Manejo Multidisciplinar del CNMP ESTADIO III Presente y
















- Slides: 50

Manejo Multidisciplinar del CNMP ESTADIO III: Presente y Futuro Dolores Isla H. Clínico Universitario Lozano Blesa ZARAGOZA #SEOM 2017

DISCLOSURE INFORMATION Ø Employment: NO Ø Consultant or Advisory Role: Hoffmann-La Roche, Eli Lilly, Astra. Zeneca, Amgen, Bristol Myers Squibb, MSD, Boehringer Ingelheim, Pierre Fabre, Pfizer, Novartis, Merck, Abbvie Ø Stock Ownership: NO Ø Research Funding: Hoffmann-La Roche, Astra. Zeneca Ø Speaking: Hoffmann-La Roche, Eli Lilly, Astra. Zeneca, Amgen, Bristol Myers Squibb, MSD, Boehringer Ingelheim, Pierre Fabre, Pfizer, Merck Ø Grant support: NO Ø Other: NO #SEOM 2017

¿ CÓMO DEFINÍAMOS EL ESTADIO III DE CPNM ? DIFÍCIL ABURRIDO #SEOM 2017

OUTLINE Ø INTRODUCTION Ø STANDARD TREATMENT: Ø SURGERY Ø RT Ø CT Ø TARGETED THERAPY Ø IMMUNOTHERAPY Ø CONCLUSIONS #SEOM 2017

Ø INTRODUCTION #SEOM 2017

INTRODUCTION Ø HETEROGENEITY: Ø Ø Tumor histopathology Tumor location / extension Patient risk profile: comorbidities, age, PS, weight loss… Inter-institution diversity: expertise !! Ø STAGING: stage migration / multiple subgroups / different treatment options Ø OPTIMAL DIAGNOSTIC WORK-UP: Ø PET-CT Ø Mediastinal staging Ø Brain image Ø DIFFERENT STRATEGIES: Ø Few head-to-head studies Ø Optimal drug and duration undefined #SEOM 2017

MULTIDISCIPLINARY MANAGEMENT #SEOM 2017

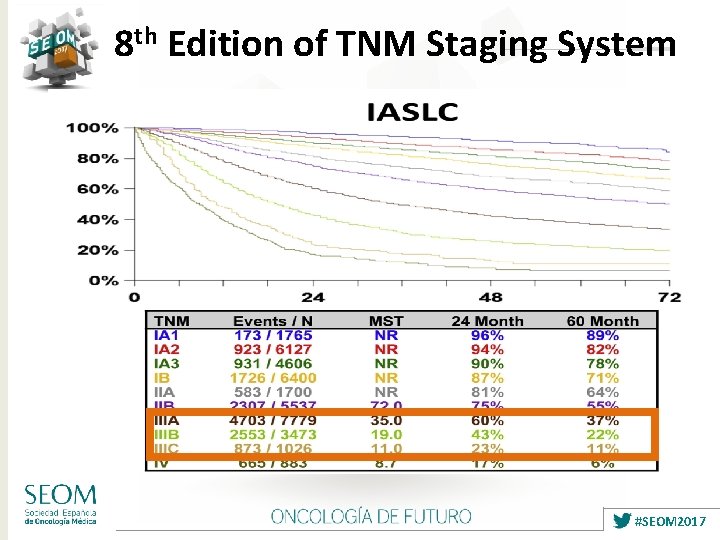
8 th Edition of TNM Staging System #SEOM 2017

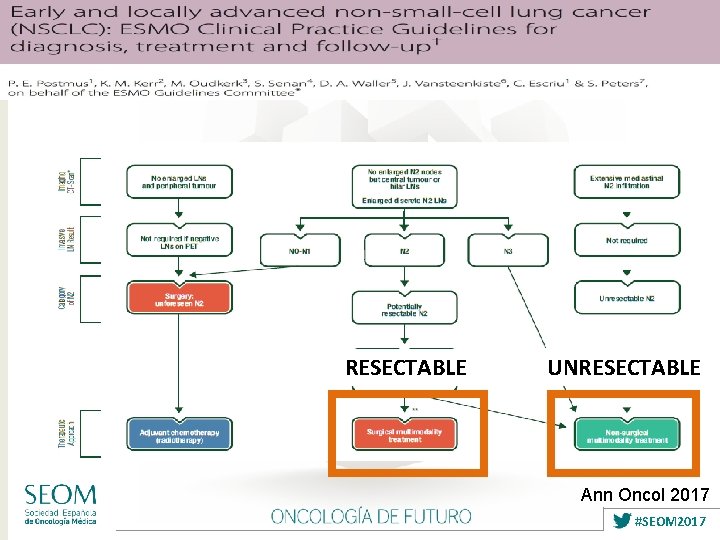
RESECTABLE UNRESECTABLE Ann Oncol 2017 #SEOM 2017

Ø STANDARD TREATMENT #SEOM 2017

1) POTENTIALLY RESECTABLE STAGE III DISEASE Ø INTRAOPERATIVE / PREOPERATIVE DIAGNOSIS OF N 2: Ø Surgery followed by Adjuvant CT +/- RT Ø Induction CT/CT-RT to Surgery: Ø One mediastinal node / No bulky / Downstaging / No pneumonectomy Ø CT-RT with better local control but similar PFS / OS Ø Definitive Concurrent CT-RT: N 2 multistation-bulky / N 3 Postmus P, Ann Oncol 2017 Santana-Davila R, JOP 2016 Guo SX, Sci Rep 2016 #SEOM 2017

