Joint Hospital Surgical Grand Round 18 July 2015

















- Slides: 52

Joint Hospital Surgical Grand Round 18 July 2015 Dr HUI Hon Cheung Rio Princess Margaret Hospital

Can all early gastric cancer be treated equally?

Content o o o Case Introduction Diagnosis and work up Management Conclusion

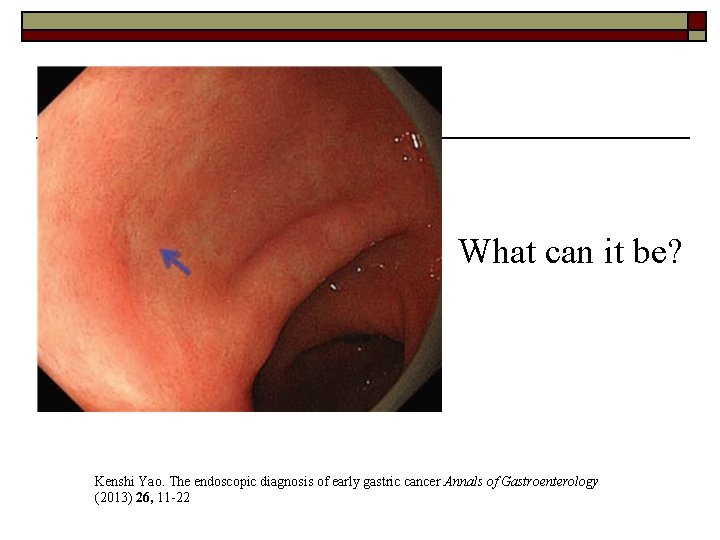
Case o o o 50/male Past history of Helicobacter pylori related gastric ulcer in 6 years before Completed a course of triple therapy Presented with on and off epigastric pain X 1 year OGD done: Vague slightly depressed lesion on the gastric antrum ~5 mm in size

What can it be? Kenshi Yao. The endoscopic diagnosis of early gastric cancer Annals of Gastroenterology (2013) 26, 11 -22

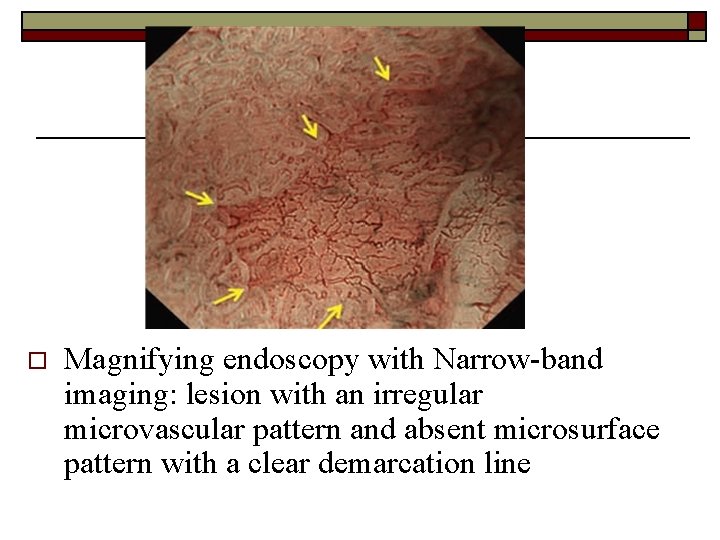
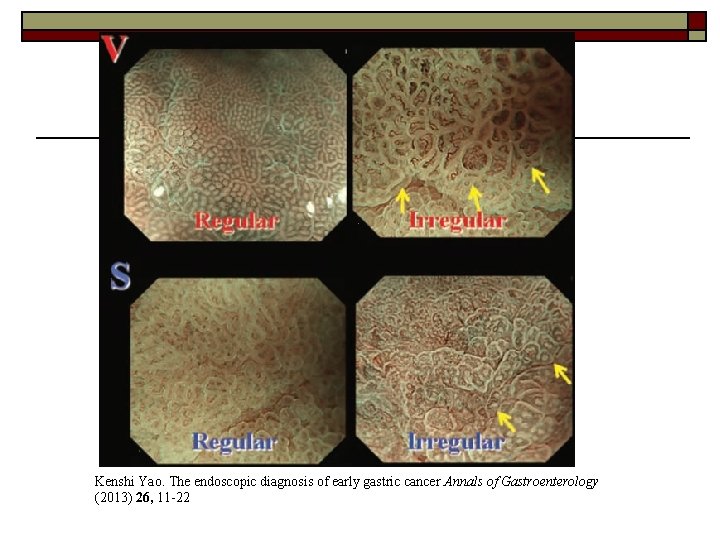
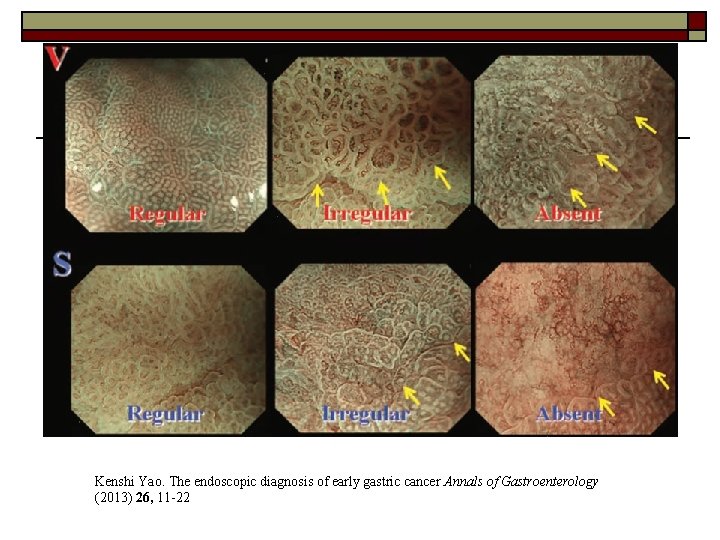
o Magnifying endoscopy with Narrow-band imaging: lesion with an irregular microvascular pattern and absent microsurface pattern with a clear demarcation line

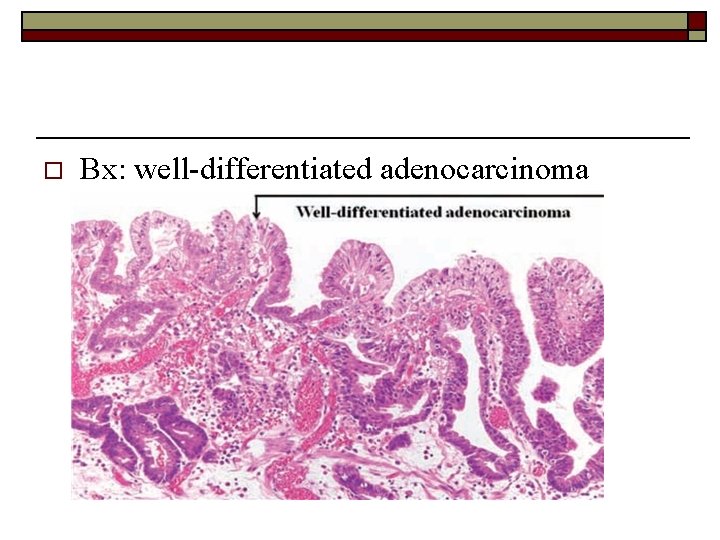
o Bx: well-differentiated adenocarcinoma

o What is the further Treatment?

