Introduction Definition of Terms Homogeneous Heterogeneous Ergotic and





- Slides: 25

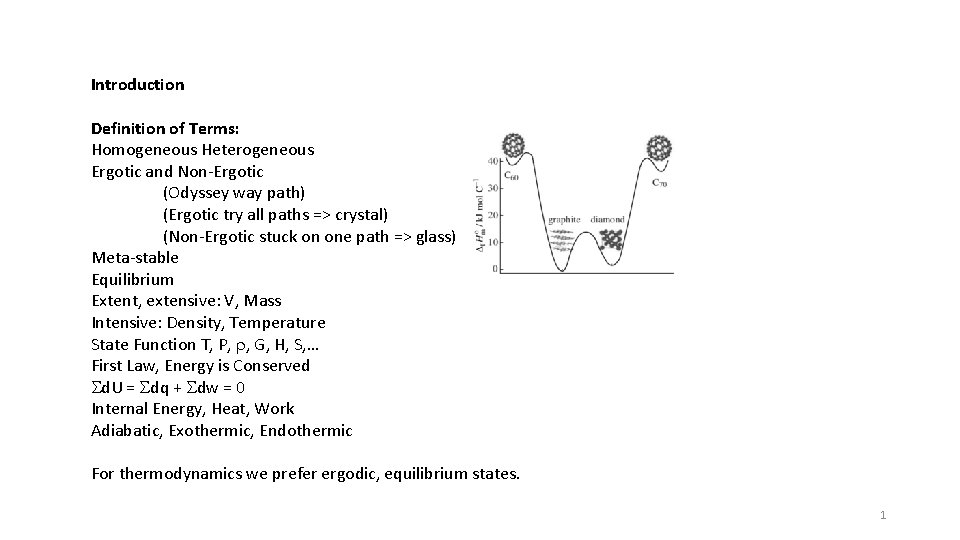
Introduction Definition of Terms: Homogeneous Heterogeneous Ergotic and Non-Ergotic (Odyssey way path) (Ergotic try all paths => crystal) (Non-Ergotic stuck on one path => glass) Meta-stable Equilibrium Extent, extensive: V, Mass Intensive: Density, Temperature State Function T, P, r, G, H, S, … First Law, Energy is Conserved Sd. U = Sdq + Sdw = 0 Internal Energy, Heat, Work Adiabatic, Exothermic, Endothermic For thermodynamics we prefer ergodic, equilibrium states. 1

2

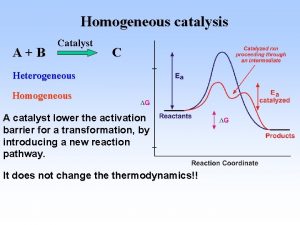
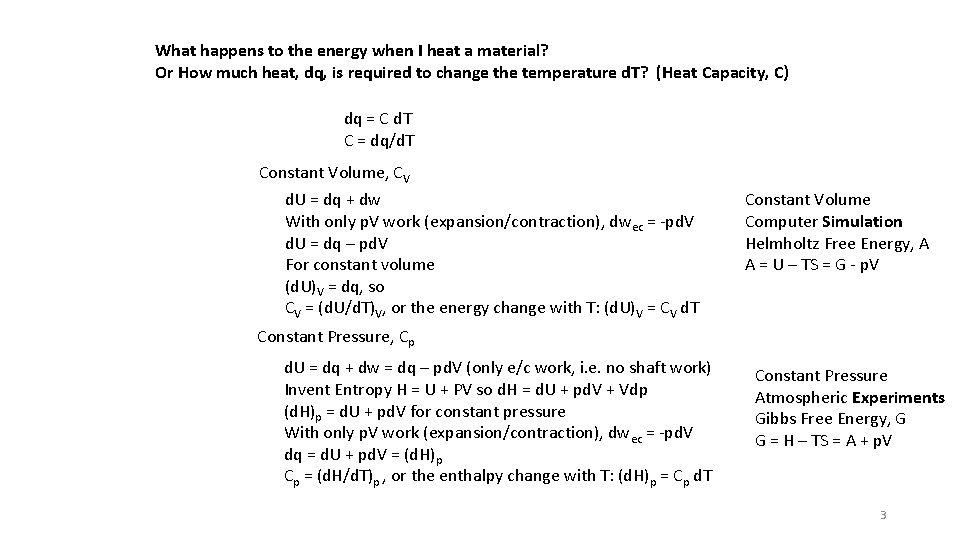
What happens to the energy when I heat a material? Or How much heat, dq, is required to change the temperature d. T? (Heat Capacity, C) dq = C d. T C = dq/d. T Constant Volume, CV d. U = dq + dw With only p. V work (expansion/contraction), dwec = -pd. V d. U = dq – pd. V For constant volume (d. U)V = dq, so CV = (d. U/d. T)V, or the energy change with T: (d. U)V = CV d. T Constant Volume Computer Simulation Helmholtz Free Energy, A A = U – TS = G - p. V Constant Pressure, Cp d. U = dq + dw = dq – pd. V (only e/c work, i. e. no shaft work) Invent Entropy H = U + PV so d. H = d. U + pd. V + Vdp (d. H)p = d. U + pd. V for constant pressure With only p. V work (expansion/contraction), dwec = -pd. V dq = d. U + pd. V = (d. H)p Cp = (d. H/d. T)p , or the enthalpy change with T: (d. H)p = Cp d. T Constant Pressure Atmospheric Experiments Gibbs Free Energy, G G = H – TS = A + p. V 3

