Alternative Minimally Invasive Treatment Options for Saphenous Vein












- Slides: 26

Alternative Minimally Invasive Treatment Options for Saphenous Vein Reflux Michael J. Singh, MD FACS RPVI UPMC Heart and Vascular Institute Director, UPMC Shadyside Vein Care Center March 2019

Michael Singh, MD I have a relevant financial relationship. Speaker’s Bureau: Medtronic Inc.

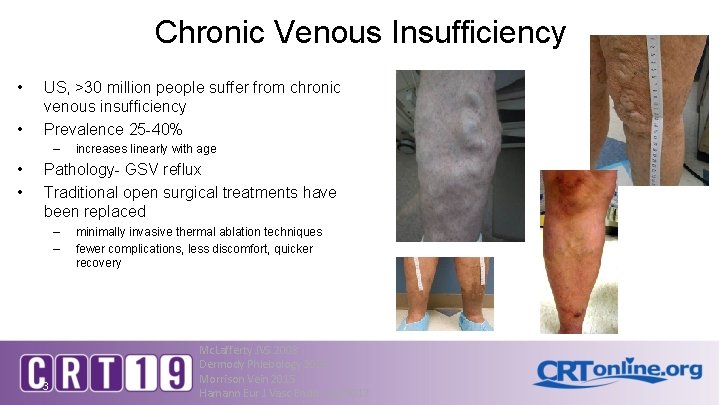
Chronic Venous Insufficiency • • US, >30 million people suffer from chronic venous insufficiency Prevalence 25 -40% – • • increases linearly with age Pathology- GSV reflux Traditional open surgical treatments have been replaced – – 3 minimally invasive thermal ablation techniques fewer complications, less discomfort, quicker recovery Mc. Lafferty JVS 2008 Dermody Phlebology 2015 Morrison Vein 2015 Hamann Eur J Vasc Endo Surg 2017

JVS Ven and Lym Dis 2017

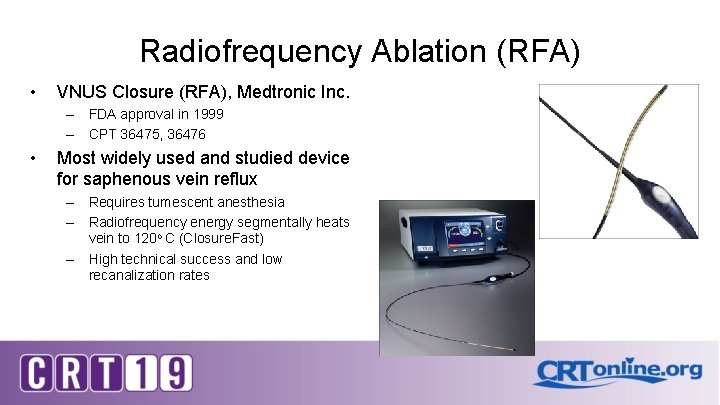
Radiofrequency Ablation (RFA) • VNUS Closure (RFA), Medtronic Inc. – FDA approval in 1999 – CPT 36475, 36476 • Most widely used and studied device for saphenous vein reflux – Requires tumescent anesthesia – Radiofrequency energy segmentally heats vein to 120 o C (Closure. Fast) – High technical success and low recanalization rates

Endovenous Laser Ablation (EVLA) • Endovenous laser- FDA approved in 2000 – • CPT 36478, 36479 Various laser fibers – wavelengths of 810 nm to 1470 nm – higher wavelengths associated with less postprocedure pain • Technique similar to RFA, requires tumescent anesthesia – – Delivery of 70 -90 joules/cm Comparable success rate

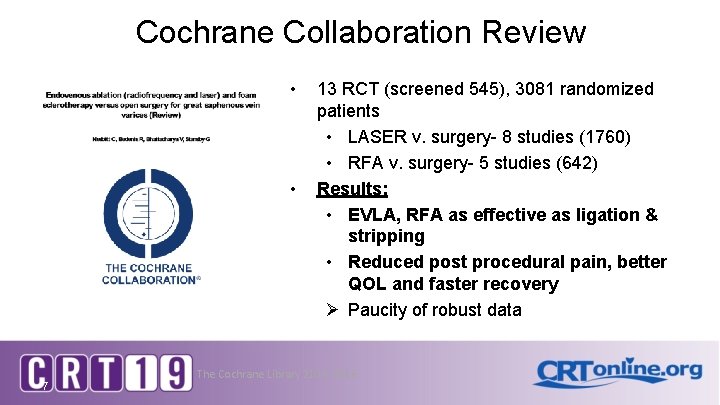
Cochrane Collaboration Review • • 7 13 RCT (screened 545), 3081 randomized patients • LASER v. surgery- 8 studies (1760) • RFA v. surgery- 5 studies (642) Results: • EVLA, RFA as effective as ligation & stripping • Reduced post procedural pain, better QOL and faster recovery Ø Paucity of robust data The Cochrane Library 2104, 2016