Introduction o o o Gastric cancer is the 6 th commonest cancer and 4 th commonest cancer death in HK Incidence of gastric cancer is higher in Asian countries than western countries Early gastric cancer (EGC) is defined as a lesion confined to the mucosa and submucosa , regardless of lymph node metastasis Japanese Research Society for Gastric Cancer

o o T 1 a: The tumor is growing into the lamina propria or muscularis mucosa. T 1 b: The tumor is growing into the submucosa. Early detection and treatment can improve survival and prognosis 5 th year survival rates for EGC are over 90% -Ono H, Kondo H, Gotoda T, et al. (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48: 225 – 229

Diagnosis of Early gastric cancer Endoscopy is the most effective method for detecting early gastric cancer Choices of endoscopy: o Conventional white light endoscopy o Chromoendoscopy (indigo-carmine) o Magnifying endoscopy-Narrow Band imaging (ME-NBI) o

o ME-NBI is proven to be more accurate than conventional white light endoscopy -Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis Qiang Zhang, Fei Wang, Zhen-Yu Chen, Zhen Wang, Fa-Chao Zhi, Si-De Liu, Yang Bai Gastric Cancer April 2015 -Kato M, Kaise M, Yonezawa J, Toyoizumi H, Yoshimura N, Yoshida Y, et al. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010; 72: 523– 9. o ME-NBI is more effective in detecting small early gastric cancer than chromoendoscopy Can we accurately diagnose minute gastric cancers (<5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (M-NBI)Shoko Fujiwara • Kenshi Yao • Takashi Nagahama • K. Uchita • Takao Kanemitsu • Kozue Tsurumi • Noritaka Takatsu • Takashi Hisabe • Hiroshi Tanabe • Akinori Iwashita • Toshiyuki Matsui Gastric Cancer 11 June 2014

Detecting the lesion—ME-NBI o o o VS classification (by Kenshi Yao) widely used in ME-NBI criteria for making a diagnosis of gastric cancer: 1) An irregular microvascular pattern with a demarcation line. 2) An irregular microsurface pattern with a demarcation line.

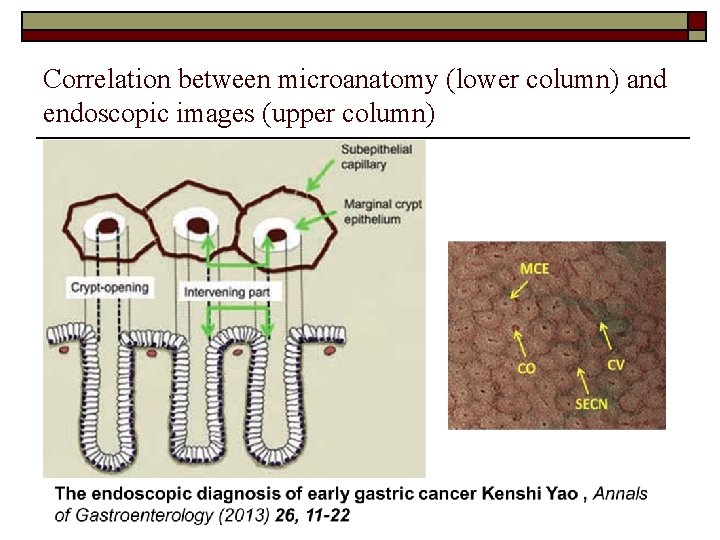
Correlation between microanatomy (lower column) and endoscopic images (upper column)

Kenshi Yao. The endoscopic diagnosis of early gastric cancer Annals of Gastroenterology (2013) 26, 11 -22

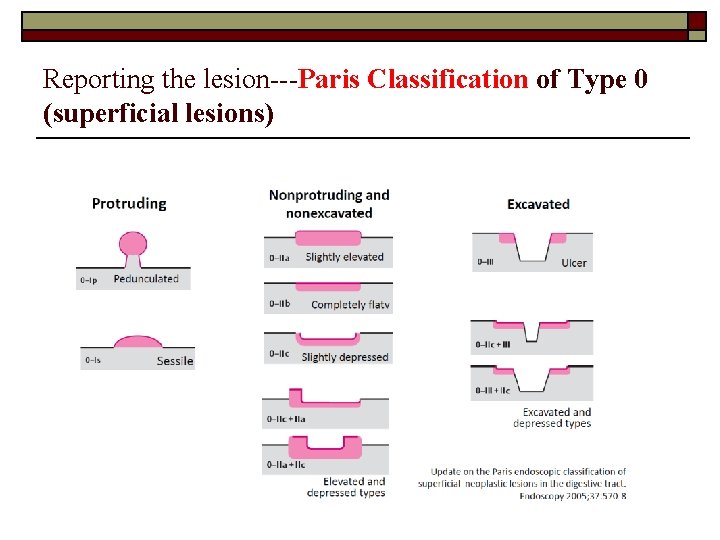
Reporting the lesion---Paris Classification of Type 0 (superficial lesions)

Work up o o History Physical examination CT scan (chest/abd/pelvis) with IV contrast: Looks for local or distant metastasis +/- Endoscopic ultrasound -H. Yanai, Y. Matsumoto, T. Harada et al. , “Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study, ” Gastrointestinal Endoscopy, vol. 46, no. 3, pp. 212– 216, 1997. -G. H. Kim, D. Y. Park, M. Kida et al. , “Accuracy of high-frequency catheter-based endoscopic ultrasonography according to the indications for endoscopic treatment of early gastric cancer, ” Journal of Gastroenterology and Hepatology, vol. 25, no. 3, pp. 506– 511, 2010. -T. Tsuzukr, H. Okada, Y. Kawahara et al. , “Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer, ” Acta Medica Okayama, vol. 65, no. 2, pp. 105– 112, 2011 -Mouri R 1, Yoshida S, Tanaka S. , et al, Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009 Apr; 43(4): 31822

Important factors affecting EGC management o o Depth of invasion of the lesion Lymph node metastasis