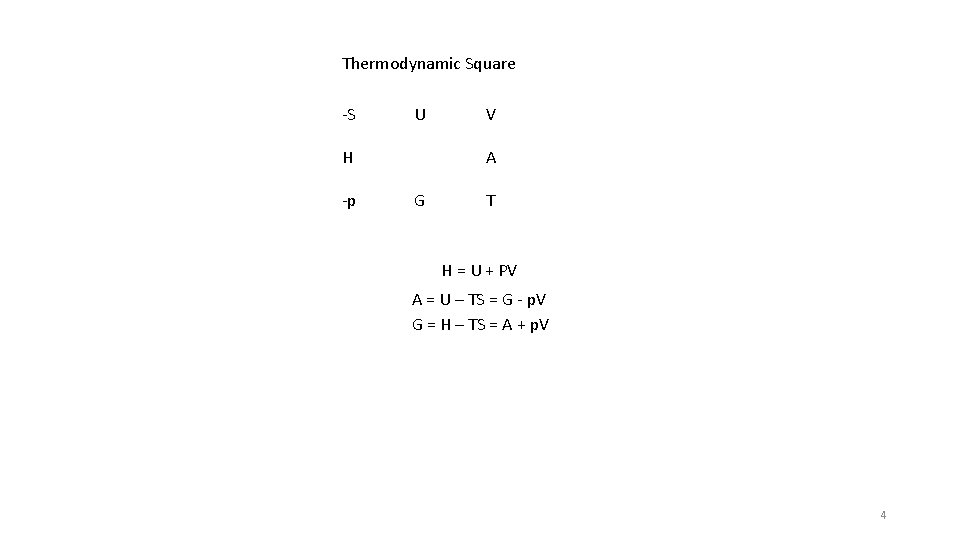
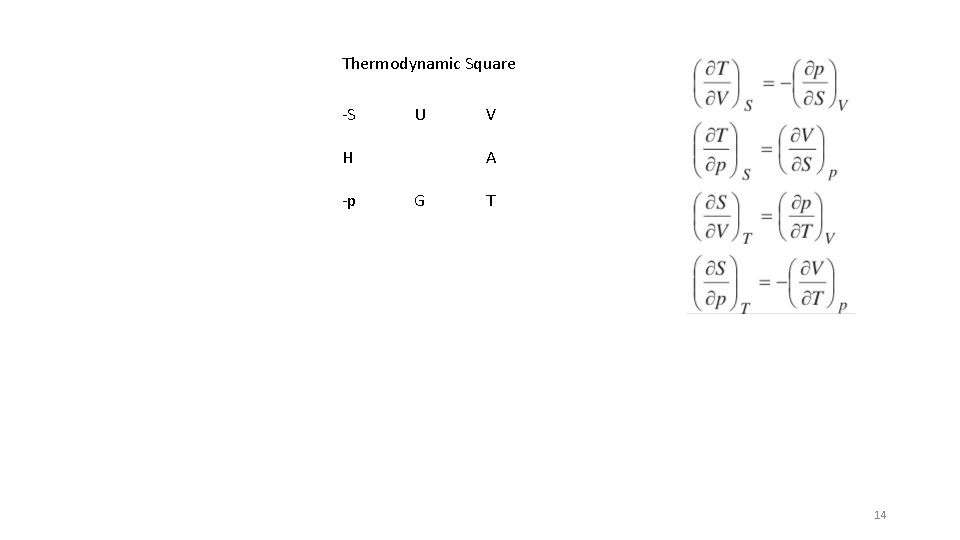
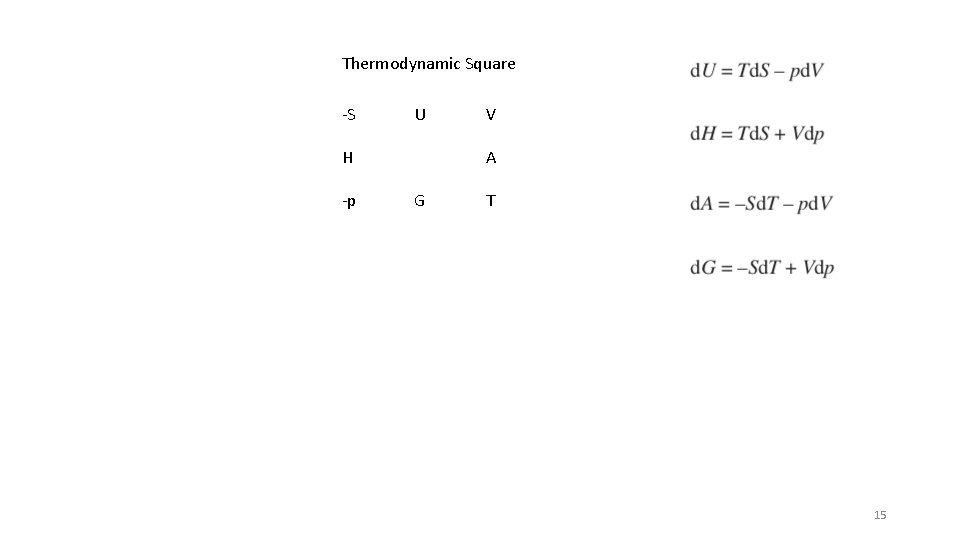
Thermodynamic Square -S U H -p V A G T H = U + PV A = U – TS = G - p. V G = H – TS = A + p. V 4