Guidelines & Recommendations 2019, Multi-societal Venous Guidelines (ACP, AVF, SVS) 2014, Clinical Practice Guidelines of the SVS and the AVF 2014, Management of Venous Leg Ulcers: clinical practice guidelines for the SVS and AVF 2013, National Institute for Health and Care Excellence (NICE) 2012, Endovenous Thermal Ablation for Varicose Vein Disease Consensus General agreement: Stripping and Ligation • grade 2 B Radiofrequency Ablation (RFA) • grade 1 B safe and effective Endovenous Laser Ablation (EVLA) • grade 1 B superior to stripping • grade 1 B not inferior to RFA 8 Gloviczki JVS 2011 Gibson EVT 2015

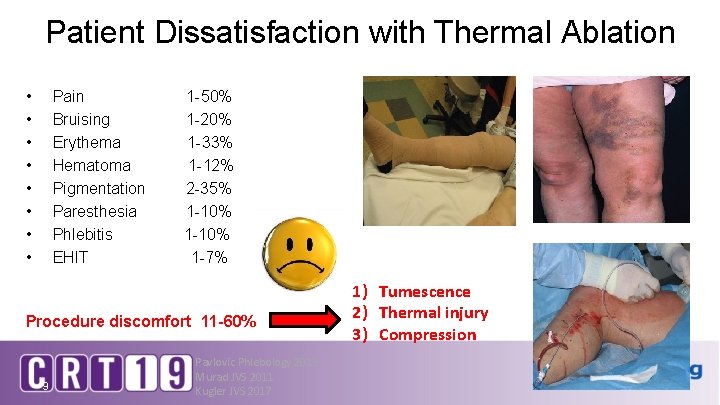
Patient Dissatisfaction with Thermal Ablation • • Pain Bruising Erythema Hematoma Pigmentation Paresthesia Phlebitis EHIT 1 -50% 1 -20% 1 -33% 1 -12% 2 -35% 1 -10% 1 -7% Procedure discomfort 11 -60% 9 Pavlovic Phlebology 2015 Murad JVS 2011 Kugler JVS 2017 1) Tumescence 2) Thermal injury 3) Compression

Nonthermal Saphenous Vein “Lunchtime” Treatments 1) Clarivein- mechanical occlusion chemical ablation 2) Vena. Seal- cyanoacrylate adhesive 3) Varithena- polidocanol microfoam Ø No heat, no tumescence, limited compression Ø Medline- 2019, >85 nonthermal ablation articles 10

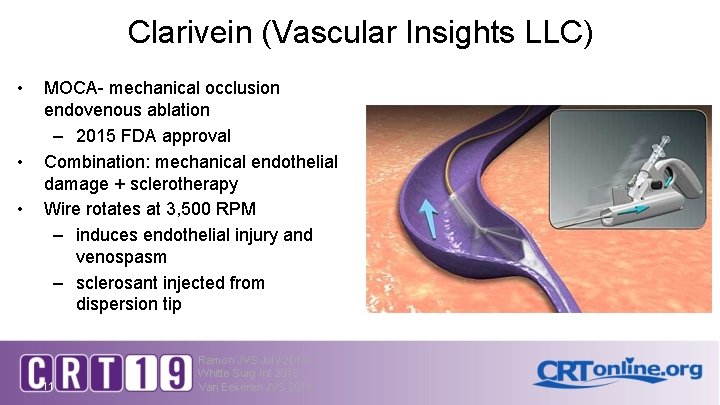
Clarivein (Vascular Insights LLC) • • • MOCA- mechanical occlusion endovenous ablation – 2015 FDA approval Combination: mechanical endothelial damage + sclerotherapy Wire rotates at 3, 500 RPM – induces endothelial injury and venospasm – sclerosant injected from dispersion tip 11 Ramon JVS July 2014 Whitte Surg Int 2015 Van Eekeren JVS 2014