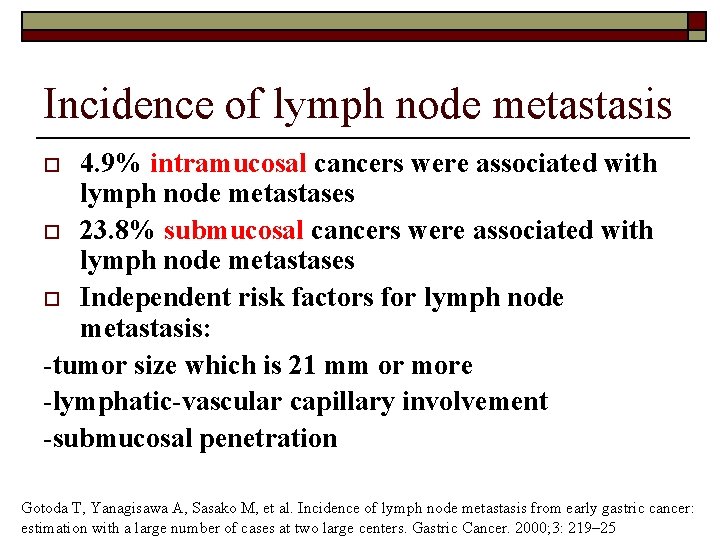
Incidence of lymph node metastasis 4. 9% intramucosal cancers were associated with lymph node metastases o 23. 8% submucosal cancers were associated with lymph node metastases o Independent risk factors for lymph node metastasis: -tumor size which is 21 mm or more -lymphatic-vascular capillary involvement -submucosal penetration o Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3: 219– 25

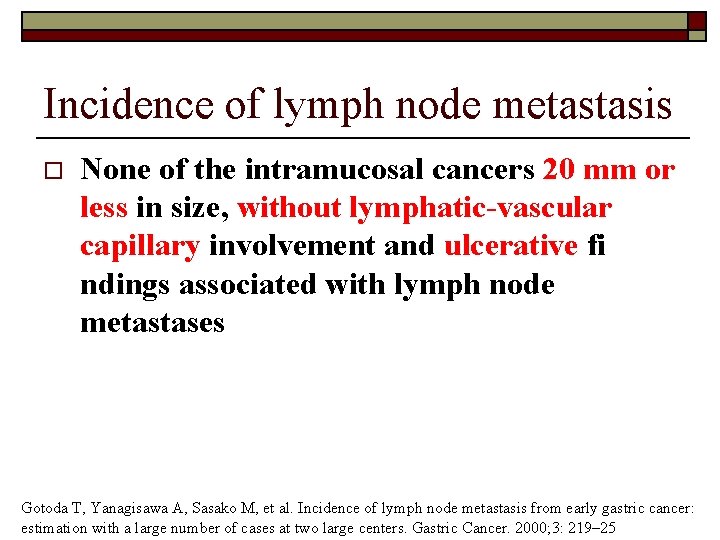
Incidence of lymph node metastasis o None of the intramucosal cancers 20 mm or less in size, without lymphatic-vascular capillary involvement and ulcerative fi ndings associated with lymph node metastases Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3: 219– 25

Management of early gastric cancer o o Management of gastric lesion Management of lymph node

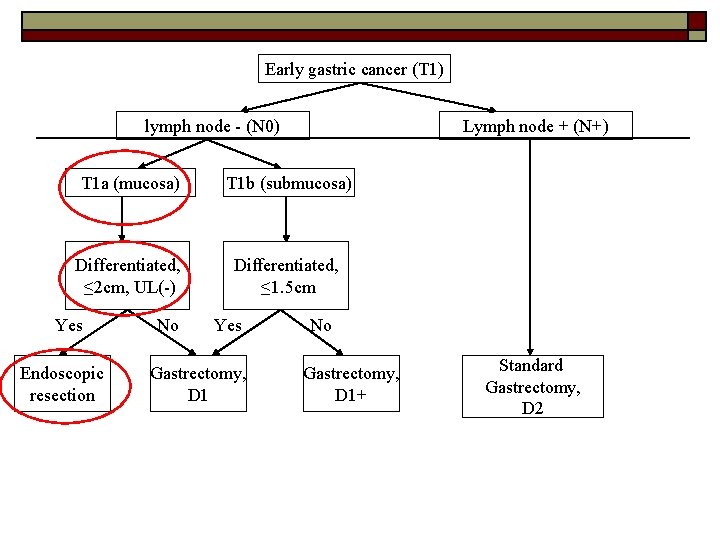
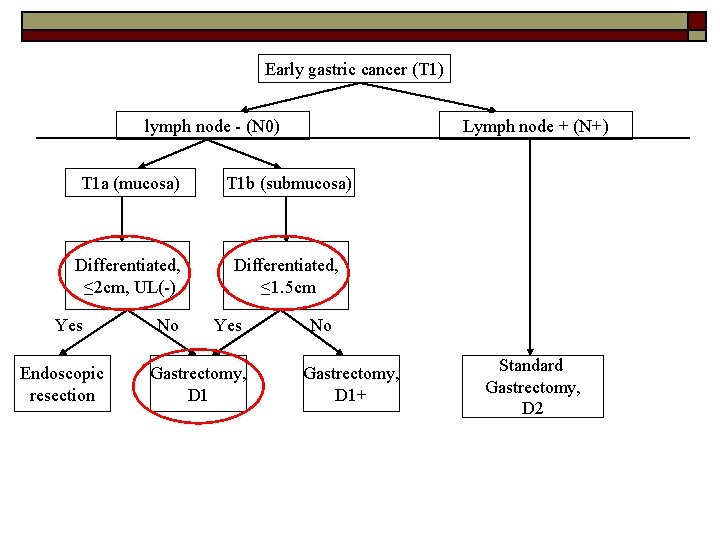
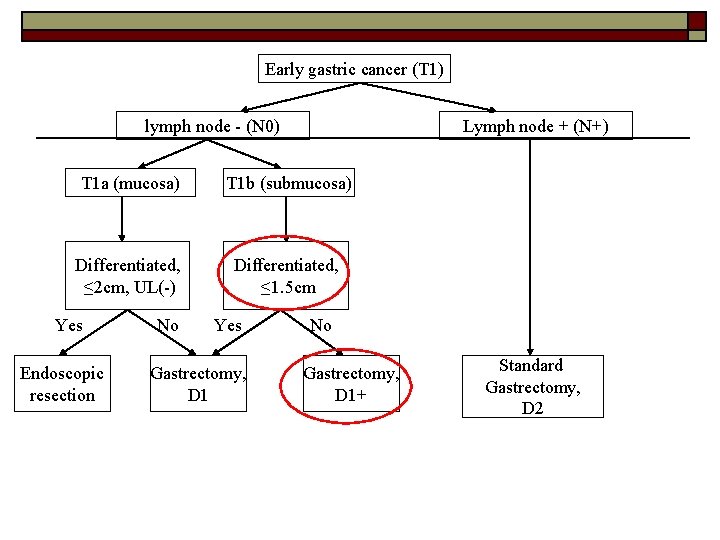
Can all early gastric cancer be treated equally? NO o Determining factors: -lymph node metastasis -Depth of invasion (T 1 a vs T 1 b) -Size of lesion -Differentiated vs undifferentiated -Ulceration o