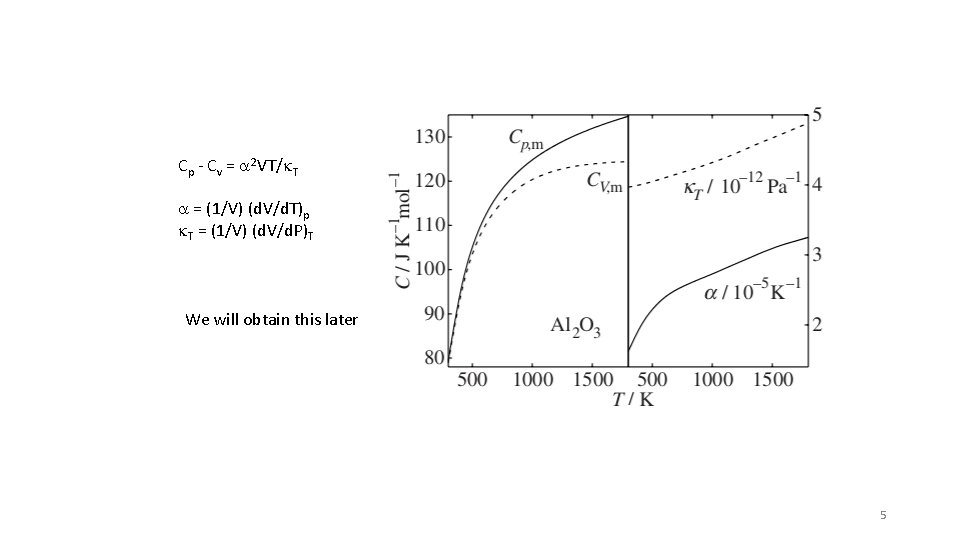
Cp - Cv = a 2 VT/k. T a = (1/V) (d. V/d. T)p k. T = (1/V) (d. V/d. P)T We will obtain this later 5

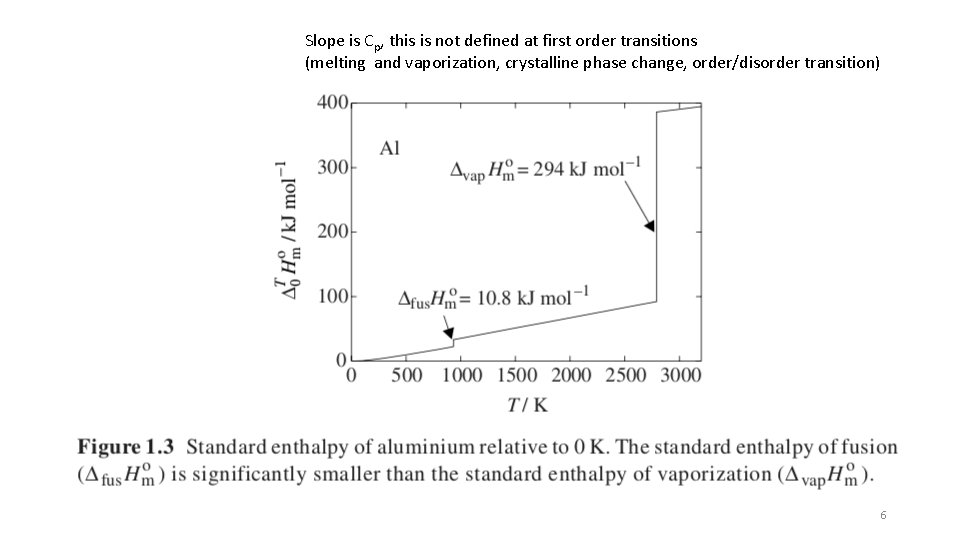
Slope is Cp, this is not defined at first order transitions (melting and vaporization, crystalline phase change, order/disorder transition) 6

Size dependent enthalpy of melting (Gibbs-Thompson Equation) For bulk materials, r = ∞, at the melting point DG = DH – T∞DS = 0 So T∞ = DH/DS Larger bonding enthalpy leads to higher T∞ , Greater randomness gain on melting leads to lower T∞. For nanoparticles there is also a surface term, (DG) V = (DH – Tr. DS)V + s. A = 0, where Tr is the melting point for size r nanoparticle If V = r 3 and A = r 2 and using DS = DH/T∞ this becomes, r = s/(DH(1– Tr/T∞)) or Tr = T∞ (1 - s/(r. DH) Smaller particles have a lower melting point and the dependence suggests a plot of Tr/T∞ against 1/r 7