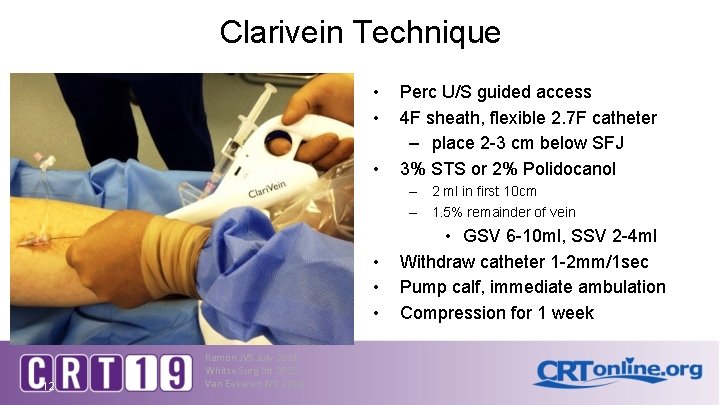
Clarivein Technique • • • Perc U/S guided access 4 F sheath, flexible 2. 7 F catheter – place 2 -3 cm below SFJ 3% STS or 2% Polidocanol – 2 ml in first 10 cm – 1. 5% remainder of vein • • • 12 Ramon JVS July 2014 Whitte Surg Int 2015 Van Eekeren JVS 2014 • GSV 6 -10 ml, SSV 2 -4 ml Withdraw catheter 1 -2 mm/1 sec Pump calf, immediate ambulation Compression for 1 week

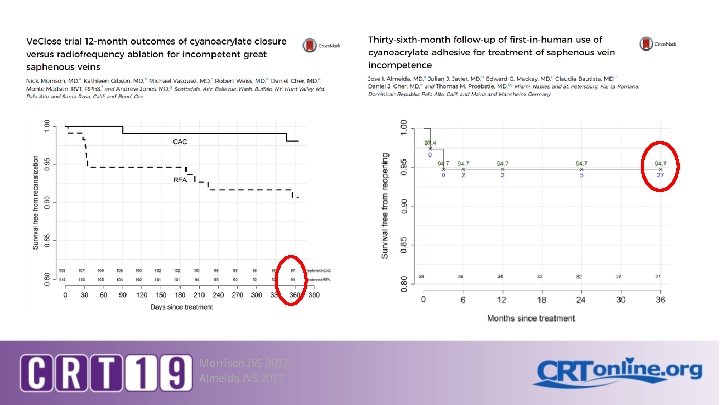
Clarivein Results • • Witte 2017, Ozen 2014 – Clarivein alone Bootun et al. 2016 – Clarivein v. RFA Van Eeken et al. 2013 – Clarivein v. EVLA Pooled data: – – – Ø Ø 13 Bootun Phlebology 2016 Van Eekeren JVS 2013 Witte JVS 2017 Ozen Phlebology 2014 1 year closure 88 -100% 2 year closure 84 -94% 3 year 88% Less pain, faster recovery, improved VCSS and AVVQ Low incidence MAE CPT 36473, 36474 January 2017 Device >$700 plus sclerosant

Vena. Seal Closure (Medtronic, Inc. ) • CAVA- cyanoacrylate vein ablation • FDA approval Feb 2015 • Implantable device • Proprietary n-butyl cyanoacrylate adhesive polymerizes when contacts blood • No thermal energy • No tumescent • No sclerosant • No compression • Disposable single use Ø CPT 36482, 36483 Ø Out of pocket expense $1, 750 14