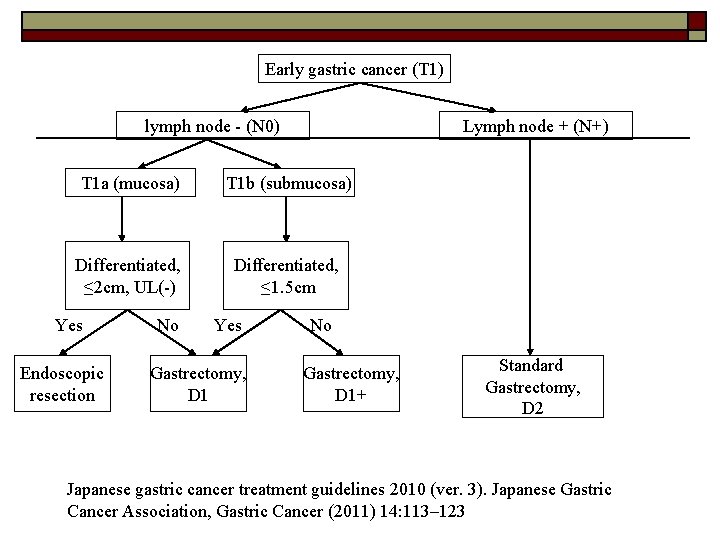
Early gastric cancer (T 1) lymph node - (N 0) Lymph node + (N+) T 1 a (mucosa) T 1 b (submucosa) Differentiated, ≤ 2 cm, UL(-) Differentiated, ≤ 1. 5 cm Yes Endoscopic resection No Yes Gastrectomy, D 1 No Gastrectomy, D 1+ Standard Gastrectomy, D 2 Japanese gastric cancer treatment guidelines 2010 (ver. 3). Japanese Gastric Cancer Association, Gastric Cancer (2011) 14: 113– 123

Early gastric cancer (T 1) lymph node - (N 0) Lymph node + (N+) T 1 a (mucosa) T 1 b (submucosa) Differentiated, ≤ 2 cm, UL(-) Differentiated, ≤ 1. 5 cm Yes Endoscopic resection No Yes Gastrectomy, D 1 No Gastrectomy, D 1+ Standard Gastrectomy, D 2

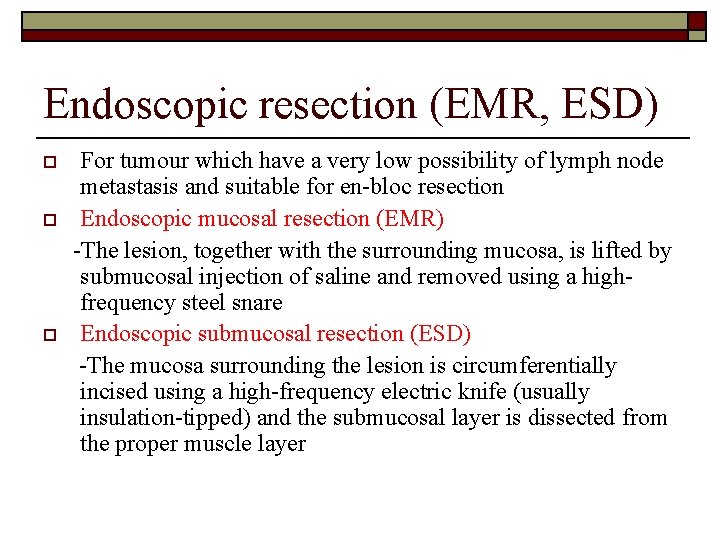
Endoscopic resection (EMR, ESD) o o o For tumour which have a very low possibility of lymph node metastasis and suitable for en-bloc resection Endoscopic mucosal resection (EMR) -The lesion, together with the surrounding mucosa, is lifted by submucosal injection of saline and removed using a highfrequency steel snare Endoscopic submucosal resection (ESD) -The mucosa surrounding the lesion is circumferentially incised using a high-frequency electric knife (usually insulation-tipped) and the submucosal layer is dissected from the proper muscle layer

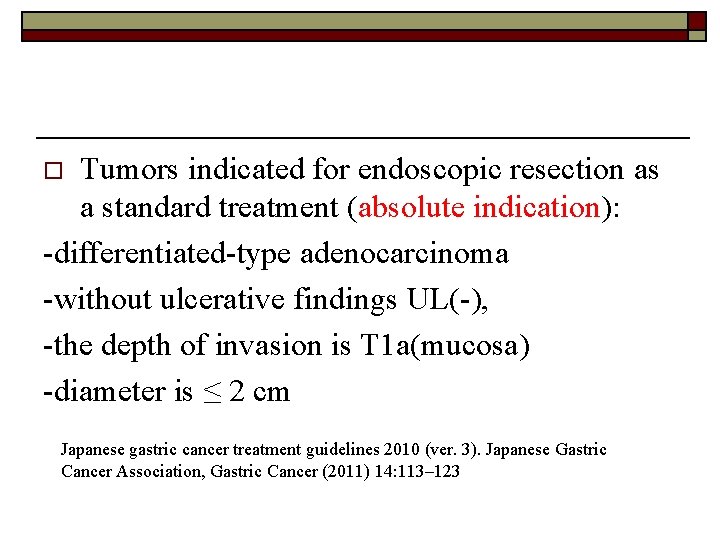
Tumors indicated for endoscopic resection as a standard treatment (absolute indication): -differentiated-type adenocarcinoma -without ulcerative findings UL(-), -the depth of invasion is T 1 a(mucosa) -diameter is ≤ 2 cm o Japanese gastric cancer treatment guidelines 2010 (ver. 3). Japanese Gastric Cancer Association, Gastric Cancer (2011) 14: 113– 123

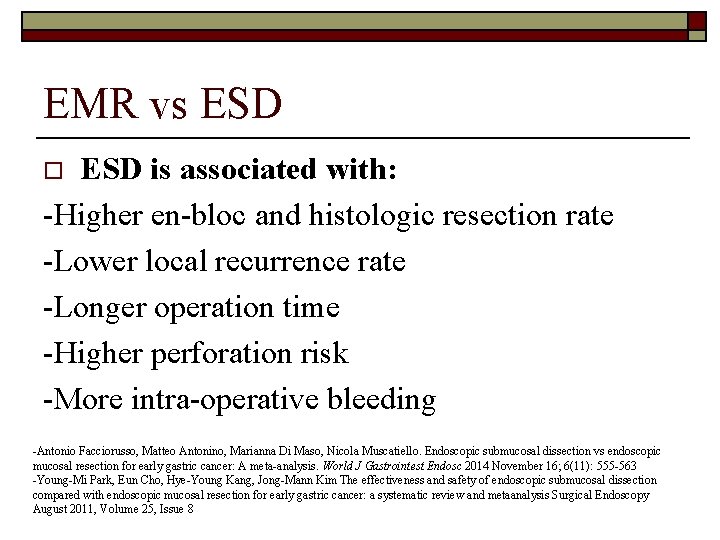
EMR vs ESD is associated with: -Higher en-bloc and histologic resection rate -Lower local recurrence rate -Longer operation time -Higher perforation risk -More intra-operative bleeding o -Antonio Facciorusso, Matteo Antonino, Marianna Di Maso, Nicola Muscatiello. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc 2014 November 16; 6(11): 555 -563 -Young-Mi Park, Eun Cho, Hye-Young Kang, Jong-Mann Kim The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis Surgical Endoscopy August 2011, Volume 25, Issue 8

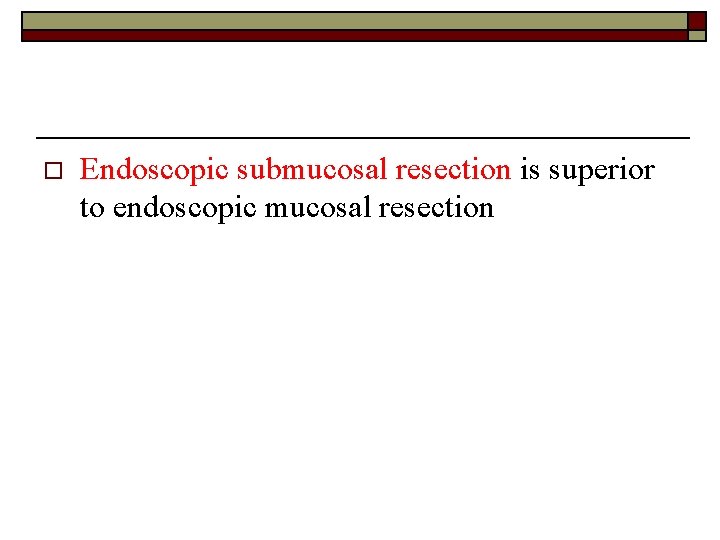
o Endoscopic submucosal resection is superior to endoscopic mucosal resection

Long term result of ESD o o 5 year overall survival: 92. 6% Local recurrence rate: 0. 2 -0. 6% -Satoshi Tanabe • Kenji Ishido • Katsuhiko Higuchi • Tohru Sasaki • Chikatoshi Katada • Mizutomo Azuma • Akira Naruke • Myungchul Kim • Wasaburo Koizumi. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center Gastric Cancer (2014) 17: 130– 136 -Li Jun Peng 1, Shu Ni Tian 1, Lin Lu 1, Hao Chen. Outcome of endoscopic submucosal dissection for early gastric cancer of conventional and expanded indications: Systematic review and meta-analysis. Journal of Digestive Diseases Volume 16, Issue 2 pages 67– 74, February 2015 -Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5 -year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2015 Jan 24.

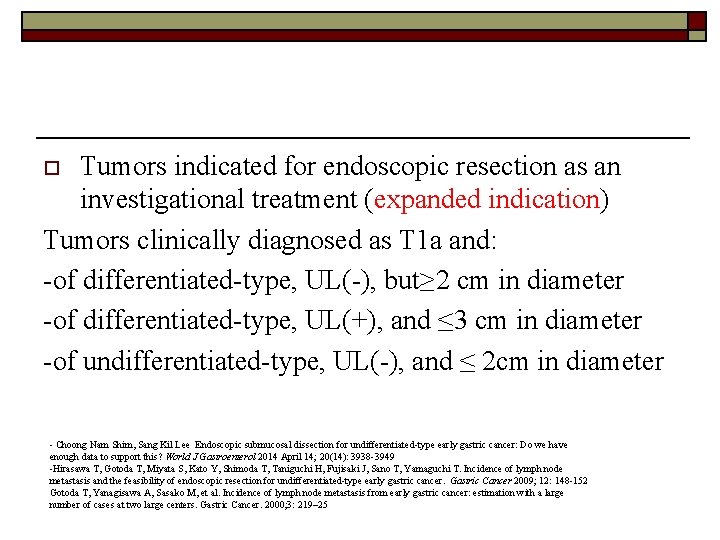
Tumors indicated for endoscopic resection as an investigational treatment (expanded indication) Tumors clinically diagnosed as T 1 a and: -of differentiated-type, UL(-), but≥ 2 cm in diameter -of differentiated-type, UL(+), and ≤ 3 cm in diameter -of undifferentiated-type, UL(-), and ≤ 2 cm in diameter o - Choong Nam Shim, Sang Kil Lee Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: Do we have enough data to support this? World J Gastroenterol 2014 April 14; 20(14): 3938 -3949 -Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12: 148 -152 Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3: 219– 25

Early gastric cancer (T 1) lymph node - (N 0) Lymph node + (N+) T 1 a (mucosa) T 1 b (submucosa) Differentiated, ≤ 2 cm, UL(-) Differentiated, ≤ 1. 5 cm Yes Endoscopic resection No Yes Gastrectomy, D 1 No Gastrectomy, D 1+ Standard Gastrectomy, D 2

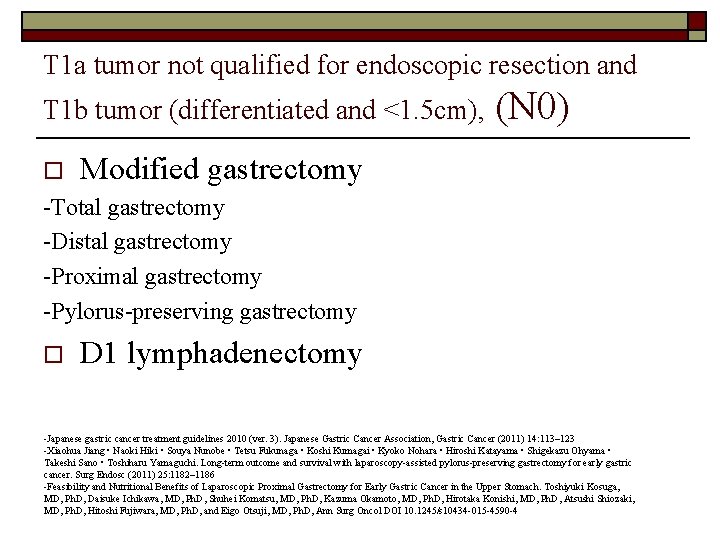
T 1 a tumor not qualified for endoscopic resection and T 1 b tumor (differentiated and <1. 5 cm), o (N 0) Modified gastrectomy -Total gastrectomy -Distal gastrectomy -Proximal gastrectomy -Pylorus-preserving gastrectomy o D 1 lymphadenectomy -Japanese gastric cancer treatment guidelines 2010 (ver. 3). Japanese Gastric Cancer Association, Gastric Cancer (2011) 14: 113– 123 -Xiaohua Jiang • Naoki Hiki • Souya Nunobe • Tetsu Fukunaga • Koshi Kumagai • Kyoko Nohara • Hiroshi Katayama • Shigekazu Ohyama • Takeshi Sano • Toshiharu Yamaguchi. Long-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc (2011) 25: 1182– 1186 -Feasibility and Nutritional Benefits of Laparoscopic Proximal Gastrectomy for Early Gastric Cancer in the Upper Stomach. Toshiyuki Kosuga, MD, Ph. D, Daisuke Ichikawa, MD, Ph. D, Shuhei Komatsu, MD, Ph. D, Kazuma Okamoto, MD, Ph. D, Hirotaka Konishi, MD, Ph. D, Atsushi Shiozaki, MD, Ph. D, Hitoshi Fujiwara, MD, Ph. D, and Eigo Otsuji, MD, Ph. D, Ann Surg Oncol DOI 10. 1245/s 10434 -015 -4590 -4