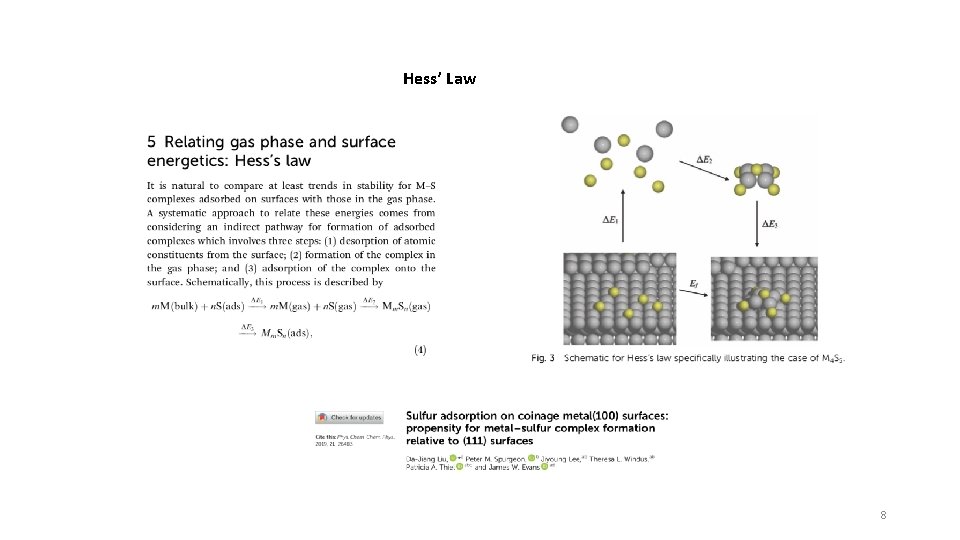
Hess’ Law 8

Second Law: Reversibility For an adiabatic reversible system DS = 0 In a process this is often used by engineers in calculations 1) Assume DS = 0, calculate DH for the process 2) Use and efficiency to modify DH to a larger value 3) Calculate the actual DS > 0 for the process The change in entropy is tied to the concept of efficiency 100% efficient process has DS = 0 Calusius Theorem is that entropy increases or stays the same but can not spontaneously decrease For a reversible process d. Srev = (dq/T)rev For any process d. S ≥ (dq/T) 9

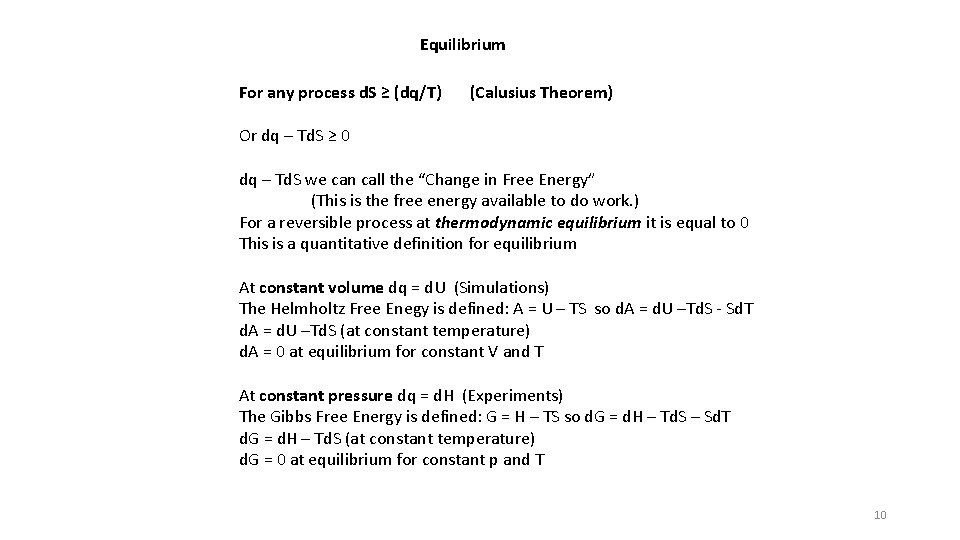
Equilibrium For any process d. S ≥ (dq/T) (Calusius Theorem) Or dq – Td. S ≥ 0 dq – Td. S we can call the “Change in Free Energy” (This is the free energy available to do work. ) For a reversible process at thermodynamic equilibrium it is equal to 0 This is a quantitative definition for equilibrium At constant volume dq = d. U (Simulations) The Helmholtz Free Enegy is defined: A = U – TS so d. A = d. U –Td. S - Sd. T d. A = d. U –Td. S (at constant temperature) d. A = 0 at equilibrium for constant V and T At constant pressure dq = d. H (Experiments) The Gibbs Free Energy is defined: G = H – TS so d. G = d. H – Td. S – Sd. T d. G = d. H – Td. S (at constant temperature) d. G = 0 at equilibrium for constant p and T 10

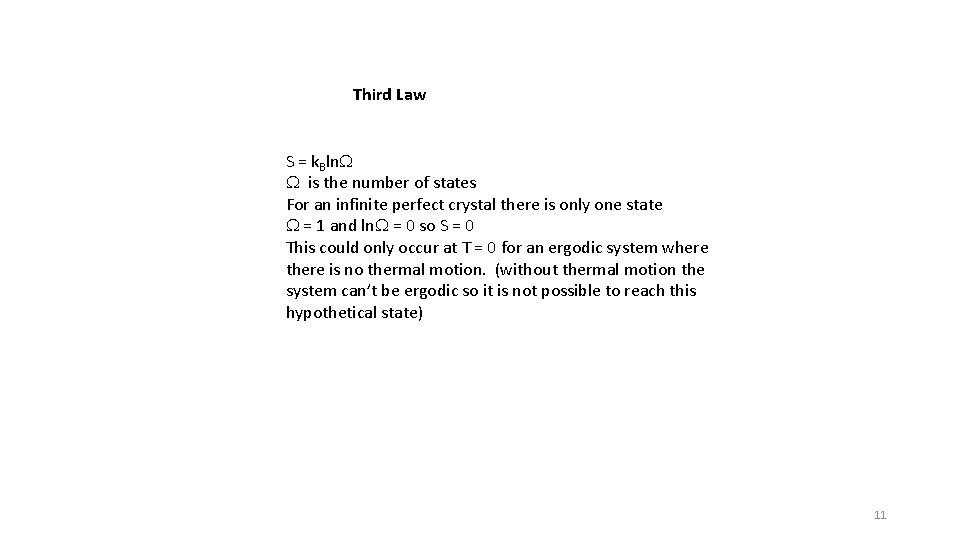
Third Law S = k. Bln. W W is the number of states For an infinite perfect crystal there is only one state W = 1 and ln. W = 0 so S = 0 This could only occur at T = 0 for an ergodic system where there is no thermal motion. (without thermal motion the system can’t be ergodic so it is not possible to reach this hypothetical state) 11

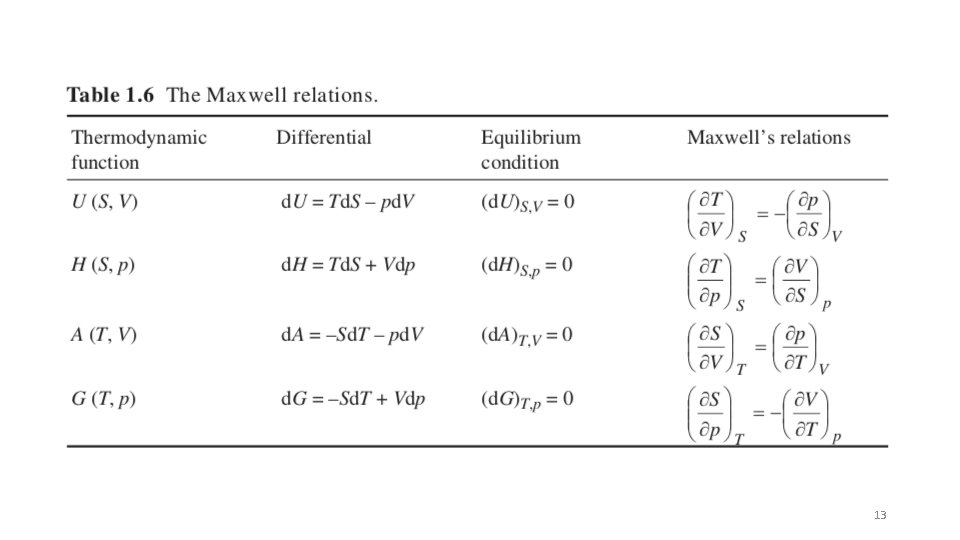
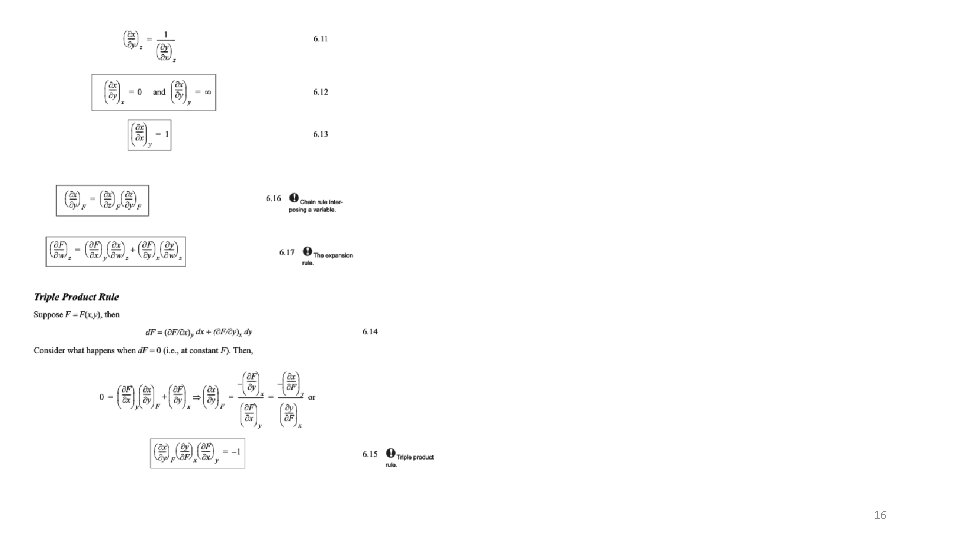
d. U = dq + dw For only ec work d. U = dq – pd. V dq = Td. S for a reversible process d. U = Td. S – pd. V So U is naturally broken into functions of S and V (d. U/d. S)V = T (d. U/d. V)S = -p d. U = (d. U/d. S)V d. S + (d. U/d. V)S d. V Take the second derivative d 2 U/(d. Sd. V) =(d/d. S(d. U/d. V)S)V = (d/d. V(d. U/d. S) V) S = d 2 U/(d. Vd. S) Using the above expressions and the middle two terms -(dp/d. S)V = (d. T/d. V)S This is a Maxwell Relationship and the process is called a Legendre transformation This can be done for all four fundamental equations, U; H; G; A 12

13

Thermodynamic Square -S U H -p V A G T 14

Thermodynamic Square -S U H -p V A G T 15

16

Derive the expression for Cp – CV Cp - Cv = a 2 VT/k. T a = (1/V) (d. V/d. T)p k. T = (1/V) (d. V/d. P)T -S U V H -p A G T CV = (d. U/d. T)V From the Thermodynamic Square d. U = Td. S – pd. V so CV = (d. U/d. T)V = T (d. S/d. T)V - p (d. V/d. T)V Second term is 0 d. V at constant V is 0 (d. S/d. T)V = CV /T Similarly Cp = (d. H/d. T)p From the Thermodynamic Square d. H = Td. S + Vdp so Cp = (d. H/d. T)p = T (d. S/d. T)p - V (dp/d. T)p Second term is 0 dp at constant p is 0 (d. S/d. T)p = Cp /T Write a differential expression for d. S as a function of T and V d. S = (d. S/d. T)Vd. T + (d. S/d. V)Td. V using expression for CV above and Maxwell for (d. S/d. V)T d. S = CV /T d. T + (dp/d. T)Vd. V use chain rule: (dp/d. T)V = -(d. V/d. T)p (d. P/d. V)T = Va / (Vk. T) Take the derivative for Cp: Cp/T = (d. S/d. T)p = CV /T (d. T/d. T)p + (a/k. T)(d. V/d. T)p = CV /T + (Va 2/k. T) Cp - Cv = a 2 VT/k. T 17

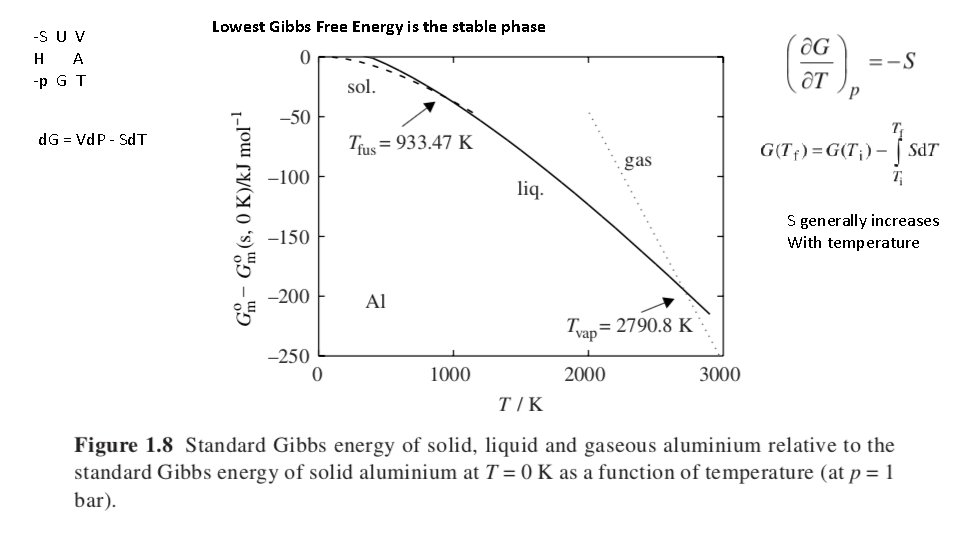
-S U V H A -p G T Lowest Gibbs Free Energy is the stable phase d. G = Vd. P - Sd. T S generally increases With temperature 18

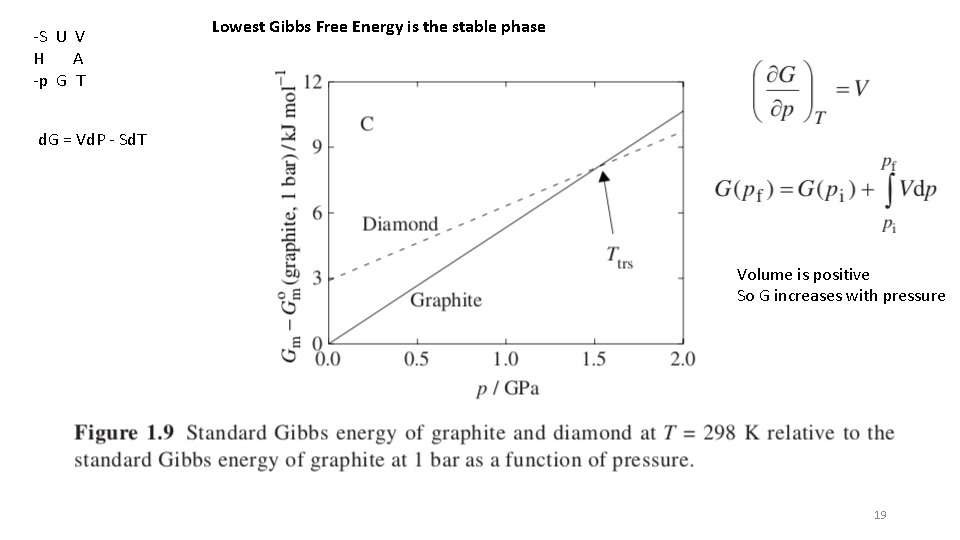
-S U V H A -p G T Lowest Gibbs Free Energy is the stable phase d. G = Vd. P - Sd. T Volume is positive So G increases with pressure 19

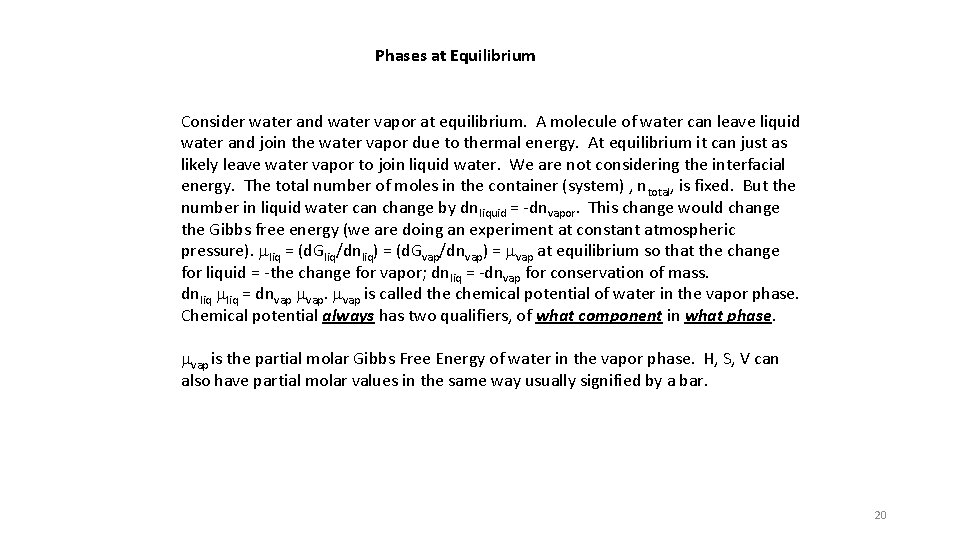
Phases at Equilibrium Consider water and water vapor at equilibrium. A molecule of water can leave liquid water and join the water vapor due to thermal energy. At equilibrium it can just as likely leave water vapor to join liquid water. We are not considering the interfacial energy. The total number of moles in the container (system) , ntotal, is fixed. But the number in liquid water can change by dnliquid = -dnvapor. This change would change the Gibbs free energy (we are doing an experiment at constant atmospheric pressure). mliq = (d. Gliq/dnliq) = (d. Gvap/dnvap) = mvap at equilibrium so that the change for liquid = -the change for vapor; dnliq = -dnvap for conservation of mass. dnliq mliq = dnvap mvap is called the chemical potential of water in the vapor phase. Chemical potential always has two qualifiers, of what component in what phase. mvap is the partial molar Gibbs Free Energy of water in the vapor phase. H, S, V can also have partial molar values in the same way usually signified by a bar. 20

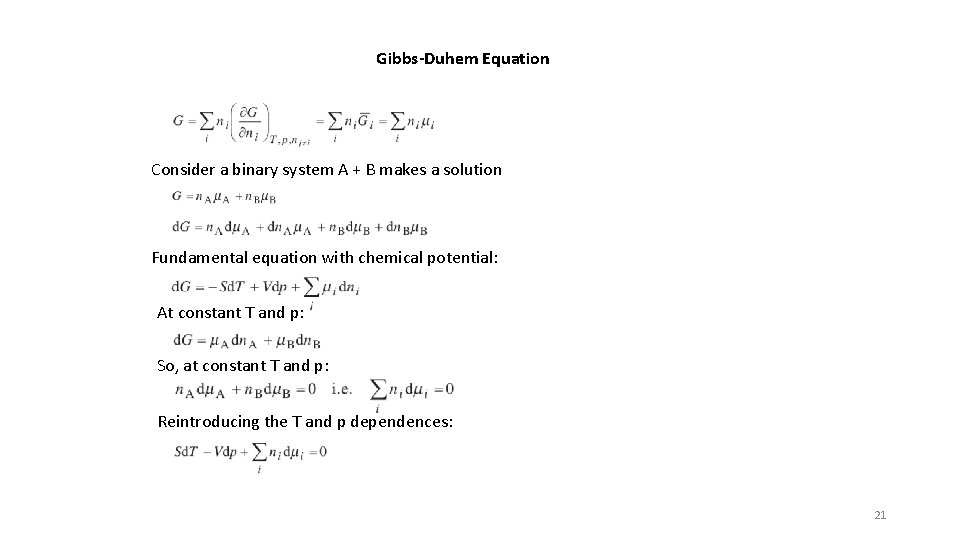
Gibbs-Duhem Equation Consider a binary system A + B makes a solution Fundamental equation with chemical potential: At constant T and p: So, at constant T and p: Reintroducing the T and p dependences: 21