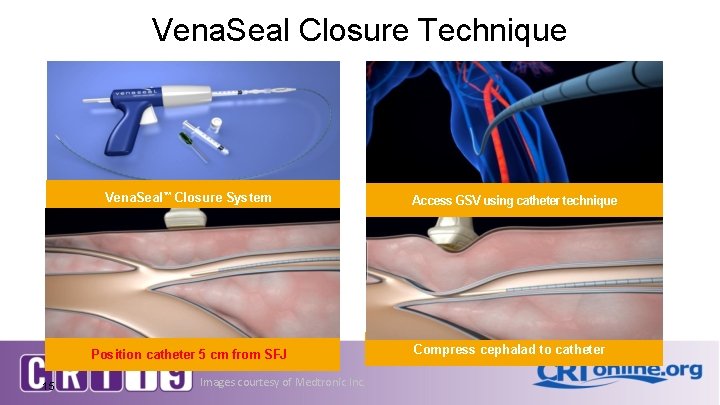
Vena. Seal Closure Technique Vena. Seal™ Closure System Position catheter 5 cm from SFJ 15 Images courtesy of Medtronic Inc. Access GSV using catheter technique Compress cephalad to catheter

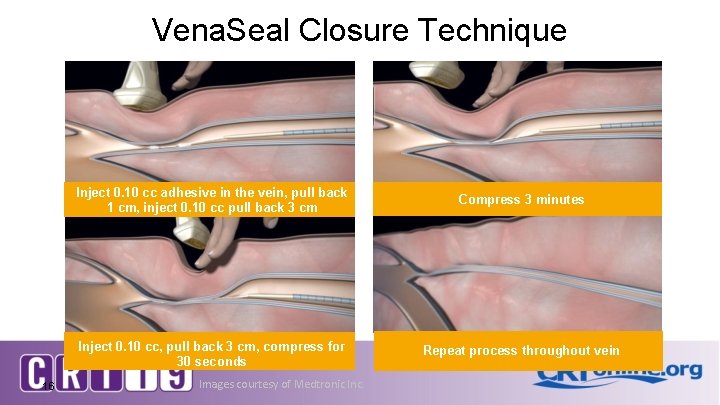
Vena. Seal Closure Technique 16 Inject 0. 10 cc adhesive in the vein, pull back 1 cm, inject 0. 10 cc pull back 3 cm Compress 3 minutes Inject 0. 10 cc, pull back 3 cm, compress for 30 seconds Repeat process throughout vein Images courtesy of Medtronic Inc.

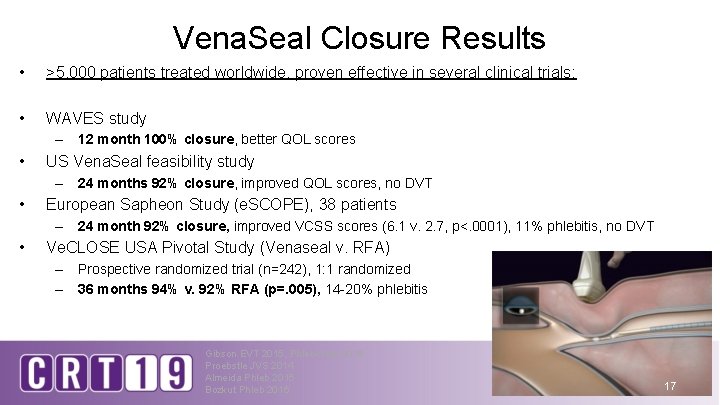
Vena. Seal Closure Results • >5, 000 patients treated worldwide, proven effective in several clinical trials: • WAVES study – 12 month 100% closure, better QOL scores • US Vena. Seal feasibility study – 24 months 92% closure, improved QOL scores, no DVT • European Sapheon Study (e. SCOPE), 38 patients – 24 month 92% closure, improved VCSS scores (6. 1 v. 2. 7, p<. 0001), 11% phlebitis, no DVT • Ve. CLOSE USA Pivotal Study (Venaseal v. RFA) – Prospective randomized trial (n=242), 1: 1 randomized – 36 months 94% v. 92% RFA (p=. 005), 14 -20% phlebitis Gibson EVT 2015, Phlebology 2018 Proebstle JVS 2014 Almeida Phleb 2015 Bozkut Phleb 2016 17

Morrison JVS 2017 Almeida JVS 2017

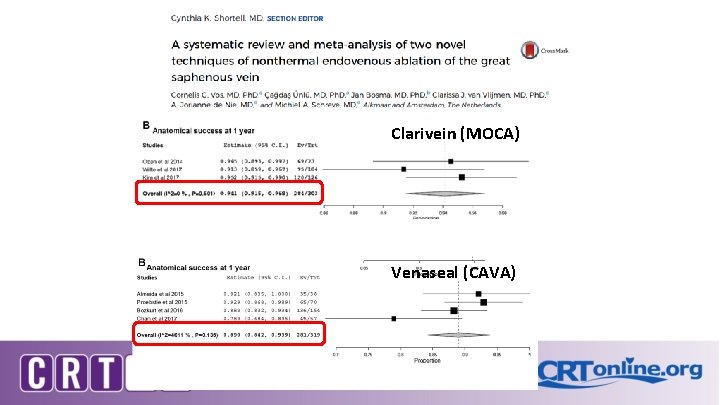
Clarivein (MOCA) Venaseal (CAVA) JVS 2017