Early gastric cancer (T 1) lymph node - (N 0) Lymph node + (N+) T 1 a (mucosa) T 1 b (submucosa) Differentiated, ≤ 2 cm, UL(-) Differentiated, ≤ 1. 5 cm Yes Endoscopic resection No Yes Gastrectomy, D 1 No Gastrectomy, D 1+ Standard Gastrectomy, D 2

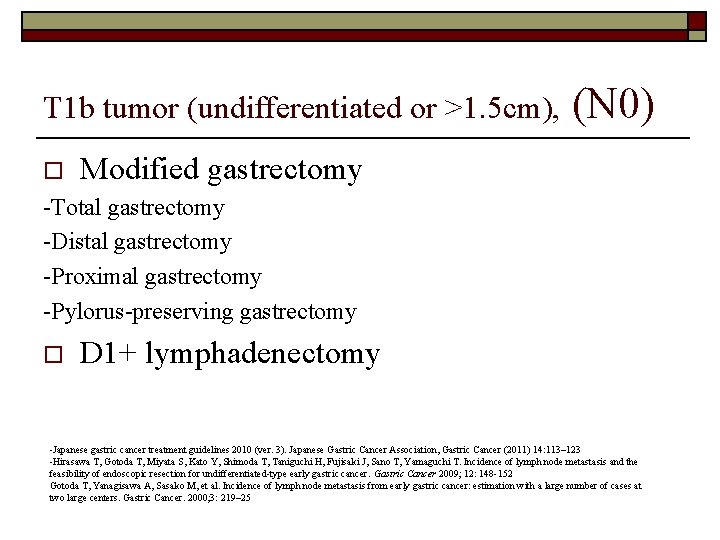
T 1 b tumor (undifferentiated or >1. 5 cm), o (N 0) Modified gastrectomy -Total gastrectomy -Distal gastrectomy -Proximal gastrectomy -Pylorus-preserving gastrectomy o D 1+ lymphadenectomy -Japanese gastric cancer treatment guidelines 2010 (ver. 3). Japanese Gastric Cancer Association, Gastric Cancer (2011) 14: 113– 123 -Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12: 148 -152 Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3: 219– 25

Early gastric cancer (T 1) lymph node - (N 0) Lymph node + (N+) T 1 a (mucosa) T 1 b (submucosa) Differentiated, ≤ 2 cm, UL(-) Differentiated, ≤ 1. 5 cm Yes Endoscopic resection No Yes Gastrectomy, D 1 No Gastrectomy, D 1+ Standard Gastrectomy, D 2

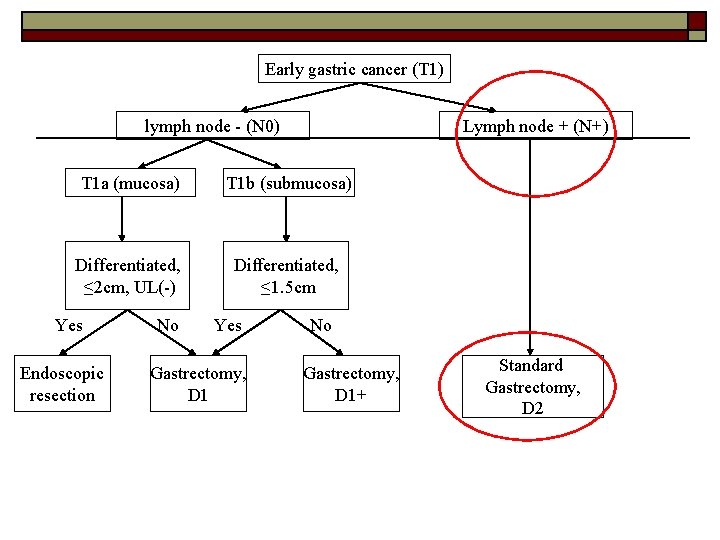
T 1 N+ tumour o Standard gastrectomy + D 2 lymphadenectomy -Japanese gastric cancer treatment guidelines 2010 (ver. 3). Japanese Gastric Cancer Association, Gastric Cancer (2011) 14: 113– 123 -Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12: 148 -152 Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3: 219– 25

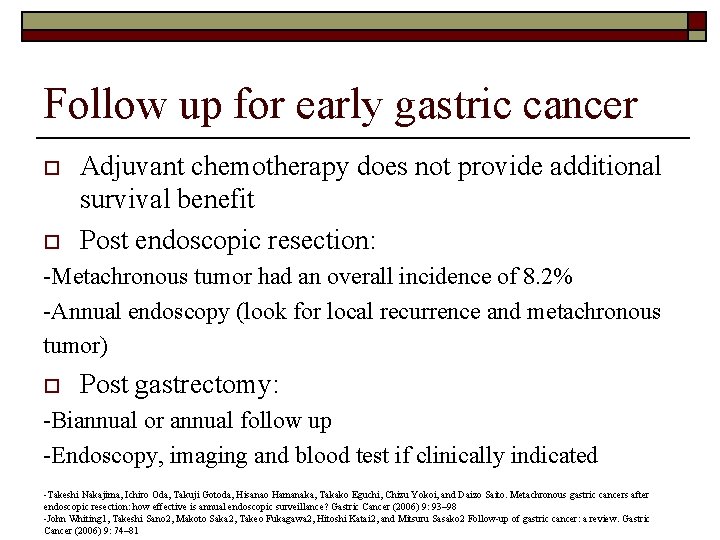
Follow up for early gastric cancer o o Adjuvant chemotherapy does not provide additional survival benefit Post endoscopic resection: -Metachronous tumor had an overall incidence of 8. 2% -Annual endoscopy (look for local recurrence and metachronous tumor) o Post gastrectomy: -Biannual or annual follow up -Endoscopy, imaging and blood test if clinically indicated -Takeshi Nakajima, Ichiro Oda, Takuji Gotoda, Hisanao Hamanaka, Takako Eguchi, Chizu Yokoi, and Daizo Saito. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer (2006) 9: 93– 98 -John Whiting 1, Takeshi Sano 2, Makoto Saka 2, Takeo Fukagawa 2, Hitoshi Katai 2, and Mitsuru Sasako 2 Follow-up of gastric cancer: a review. Gastric Cancer (2006) 9: 74– 81

Conclusion o o Early gastric cancer has good long term prognosis Early diagnosis and treatment is important Endoscopy is the only effective method for diagnosis Endoscopic resection is a proven and standard treatment for lesion under absolute indication

References o o o -Ono H, Kondo H, Gotoda T, et al. (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48: 225 – 229 -Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis Qiang Zhang, Fei Wang, Zhen-Yu Chen, Zhen Wang, Fa-Chao Zhi, Si-De Liu, Yang Bai Gastric Cancer April 2015 -Kato M, Kaise M, Yonezawa J, Toyoizumi H, Yoshimura N, Yoshida Y, et al. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010; 72: 523– 9. Can we accurately diagnose minute gastric cancers (<5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (MNBI)Shoko Fujiwara • Kenshi Yao • Takashi Nagahama • K. Uchita • Takao Kanemitsu Kozue Tsurumi • Noritaka Takatsu • Takashi Hisabe • Hiroshi Tanabe • Akinori Iwashita • Toshiyuki Matsui. Gastric Cancer 11 June 2014 Update on the Paris endoscopic classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005; 37: 570 -8 H. Yanai, Y. Matsumoto, T. Harada et al. , “Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study, ” Gastrointestinal Endoscopy, vol. 46, no. 3, pp. 212– 216, 1997. -G. H. Kim, D. Y. Park, M. Kida et al. , “Accuracy of high-frequency catheter-based endoscopic ultrasonography according to the indications for endoscopic treatment of early gastric cancer, ” Journal of Gastroenterology and Hepatology, vol. 25, no. 3, pp. 506– 511, 2010. -T. Tsuzukr, H. Okada, Y. Kawahara et al. , “Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer, ” Acta Medica Okayama, vol. 65, no. 2, pp. 105– 112, 2011 Gotoda T. , Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3: 219– 25 Facciorusso, Matteo Antonino, Marianna Di Maso, Nicola Muscatiello. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc 2014 November 16; 6(11): 555563 -Young-Mi Park, Eun Cho, Hye-Young Kang, Jong-Mann Kim The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis Surgical Endoscopy August 2011, Volume 25, Issue 8

o o o -Satoshi Tanabe • Kenji Ishido • Katsuhiko Higuchi • Tohru Sasaki • Chikatoshi Katada • Mizutomo Azuma • Akira Naruke • Myungchul Kim • Wasaburo Koizumi. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center Gastric Cancer (2014) 17: 130– 136 Li Jun Peng 1, Shu Ni Tian 1, Lin Lu 1, Hao Chen. Outcome of endoscopic submucosal dissection for early gastric cancer of conventional and expanded indications: Systematic review and meta-analysis. Journal of Digestive Diseases Volume 16, Issue 2 pages 67– 74, February 2015 -Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5 -year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2015 -Japanese gastric cancer treatment guidelines 2010 (ver. 3). Japanese Gastric Cancer Association, Gastric Cancer (2011) 14: 113– 123 -Xiaohua Jiang • Naoki Hiki • Souya Nunobe • Tetsu Fukunaga • Koshi Kumagai • Kyoko Nohara • Hiroshi Katayama • Shigekazu Ohyama • Takeshi Sano • Toshiharu Yamaguchi. Long-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc (2011) 25: 1182– 1186 -Feasibility and Nutritional Benefits of Laparoscopic Proximal Gastrectomy for Early Gastric Cancer in the Upper Stomach. Toshiyuki Kosuga, MD, Ph. D, Daisuke Ichikawa, MD, Ph. D, Shuhei Komatsu, MD, Ph. D, Kazuma Okamoto, MD, Ph. D, Hirotaka Konishi, MD, Ph. D, Atsushi Shiozaki, MD, Ph. D, Hitoshi Fujiwara, MD, Ph. D, and Eigo Otsuji, MD, Ph. D, Ann Surg Oncol DOI 10. 1245/s 10434 -015 -4590 -4

o Thank you.



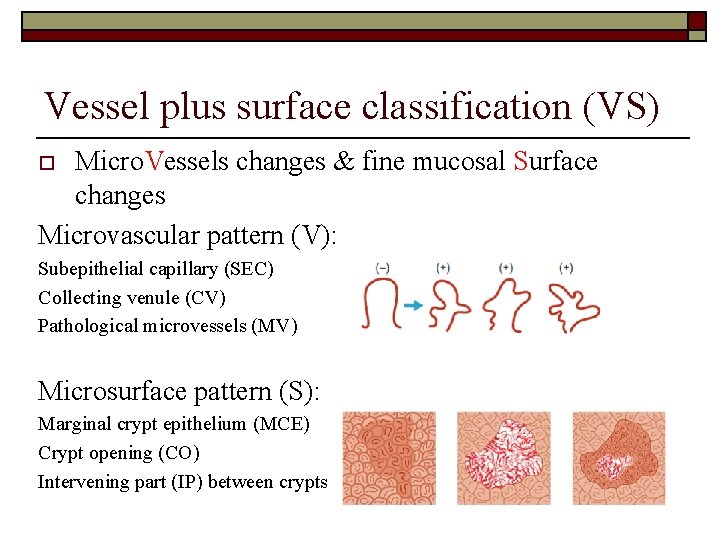
Vessel plus surface classification (VS) Micro. Vessels changes & fine mucosal Surface changes Microvascular pattern (V): o Subepithelial capillary (SEC) Collecting venule (CV) Pathological microvessels (MV) Microsurface pattern (S): Marginal crypt epithelium (MCE) Crypt opening (CO) Intervening part (IP) between crypts

Kenshi Yao. The endoscopic diagnosis of early gastric cancer Annals of Gastroenterology (2013) 26, 11 -22

Is EUS useful for early gastric cancer? o o Accuracy of depth diagnosis is 65%– 86% Accuracy can reach 92% when combined with endoscopic findings -H. Yanai, Y. Matsumoto, T. Harada et al. , “Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study, ” Gastrointestinal Endoscopy, vol. 46, no. 3, pp. 212– 216, 1997. -G. H. Kim, D. Y. Park, M. Kida et al. , “Accuracy of high-frequency catheter-based endoscopic ultrasonography according to the indications for endoscopic treatment of early gastric cancer, ” Journal of Gastroenterology and Hepatology, vol. 25, no. 3, pp. 506– 511, 2010. -T. Tsuzukr, H. Okada, Y. Kawahara et al. , “Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer, ” Acta Medica Okayama, vol. 65, no. 2, pp. 105– 112, 2011 -Mouri R 1, Yoshida S, Tanaka S. , et al, Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009 Apr; 43(4): 31822

Is EUS useful for early gastric cancer? o o o Objective method for determining depth of EGC Determine feasibility of endoscopic therapy Diagnostic accuracy decreased for: -depressed lesions -undifferentiated cancers -lesions with ulcers -minute submucosal invasion in a large lesion -type 0 -I lesions -lesions located in the upper-third of the stomach

Is EUS useful for early gastric cancer? o o Diagnostic accuracy of ME-NBI can be up to 85% EUS is not a routine examination for EGC