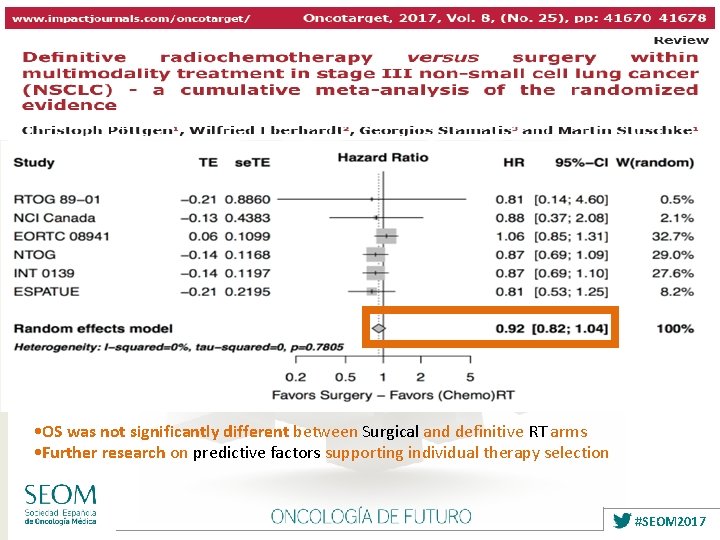
• OS was not significantly different between Surgical and definitive RT arms • Further research on predictive factors supporting individual therapy selection #SEOM 2017

1) POTENTIALLY RESECTABLE STAGE III DISEASE Ø SUPERIOR SULCUS TUMOUR / SELECTED T 3 -T 4 CENTRAL TUMOURS: Ø Induction CT-RT to Surgery Ø INDUCTION CT: Ø Platinum-based, preferably Cisplatin Multidisciplinary Team: VERY IMPORTANT Postmus P, Ann Oncol 2017 Santana-Davila R, JOP 2016 #SEOM 2017

2) UNRESECTABLE STAGE III DISEASE Ø CONCURRENT DEFINITIVE CT-RT: Fit Patients Ø SEQUENTIAL CT-RT: Un. Fit / Elderly Patients Postmus P, Ann Oncol 2017 Santana-Davila R, JOP 2016 #SEOM 2017

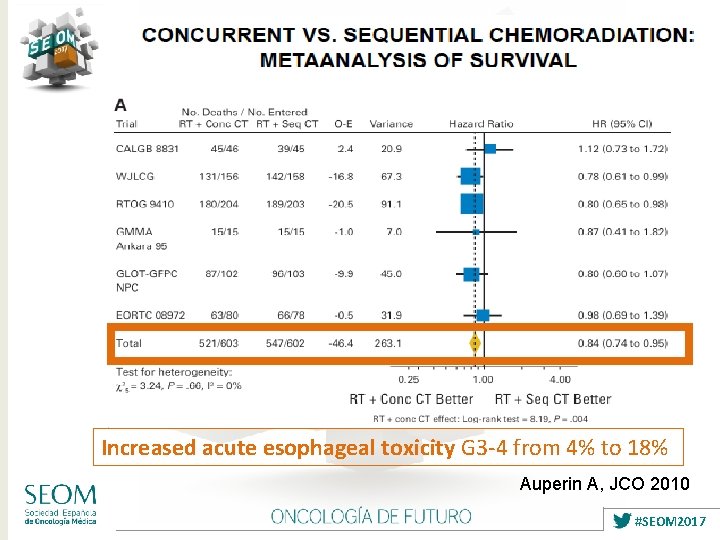
Increased acute esophageal toxicity G 3 -4 from 4% to 18% Auperin A, JCO 2010 #SEOM 2017

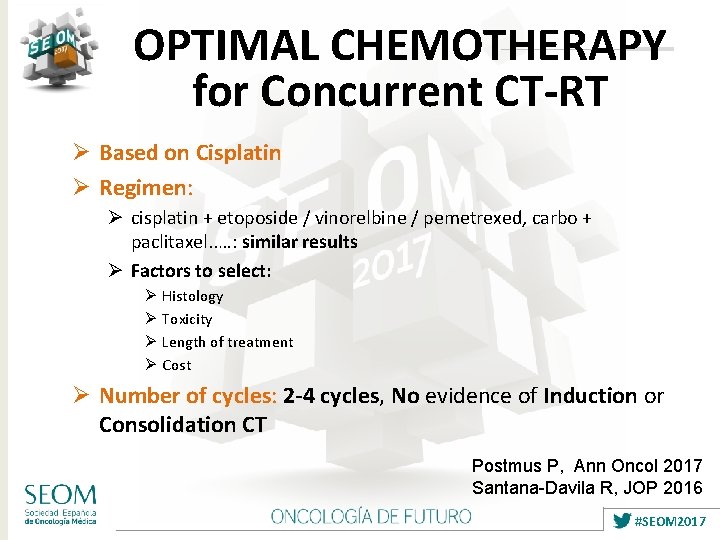
OPTIMAL CHEMOTHERAPY for Concurrent CT-RT Ø Based on Cisplatin Ø Regimen: Ø cisplatin + etoposide / vinorelbine / pemetrexed, carbo + paclitaxel. . …: similar results Ø Factors to select: Ø Histology Ø Toxicity Ø Length of treatment Ø Cost Ø Number of cycles: 2 -4 cycles, No evidence of Induction or Consolidation CT Postmus P, Ann Oncol 2017 Santana-Davila R, JOP 2016 #SEOM 2017

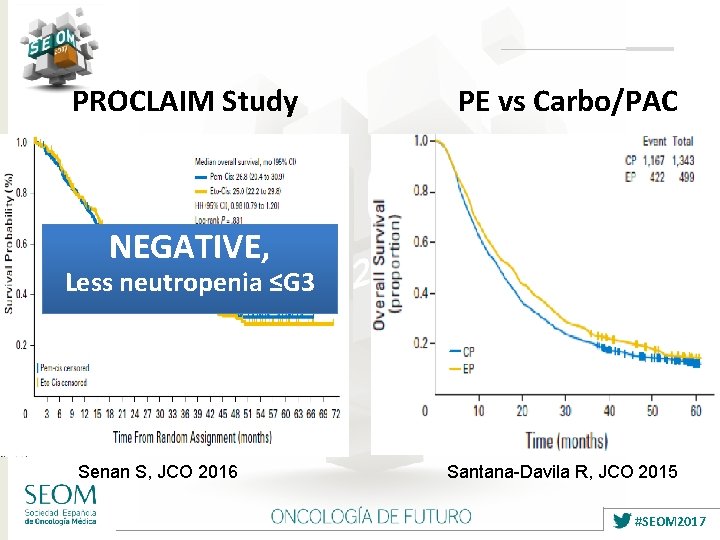
PROCLAIM Study PE vs Carbo/PAC NEGATIVE, Less neutropenia ≤G 3 Senan S, JCO 2016 Santana-Davila R, JCO 2015 #SEOM 2017

BETTER SAFETY PROFILE FOR OVNR ARM Phase II NORA Study (GECP): Oral VNR with Metronomic approach

OPTIMAL RADIOTHERAPY Ø Dose: Ø 60 -63 -66 Gy / 30 -33 fractions (74 Gy vs 60 Gy in RTOG 0617 Study was detrimental 1) Ø 40 -50 Gy as Induction CT-RT NEGATIVE Ø Investigational Areas : Ø Ø Ø Accelerated RT: sequential or RT alone, promising ? (No Phase III studies) Proton / Carbon-ion RT-CT 2: less toxicity ? , RTOG 1308 Study (60 vs 74 Gy) IMRT: to protect normal tissues, RTOG 06173 (less pneumonitis, better Qo. L) Adaptive RT: PET to personalize treatment, RTOG 0116 Study (60 vs 80 Gy) SBRT 4: a tool to increase RT dose Postmus P, Ann Oncol 2017 Santana-Davila R, JOP 2016 1 Bradley J, Lancet Oncol 2015 2 Chang J, JAMA Oncol 2017 3 Movsas B, JAMA Oncol 2016 4 Tekati H, JTO 2016 #SEOM 2017

PCI There is currently NO role Postmus P, Ann Oncol 2017 Santana-Davila R, JOP 2016 #SEOM 2017

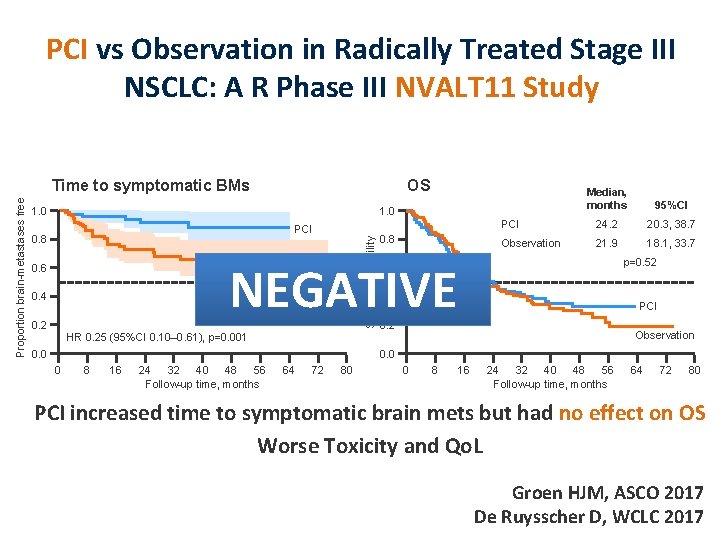
PCI vs Observation in Radically Treated Stage III NSCLC: A R Phase III NVALT 11 Study OS 1. 0 Median, months 95%CI PCI 24. 2 20. 3, 38. 7 Observation 21. 9 18. 1, 33. 7 1. 0 PCI 0. 8 Survival probability Proportion brain-metastases free Time to symptomatic BMs 0. 8 p=0. 52 NEGATIVE Observation 0. 6 0. 4 0. 2 HR 0. 25 (95%CI 0. 10– 0. 61), p=0. 001 0. 0 0. 6 0. 4 PCI 0. 2 Observation 0. 0 0 8 16 24 32 40 48 56 Follow-up time, months 64 72 80 PCI increased time to symptomatic brain mets but had no effect on OS Worse Toxicity and Qo. L Groen HJM, ASCO 2017 De Ruysscher D, WCLC 2017

Ø TARGETED THERAPY #SEOM 2017

INVESTIGATIONAL DRUGS Veliparib Olaparib #SEOM 2017

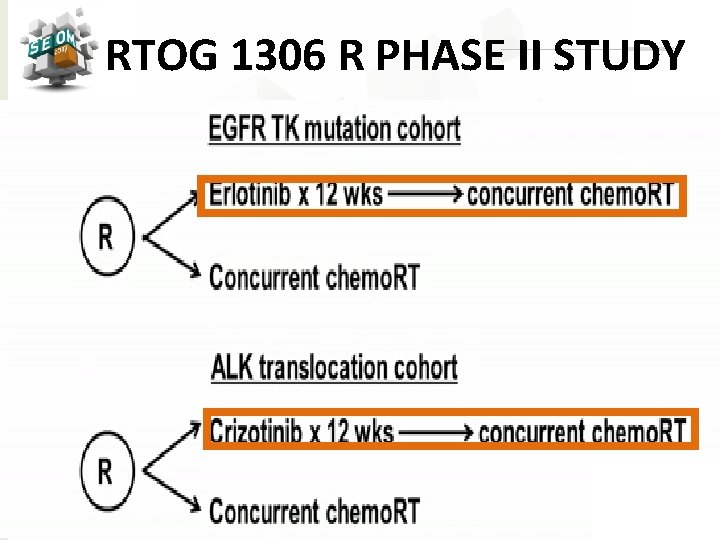
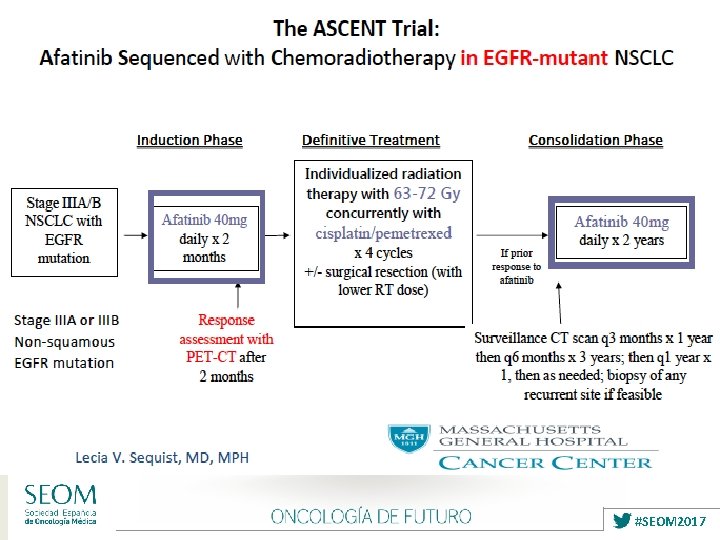
TARGETED THERAPY Ø Unselected Patients: ØNegative results: ØGefitinib (SWOG 0023 Study) ØCetuximab (RTOG 0617 Study) ØVeliparib ØSelumetinib Ø Selected Patients: Ongoing Trials ØErlotinib / Crizotinib (RTOG 1306 Study) ØAfatinib (ASCENT Trial) #SEOM 2017

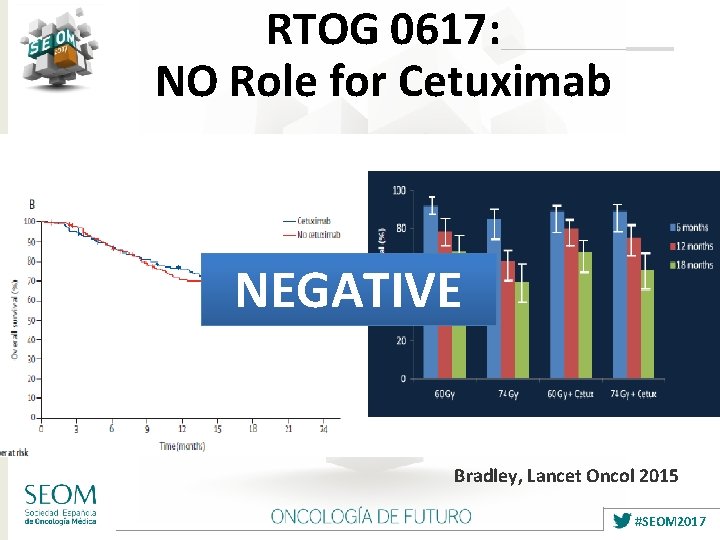
RTOG 0617: NO Role for Cetuximab NEGATIVE Bradley, Lancet Oncol 2015 #SEOM 2017

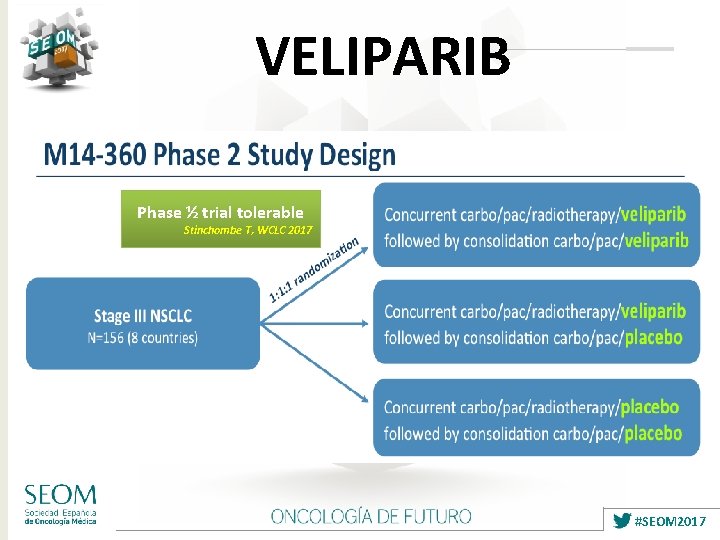
VELIPARIB Phase ½ trial tolerable Stinchombe T, WCLC 2017 #SEOM 2017

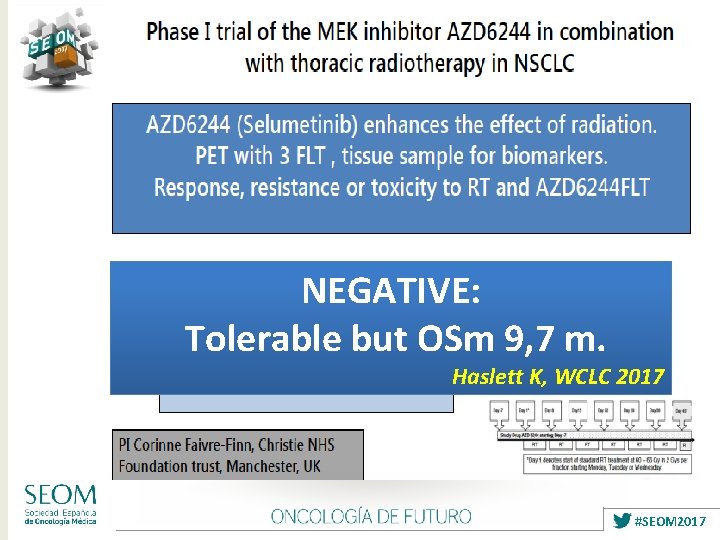
NEGATIVE: Tolerable but OSm 9, 7 m. Haslett K, WCLC 2017 #SEOM 2017

RTOG 1306 R PHASE II STUDY #SEOM 2017

#SEOM 2017

Ø IMMUNOTHERAPY #SEOM 2017

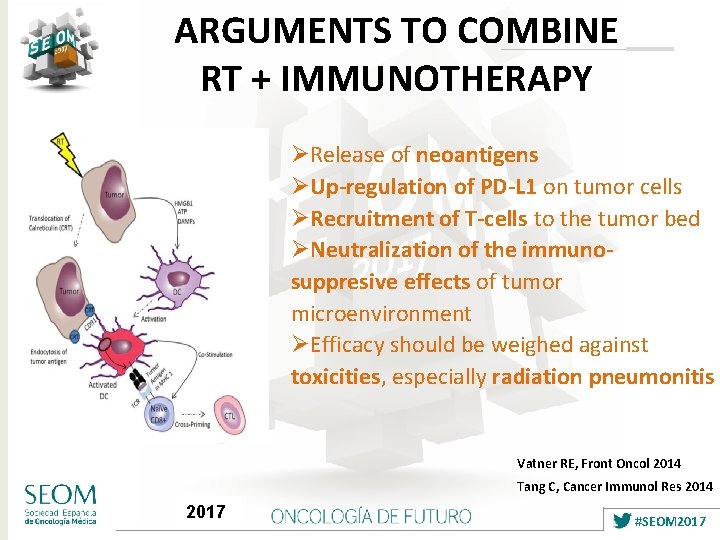
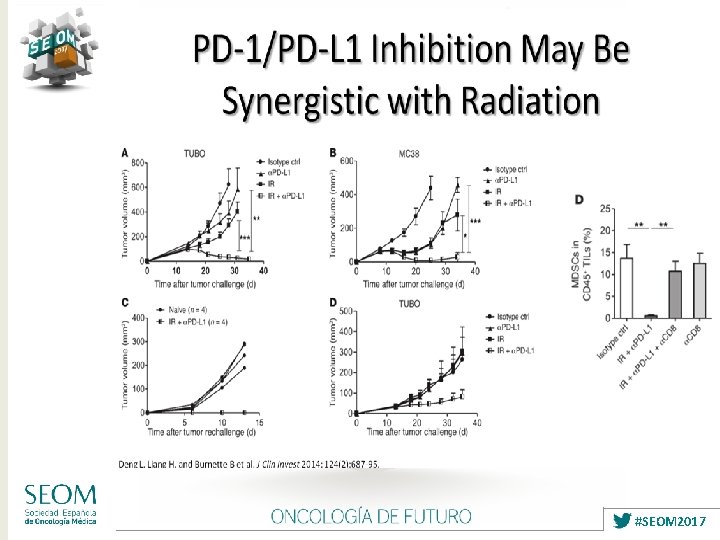
ARGUMENTS TO COMBINE RT + IMMUNOTHERAPY ØRelease of neoantigens ØUp-regulation of PD-L 1 on tumor cells ØRecruitment of T-cells to the tumor bed ØNeutralization of the immunosuppresive effects of tumor microenvironment ØEfficacy should be weighed against toxicities, especially radiation pneumonitis Vatner RE, Front Oncol 2014 Tang C, Cancer Immunol Res 2014 2017 #SEOM 2017

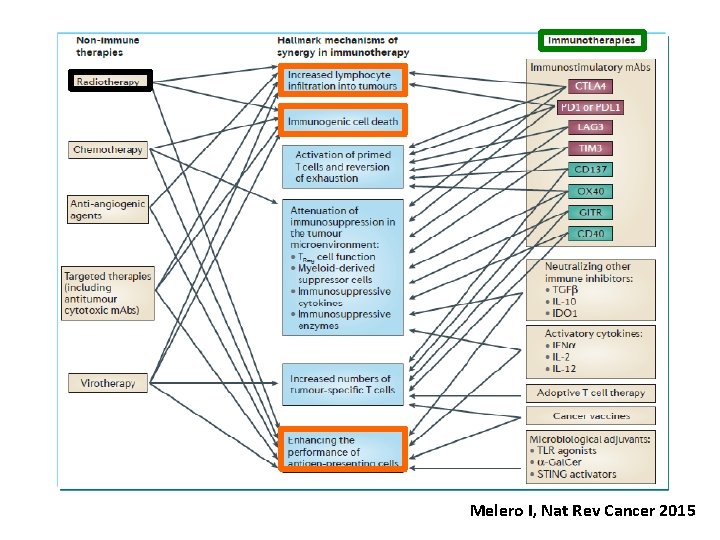
Melero I, Nat Rev Cancer 2015

#SEOM 2017

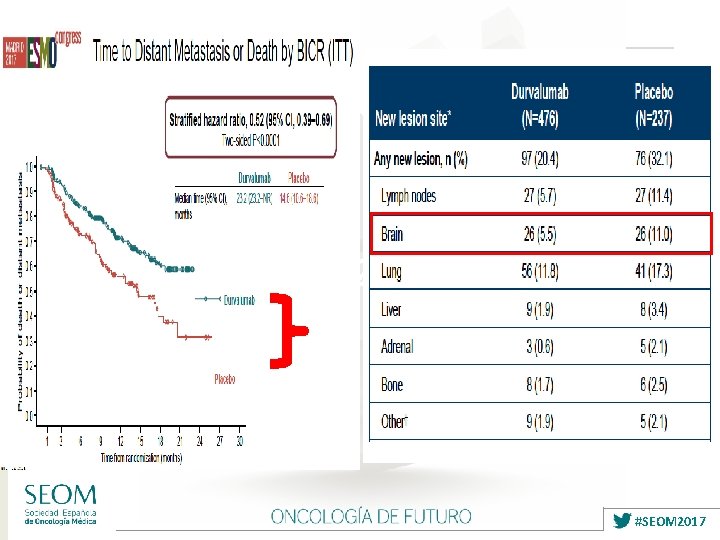
Antonia S, NEJM 2017 #SEOM 2017

T I S O P G ! ! N ! I S! D N T L A T SU S T E U R O E IV #SEOM 2017

#SEOM 2017

#SEOM 2017

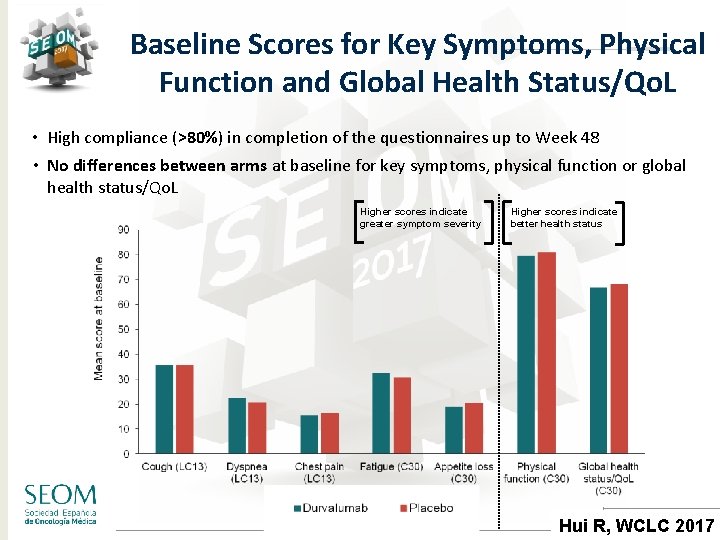
Baseline Scores for Key Symptoms, Physical Function and Global Health Status/Qo. L • High compliance (>80%) in completion of the questionnaires up to Week 48 • No differences between arms at baseline for key symptoms, physical function or global health status/Qo. L Higher scores indicate greater symptom severity Higher scores indicate better health status #SEOM 2017 Hui R, WCLC 2017

t d e d R A n L i I l S P M b I Ue S s O S Gi. Rm n A i W Hs. E t a Y m ET Et. Nh. Ti F e r SA Wt. E y Ta d E u B t S S O o #SEOM 2017

N I E C V I L T SC C F A EN I O C I AG D T R S g T A R n S i D FI LY d N n R A e EA ST S p n O W e : E E p N R o s i CA P EA #SEOM 2017

