 $111, 111 Direct $96, 820 $72, 843 Indirect $24, 321 $38, 267 Ileal conduit Continent cutaneous diversion Neobladder

Cost Conclusions • Robotic cystectomy appears more cost-effective than open cystectomy for treatment of bladder cancer – Majority of improvement driven by lower LOS – High initial materials cost of robotic surgery defrayed by subsequent cost savings during hospitalization • Annual robotic volume does not need to be high (<25 cases per year) to justify use of robotic cystectomy • Cost savings of robotic cystectomy however is diminished with decreased open cystectomy LOS (2 to 9 days)

Conclusions: Robotic Cystectomy • Increased operative time – significantly longer operative time in the robotic neobladder cohort (p=0. 01) • Decreased operative time with increased experience – 450 to 338 min (p=0. 007)

Conclusions: Robotic Cystectomy • Decreased – Blood loss – Transfusion requirement – Time to regular diet – Length of hospital stay

Conclusions: Robotic Cystectomy • Equivalent lymph node yield – 17. 4 (robotic) vs. 18. 9 (open), p=0. 6 • Equivalent margin rate – 2% (robotic) vs. 8% (open), p=0. 2 • Long term oncologic and functional outcomes are required Stein JP et al: J Urol (2003) 170: 35 Herr H et al: J Urol (2004) 171: 1823

Minimally Invasive Cystectomy • Minimally Invasive = Cancer Sparing

Future Directions • Prostate Sparing? • Improved Diagnostics

Prostate Sparing Cystectomy • Role for improved continence and potency • Need to rule out prostate cancer or TCC of prostatic urethra • Functional Results are good: a. ) 97% complete continence b. ) No episodes of retention c. ) 82% maintained potency Vallancien et al. J Urol, 168: 2413, 2002

Prostate Sparing Cystectomy • Incidence of Pca is 30 -50% with approx. 48% are clinically significant • 60% of Ca. P involve the apex (79% significant and 42% insignificant) • 48% of prostates had urothelial ca involvement of which 33% had apical involvement

Multiphoton Images

Multiphoton Images
 Minimally invasive surgery
Minimally invasive surgery Minimally conscious state
Minimally conscious state Cas treatment approaches
Cas treatment approaches Doc for invasive aspergillosis
Doc for invasive aspergillosis Ipane
Ipane Exotic species meaning
Exotic species meaning Least invasive intervention teach like a champion
Least invasive intervention teach like a champion Non invasive ventilation
Non invasive ventilation Non invasive ventilation
Non invasive ventilation Invasive species exponential growth
Invasive species exponential growth Invasive species characteristics
Invasive species characteristics Invasive candidiasis
Invasive candidiasis Lerman halo
Lerman halo Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features Invasive species investigator worksheet
Invasive species investigator worksheet Antt safeguards
Antt safeguards Lerman non invasive halo
Lerman non invasive halo Indiana invasive species council
Indiana invasive species council Are invasive species always bad
Are invasive species always bad Invasive beatmung über tracheostoma
Invasive beatmung über tracheostoma Invasive species compendium
Invasive species compendium Ontario invasive fish
Ontario invasive fish Characteristics of invasive species
Characteristics of invasive species Invasive fungi
Invasive fungi Invasive species act ontario
Invasive species act ontario Non invasive cgm
Non invasive cgm Invasive species characteristics
Invasive species characteristics Invasive meningococcal disease
Invasive meningococcal disease Sesleria autumnalis invasive
Sesleria autumnalis invasive Non invasive health monitor
Non invasive health monitor Invasive species characteristics
Invasive species characteristics Invasive species investigator worksheet
Invasive species investigator worksheet Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Kể tên các môn thể thao
Kể tên các môn thể thao Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Dot
Dot Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Gấu đi như thế nào
Gấu đi như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau