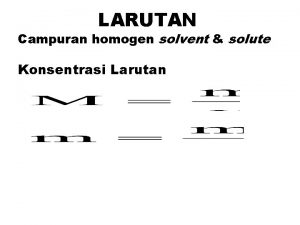
Karakteristik larutan Penyusun larutan solut dan solvent Keuntungan































- Slides: 37


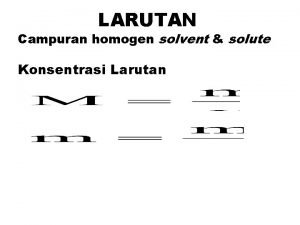
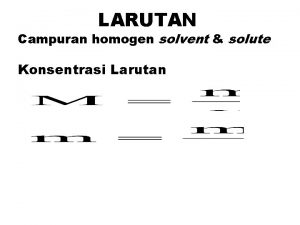
� Karakteristik larutan… � Penyusun larutan : solut dan solvent � Keuntungan larutan; 1. 2. 3. 4. 5. Nyaman utk pediatric dan geriatric Efek lebih cepat dibandingkan sediaan… lebih homogen dibandingkan suspensi atau emulsi Mengurangi efek samping obat yg iritatif thd sal cerna jika tdk diberikan dalam btk larutan (aspirin, KBr) Flexible dose

� 1. bulky � 2. sulit menutupi rasa/bau yg tidak enak utk obat tertentu � 3. takaran kdg 2 x sulit utk tepat dosis � 4. stabilitas < sediaan padat

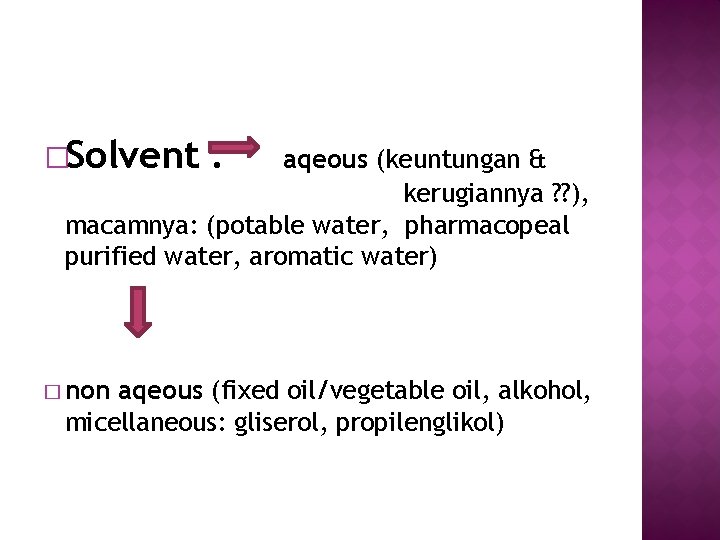
�Solvent : aqeous (keuntungan & kerugiannya ? ? ), macamnya: (potable water, pharmacopeal purified water, aromatic water) � non aqeous (fixed oil/vegetable oil, alkohol, micellaneous: gliserol, propilenglikol)

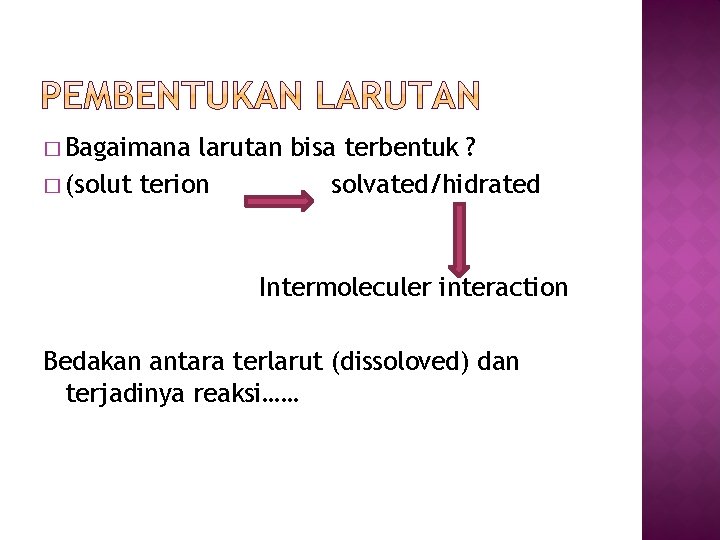
� Bagaimana larutan bisa terbentuk ? � (solut terion solvated/hidrated Intermoleculer interaction Bedakan antara terlarut (dissoloved) dan terjadinya reaksi……

� Faktor penting yang perlu diperhatikan dlm pembentukan larutan : solubility �stability �

� dissolution rates generally increase with: a. smaller particle sizes b. effective stirring c. lower viscosities d. increased temperature.

1. Buffers 2. Viscosity enhancer 3. Preservative (syarat nya: …… 1. effective against a wide spectrum of microorganisms 2. stable for its shelf life 3. non toxic, non sensitizing 4. compatible with the ingredients in the dosage form 5. free of taste and odour

� 1. gol. Alkohol (etanol, propilenglikol) � 2. acid (as. Benzoat, as. Sorbat) � 3. ester (paraben = ester dari parahidroksibenzoic acid, metil, propil, butil), perhatikan p. H efektifnya…. . � 4. gol. Ammonium kuarterner (Benzalkonium chloride )

Agent p. H Range Benzoic acid 2. 5 -4. 0 Sorbic acid 3. 0 -6. 5 Proprionic acid 2. 5 -5. 0 Acetic acid 3. 0 -5. 0 Parabens 3. 0 -9. 0 Sulfites 2. 5 -5. 0 Nitrites 4. 0 -5. 5

4. Antioxidants (propil &oktil ester dr asam galat, tokoferol, sod sulfit, ascorbic acid) oksidasi dpt diinisiasi oleh heat, light , heavy metal (solusi……) 5. Sweetening agents � ex: Sucrose Advantages: Colourless, highly water soluble, stable over a wide p. H range (4 -8), increase the viscosity, masks both salty and bitter taste, has soothing effect on throat. Polyhydric alcohols (sorbitol, mannitol and glycerol) possess sweetening power and can be used for diabetic preparations.

6. Flavours and perfumes Natural products: fruit juices, aromatic oil (peppermint, lemon) Artificial perfumes are cheaper, more readily available and more stable than natural products.

� physical � clarity, and chemical stability colour, odour, taste and viscosity over its shelf life.

� 1 - Loss of flavour � 2 - Change in taste � 3 - Presence of off flavours due to interaction with plastic bottle � 4 - Loss of dye � 5 - Precipitation � 6 - discoloration The effect: Change in smell or feel or taste




� Dissolved O 2 from air �Catalyzed by trace transition metals Cu, Ni, Fe, Mn, Co, etc. Contaminants from drug and solutes from which solution was made �Ex: captopril � Strategies �Purge with inert gas, usually N 2 �Antioxidants, EDTA (chelates free metal ions)



Dapat dicegah dengan: 1 - suitable packing in amber coloured bottles 2 - cardboard outers 3 - aluminium foil over wraps

1 - p. H - - The acidity or the alkalinity of a solution has a profound influence on the decomposition of drug compound. Aspirin buffered solution is maximum stable at a p. H of 2. 4, above a p. H of 10 the decomposition rate rapidly increases. p. H can also influence the rate of oxidation. The system is less readily oxidized when the p. H is low.

2 - Complexation Complex formation reduces the rate of hydrolysis and oxidation. e. g. caffeine complexes with local anesthetics, such as benzocaine, procaine and tetracaime to cause a reduction in their rate of hydrolytic degradation.

3 - Surfactants Nonionic, cationic and anionic surfactants when added to solutions containing drugs form micelle and the drug particles become trapped in the micelle. The hydrolytic groups such as OH cannot penetrate this micelle cover and reach the drug particles, hence hydrolysis rate is decreased.

4 - Presence of heavy metals Heavy metals, such as copper, iron, cobalt and nickel increase the rate of formation of free radicals and enhance oxidative decomposition. 5 - Light and humidity Light, especially ultraviolet light enhances photolysis and humidity enhances hydrolytic decomposition.

1 - Temperature All the drug products are stored at suitable temperatures to avoid thermal acceleration of decomposition. Three varieties of temperatures are suggested for storage of drug products. Room temperature, cool storage and cold storage. 2 - Light sensitive materials are stored in ambered colour bottles.

3 - Humidity Packing materials are chosen (usually glass and plastic) to prevent exposure of drug products to high humid condition. 4 - Oxygen Proper packing keeping the oxygen content of the solution less and leaving very little head space in the bottle above the drug products are methods to fight against oxidation.