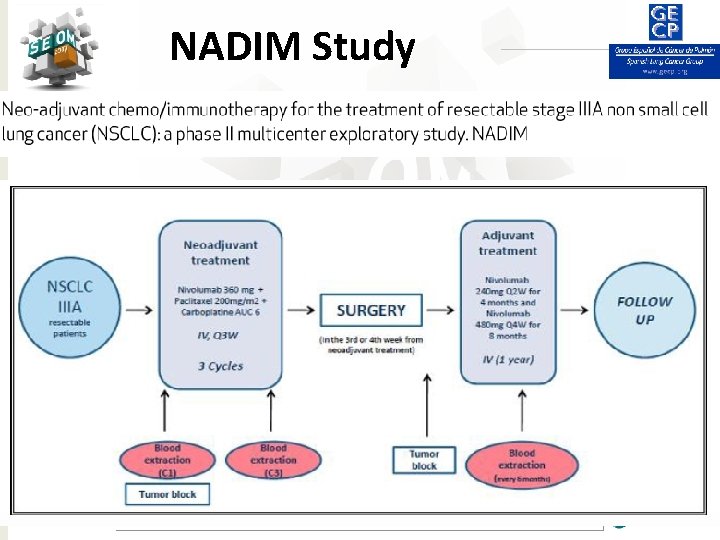
NADIM Study #SEOM 2017

#SEOM 2017

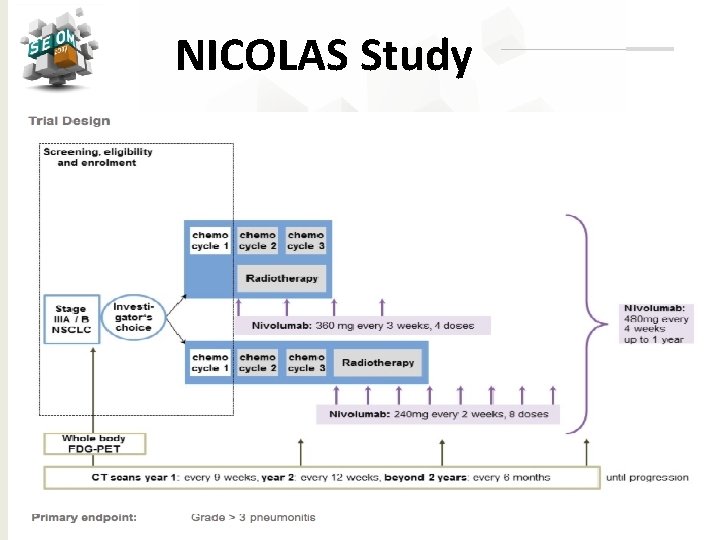
NICOLAS Study #SEOM 2017

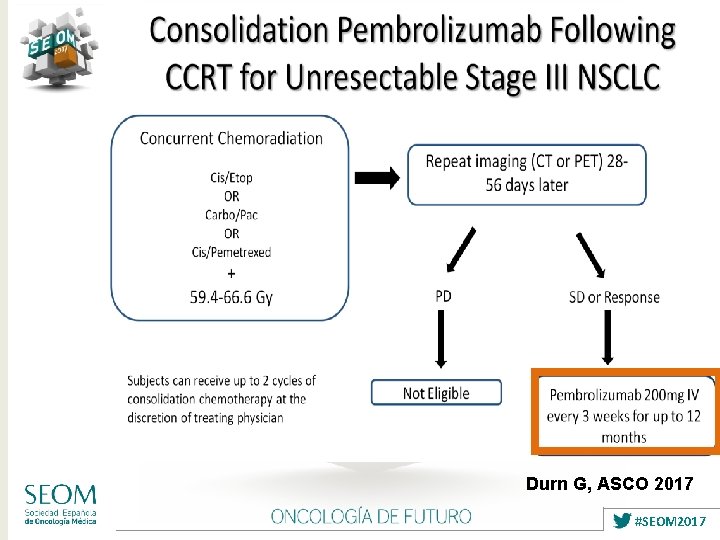
Durn G, ASCO 2017 #SEOM 2017

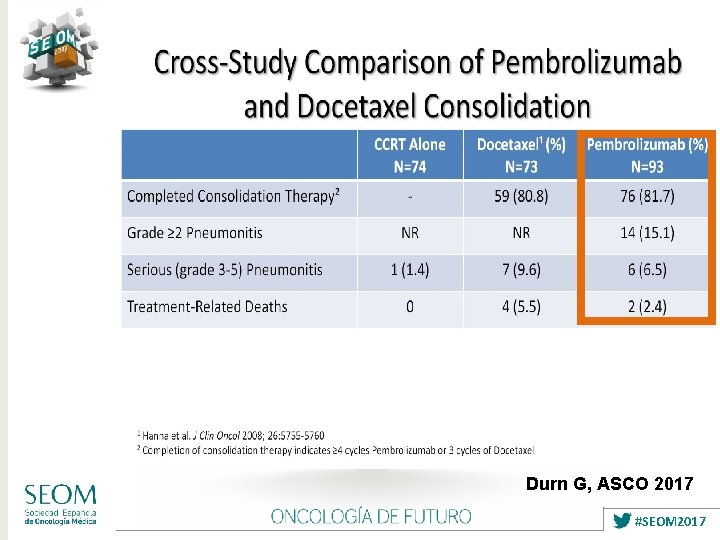
Durn G, ASCO 2017 #SEOM 2017

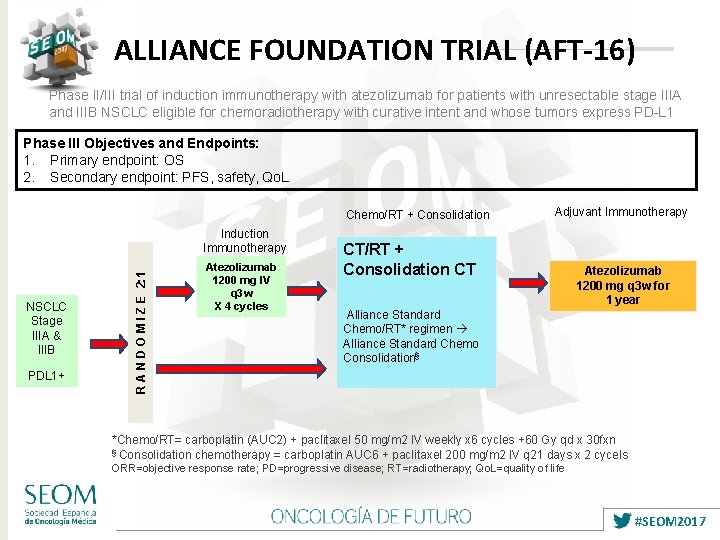
ALLIANCE FOUNDATION TRIAL (AFT-16) Phase II/III trial of induction immunotherapy with atezolizumab for patients with unresectable stage IIIA and IIIB NSCLC eligible for chemoradiotherapy with curative intent and whose tumors express PD-L 1 Phase III Objectives and Endpoints: 1. Primary endpoint: OS 2. Secondary endpoint: PFS, safety, Qo. L Chemo/RT + Consolidation NSCLC Stage IIIA & IIIB PDL 1+ R A N D O M I Z E 2: 1 Induction Immunotherapy Atezolizumab 1200 mg IV q 3 w X 4 cycles Adjuvant Immunotherapy CT/RT + Consolidation CT Atezolizumab 1200 mg q 3 w for 1 year Alliance Standard Chemo/RT* regimen Alliance Standard Chemo Consolidation§ *Chemo/RT= carboplatin (AUC 2) + paclitaxel 50 mg/m 2 IV weekly x 6 cycles +60 Gy qd x 30 fxn § Consolidation chemotherapy = carboplatin AUC 6 + paclitaxel 200 mg/m 2 IV q 21 days x 2 cycels ORR=objective response rate; PD=progressive disease; RT=radiotherapy; Qo. L=quality of life #SEOM 2017

#SEOM 2017

Ø CONCLUSIONS #SEOM 2017

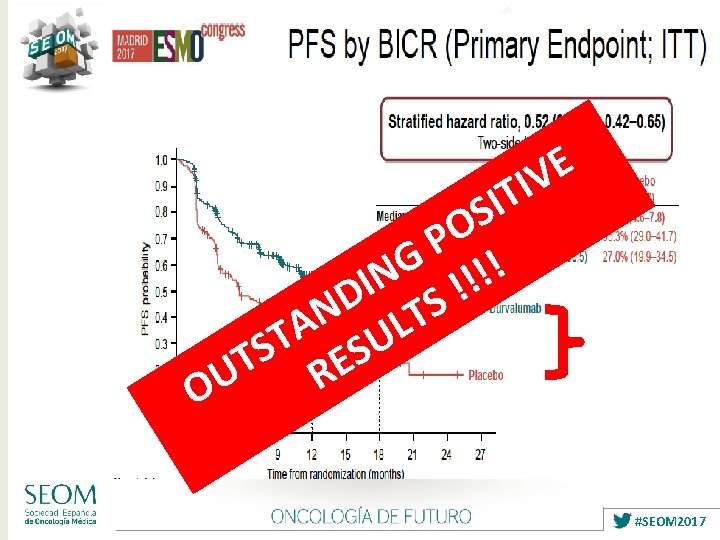
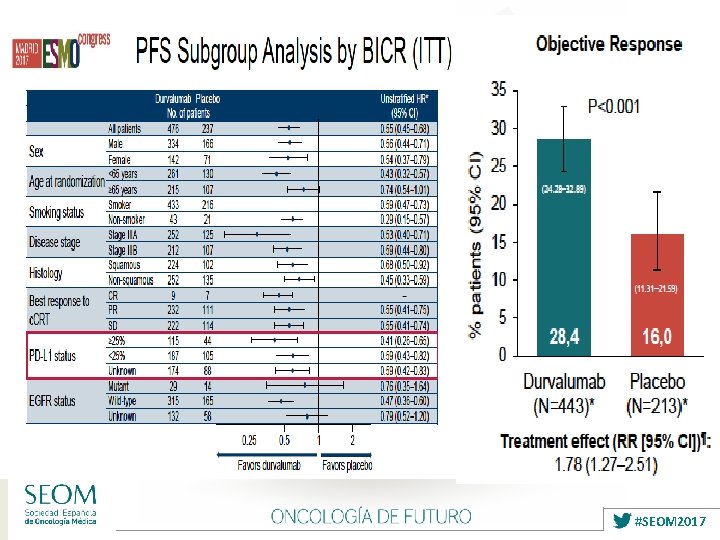
Ø Stage III NSCLC remains a Heterogeneus Disease Ø Concurrent CT-RT is Standard treatment but not always in clinical practice: FIT PATIENTS Ø Therapeutic plateau reached regarding CT with RT: 5 -ys OS 30 -35% Ø No role for available Targeted Agents yet Ø IMMUNOTHERAPY offers now a POSITIVE Phase III study (PACIFIC): ✔ ✔ ✔ Ø First step after many years…. . . Ø Toxicity no relevant / OS ? Ø Need of more INDIVIDUALIZED THERAPIES: clinical factors / biomarkers …. . Ø MULTIDISCIPLINARY and EXPERT TEAM ? ✔ #SEOM 2017

Gracias lola. isla@gmail. com #SEOM 2017