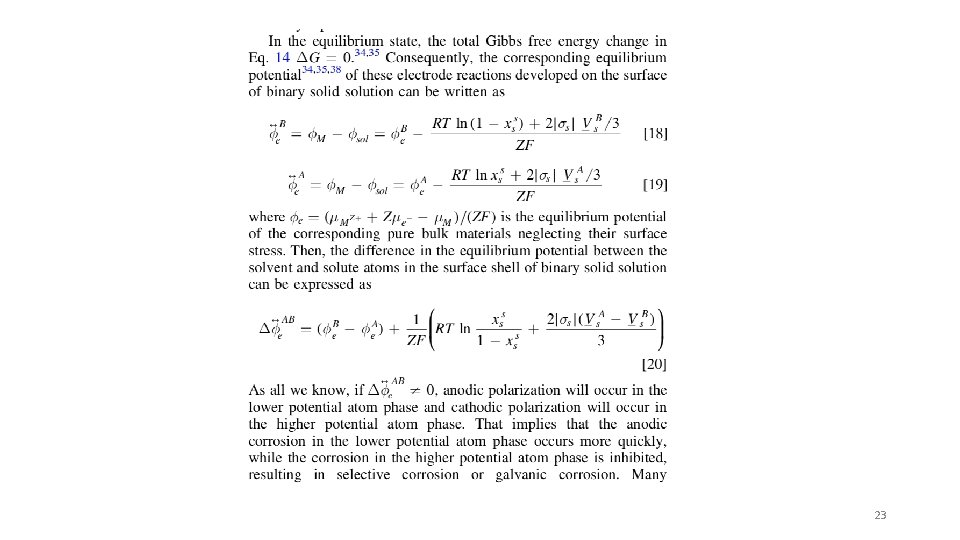
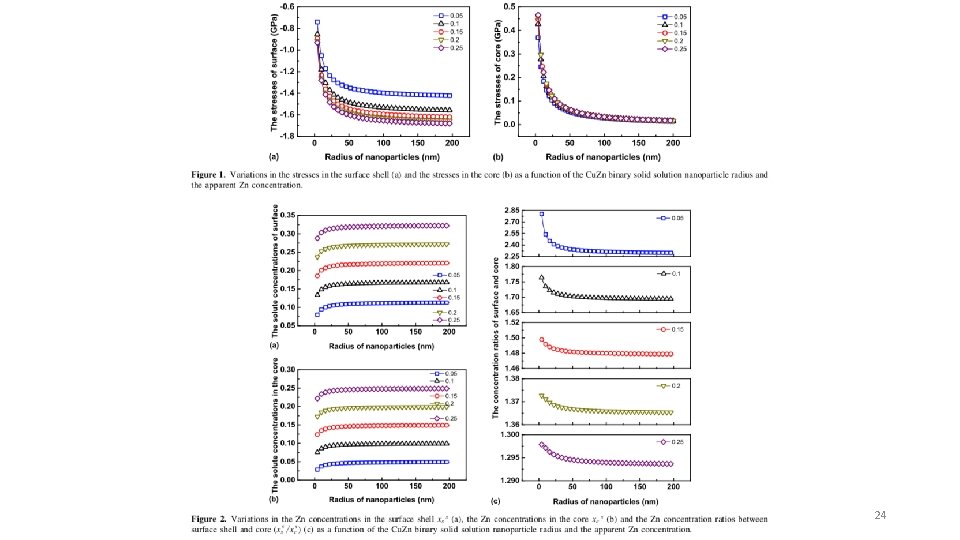
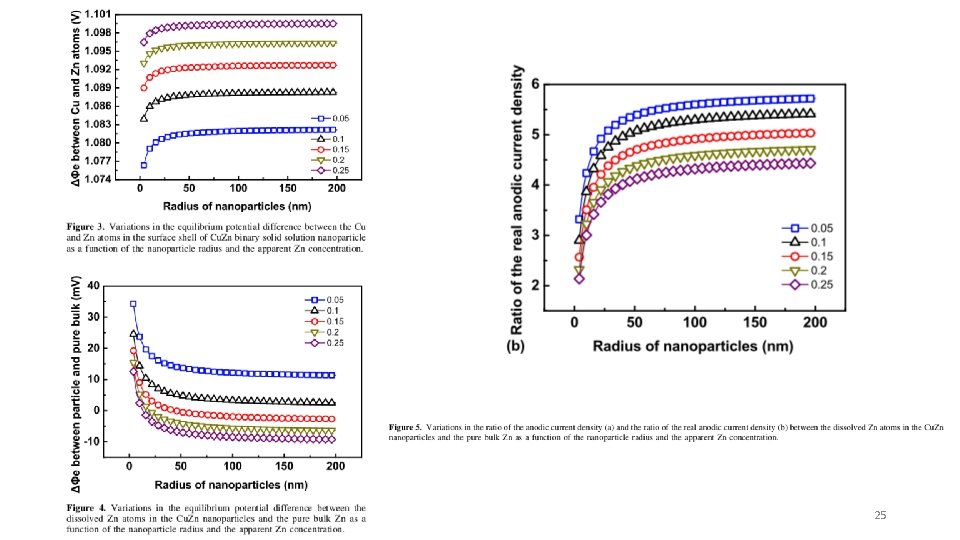
Corrosion in nanoparticles can be higher or lower Consider a binary solution in a nanoparticle. There Gibbs-Duhem Equation is significant stress on the surface, s, compared to the core, c. Break particle into core and surface regions with different stress, sc, ss, molar composition of the solute in core, xc, and surface, xs. Assume equilibrium, mc = ms. Ln term is gaseous mixing entropy, 0 terms are for infinite dilution. For the two components with an electric potential the equilibrium corrosion potential can be calculated for each component which changes with particle size. s = the stress (DP) so the contribution to chemical potential from stress is proportional to s V depending on geometry 22

23

24

25
 Homogeneous mixture and heterogeneous mixture class 9
Homogeneous mixture and heterogeneous mixture class 9 What is homogeneous distributed database
What is homogeneous distributed database Non homogeneous differential equation definition
Non homogeneous differential equation definition Is chunky peanut butter homogeneous or heterogeneous
Is chunky peanut butter homogeneous or heterogeneous Homogeneous vs heterogeneous reactions
Homogeneous vs heterogeneous reactions Homogeneous vs heterogeneous
Homogeneous vs heterogeneous How do you classify uniform and non-uniform mixtures?
How do you classify uniform and non-uniform mixtures? Heterogeneous vs homogeneous groups
Heterogeneous vs homogeneous groups Tap water homogeneous or heterogeneous
Tap water homogeneous or heterogeneous Different types of mixtures
Different types of mixtures Vinegar homogenous
Vinegar homogenous Substances and mixtures
Substances and mixtures Is jello homogeneous or heterogeneous
Is jello homogeneous or heterogeneous Is italian salad dressing homogeneous or heterogeneous
Is italian salad dressing homogeneous or heterogeneous Is bird seed a pure substance or mixture
Is bird seed a pure substance or mixture Is flat soda homogeneous or heterogeneous
Is flat soda homogeneous or heterogeneous Is supreme pizza homogeneous or heterogeneous
Is supreme pizza homogeneous or heterogeneous Is cake batter a mixture or pure substance
Is cake batter a mixture or pure substance A pile of rusty iron filings homogeneous or heterogeneous
A pile of rusty iron filings homogeneous or heterogeneous Is ketchup homogeneous or heterogeneous
Is ketchup homogeneous or heterogeneous Rice pudding homogeneous or heterogeneous
Rice pudding homogeneous or heterogeneous Is orange juice a homogeneous mixture
Is orange juice a homogeneous mixture Blood is a homogeneous mixture
Blood is a homogeneous mixture Is rice pudding a homogeneous mixture
Is rice pudding a homogeneous mixture Like terms and unlike terms in polynomials
Like terms and unlike terms in polynomials What are like terms
What are like terms