Varithena (BTG International Ltd. ) • • FDA approval 2014 Treats: GSV, SSV, varicies 1% Polidocanol- low density microfoam displaces blood and ablates endothelium Soluble gas mixture • • 20 65% O 2: 35% CO 2 nitrogen content <0. 8% 1: 7 liquid to gas ratio small consistent bubble size <100 μm King Eur J Vasc Endo Surg 2015 Todd Phlebology 2014 Todd JVS 2015

Varithena Facts • • • Varithena canister- 45 m. L of usable foam Must dispense within 30 days Treat vein diameters <25. 9 mm • 5 m. L per injection (max 15 m. L/session) • elevate leg 45 degree • perforator and SFJ compression • inject 0. 5– 1 ml/sec • apply compression, no tumescent needed Ø CPT 36465, 36466 Ø Cost $3, 195/45 m. L canister (less cost effective) 21

Varithena Durability- VANISH Studies • Todd 2015 - randomized blinded trial (polidocanol 0. 5 v. 1%) • • • Vein closure 89% 8 weeks, 73% 1 year Varithena demonstrated consistent, durable, clinically meaningful improvement of symptoms King 2015 - validated VANISH 2 results • • • Technical success 80% No difference in concentrations Improved patient satisfaction scores, vein appearance, VCSS and VEINES-QOL scores at 1 year Ø MAE 60 -78%, pain, tenderness, phlebitis, DVT (Todd 8. 6%, King 2%) • Gibson 2017 - multicenter study • Ø 77 randomized patients, 39 Varithena DVT 5. 1 -7. 1%, 92% any adverse event, DUS response 90% at 4 weeks Todd JVS 2015 King Eur J Vasc Endovasc Surg 2015 Kugler JVS 2017 Gibson Phlebology 2017

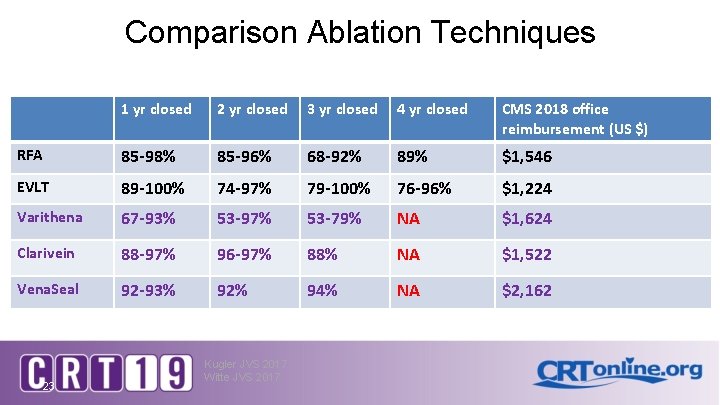
Comparison Ablation Techniques 1 yr closed 2 yr closed 3 yr closed 4 yr closed CMS 2018 office reimbursement (US $) RFA 85 -98% 85 -96% 68 -92% 89% $1, 546 EVLT 89 -100% 74 -97% 79 -100% 76 -96% $1, 224 Varithena 67 -93% 53 -97% 53 -79% NA $1, 624 Clarivein 88 -97% 96 -97% 88% NA $1, 522 Vena. Seal 92 -93% 92% 94% NA $2, 162 23 Kugler JVS 2017 Witte JVS 2017

Conclusion 24 • Thermal ablation remains the standard of care for symptomatic saphenous vein reflux • Nonthermal ablation has demonstrated improvements in technique, success and durability in the past 5 years • Nonthermal ablation reduces procedure related discomfort, improves patient satisfaction scores and lowers DVT risk • Recent CMS approval has increased utilization of nonthermal ablation techniques

Thank you Pittsburgh, PA 25

JVS 2017 JVS 2018