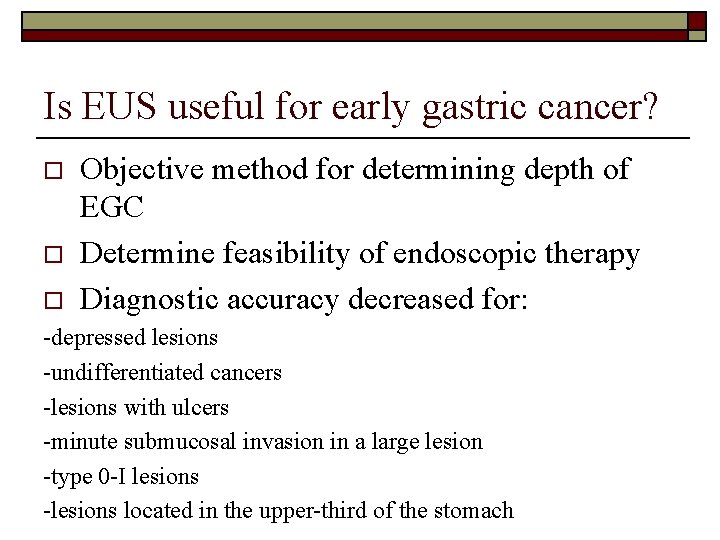
Type 0 -1 and Type IIa o The distinction between a sessile (protruding) lesion (Type 0 -1) and a slightly elevated (nonprotruding) lesion (Type II-a) is based on the extent of the elevation from the adjacent mucosa, cut off 2. 5 mm Type 0 -1 Type II-a 2. 5 mm Update on the Paris endoscopic classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005; 37: 570 -8

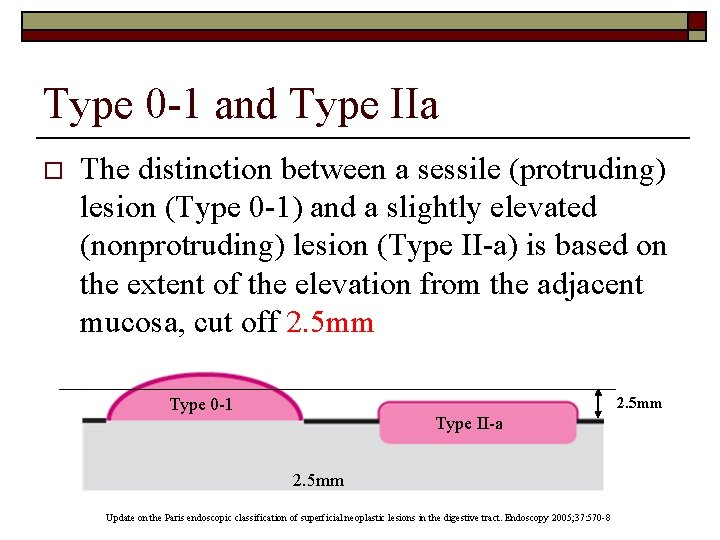
Type 0 -IIc and 0 -III o The distinction between a slightly depressed lesion (Type 0 -IIc) and an excavated lesion (Type 0 -III) is based on the depth of the depression from the adjacent mucosa, cut off 1. 2 mm Type 0 -IIc Type 0 -III 1. 2 mm

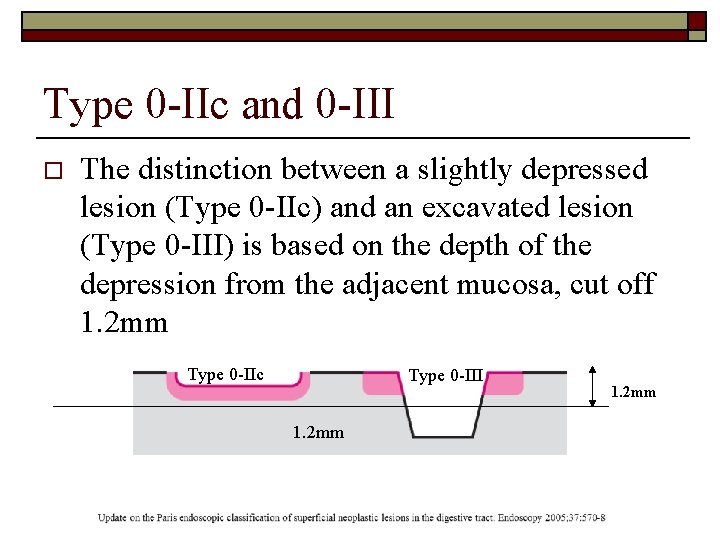
Measurement of lesions o Biopsy forceps as reference standard

 Joint hospital surgical grand round
Joint hospital surgical grand round Joint hospital surgical grand round
Joint hospital surgical grand round Joint hospital surgical grand round
Joint hospital surgical grand round Joint hospital surgical grand round
Joint hospital surgical grand round Joint hospital surgical grand round
Joint hospital surgical grand round Joint hospital surgical grand round
Joint hospital surgical grand round Joint hospital surgical grand round
Joint hospital surgical grand round Wellspan surgical and rehab hospital
Wellspan surgical and rehab hospital Imaging at lafayette surgical specialty hospital
Imaging at lafayette surgical specialty hospital Iuss health facility guidelines
Iuss health facility guidelines Round and round we go burning burning all aglow
Round and round we go burning burning all aglow Ground round hospital
Ground round hospital Define hospital and hospital pharmacy
Define hospital and hospital pharmacy What cartilage is found between the vertebrae
What cartilage is found between the vertebrae Memorandum joint venture account format
Memorandum joint venture account format Lamb carcass grading
Lamb carcass grading Break joint lamb
Break joint lamb Condyloid joint
Condyloid joint What is a permanent joint
What is a permanent joint Monday 13th july
Monday 13th july July 30 2009 nasa
July 30 2009 nasa The mysteries of harris burdick the harp
The mysteries of harris burdick the harp July 16 1776
July 16 1776 July 2 1937 amelia earhart
July 2 1937 amelia earhart Ctdssmap payment schedule july 2021
Ctdssmap payment schedule july 2021 Criciúma ec
Criciúma ec Super saturday tribal bingo july 4
Super saturday tribal bingo july 4 Malaga in july
Malaga in july On july 18 2001 a train carrying hazardous chemicals
On july 18 2001 a train carrying hazardous chemicals The cuban melodrama
The cuban melodrama Sources nso july frenchhowell neill technology...
Sources nso july frenchhowell neill technology... Harris burdick captain tory
Harris burdick captain tory June 22 to july 22
June 22 to july 22 July 1969
July 1969 2001 july 15
2001 july 15 Leaf yeast
Leaf yeast July 4 sermon
July 4 sermon Imagery in poppies in july
Imagery in poppies in july What is the significance of july 4 1776 brainpop
What is the significance of july 4 1776 brainpop July 1-4 1863
July 1-4 1863 January february march april june july
January february march april june july July 12 1776
July 12 1776 Slidetodoc.com
Slidetodoc.com 2003 july 17
2003 july 17 July 26 1953
July 26 1953 June too soon july stand by
June too soon july stand by July 10 1856
July 10 1856 I am silver and exact i have no preconceptions
I am silver and exact i have no preconceptions Tender definition
Tender definition July 14 1789
July 14 1789 Sensory language definition
Sensory language definition Prevention of vte in nonorthopedic surgical patients
Prevention of vte in nonorthopedic surgical patients Congenital flat foot
Congenital flat foot