5 - Chelating Agents Chelating agents form complexes with heavy metal ions and prevent them from catalyzing oxidative decomposition. e. g. ethylenediamine tetracetic acid (EDTA) derivatives and salts, citric acid and tartaric acid. 6 - Solvents By the addition of a suitable solvent hydrolysis rate may be decreased


(1) suitably designing the containers (2) usually usingle dose containers (3) sticking to proper storage conditions (4) adding an antimicrobial substance as preservative.

� 1. gelas � 2. plastik Plastics The problems with plastic are: 1. Migration of the drug through the plastic into the environment. 2. Transfer of environmental moisture, oxygen, and other elements into the pharmaceutical product. 3. Leaching of container ingredients into the drug. 4. Adsorption of the active drug or excipients by the plastic.

3. Metals - Various alloys and aluminium tubes may be utilized as containers for emulsions, ointments, creams and pastes. - Limitation: They may cause corrosion and precipitation in the drug product. - Overcome: Coating the tubes with polymers may reduce these tendencies.

4. Rubber - Rubber also has the problems of extraction of drug ingredients and leaching of container ingredients. - The pretreatment of rubber vial stoppers and closures with water and steam reduces potential leaching

� No dissolution phase � Major concern is physicochemical stability and interaction with other substances in GI fluid �Change in p. H �Dilution of cosolvent �Formation of insoluble complexes Ciprofloxacin with Fe 2+, Ca 2+, Mg 2+ etc. � Viscosity of solution may slow absorption �Assuming spherical drug molecule


1. Simple Solution (by stirring or heating) 2. Solution by Chemical Reaction (by reacting two or more solutes with each other in a suitable solvent) 3. Solution by Extraction (Plant or animal products)
 Contoh soal keuntungan mutlak dan keuntungan berbanding
Contoh soal keuntungan mutlak dan keuntungan berbanding Siittiö vararavinto
Siittiö vararavinto Solut
Solut Keuntungan dan kerugian sediaan emulsi
Keuntungan dan kerugian sediaan emulsi Campuran larutan berikut bersifat buffer, kecuali
Campuran larutan berikut bersifat buffer, kecuali Larutan penyangga dalam obat tetes mata
Larutan penyangga dalam obat tetes mata Rumus basa kuat
Rumus basa kuat Mengamati organ penyusun sistem gerak
Mengamati organ penyusun sistem gerak Organ penyusun sistem transportasi
Organ penyusun sistem transportasi Contoh solute dan solvent
Contoh solute dan solvent Apa itu persekutuan komanditer
Apa itu persekutuan komanditer Keunggulan metode observasi adalah
Keunggulan metode observasi adalah Sedian solid
Sedian solid Keuntungan dan kerugian pendekatan direktif
Keuntungan dan kerugian pendekatan direktif Perbedaan laba dan keuntungan
Perbedaan laba dan keuntungan Jamur chytridiomycota
Jamur chytridiomycota Keuntungan dan kerugian tata ruang kantor tertutup
Keuntungan dan kerugian tata ruang kantor tertutup Tujuan erd
Tujuan erd Sifat bahan yang digunakan untuk membuat jas hujan adalah
Sifat bahan yang digunakan untuk membuat jas hujan adalah Organ penyusun sistem gerak adalah …. *
Organ penyusun sistem gerak adalah …. * Penyusun dinding sel
Penyusun dinding sel Bagian terkecil penyusun tubuh makhluk hidup
Bagian terkecil penyusun tubuh makhluk hidup Penyusun enzim adalah
Penyusun enzim adalah Kelompok tulang pipa
Kelompok tulang pipa Tulang penyusun rangka manusia
Tulang penyusun rangka manusia Bahan dasar penyusun dna
Bahan dasar penyusun dna Unsur hara penyusun tanaman
Unsur hara penyusun tanaman Zona akar
Zona akar Unit tekecil penyusun tubuh kita adalah …….. *
Unit tekecil penyusun tubuh kita adalah …….. * Batu lempung
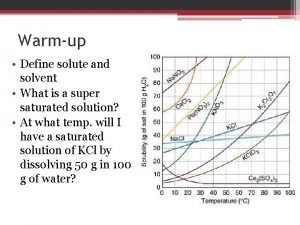
Batu lempung Define solute and solvent
Define solute and solvent Solute vs solvent
Solute vs solvent Ultra shine company manufactures a cleaning solvent
Ultra shine company manufactures a cleaning solvent Solvent vs solute
Solvent vs solute Solute
Solute Which substance
Which substance Solute and solvent
Solute and solvent A homogeneous mixture of a solute and solvent
A homogeneous mixture of a solute and solvent