Interaction of Ionizing Radiation with Matter charged particles
- Slides: 43

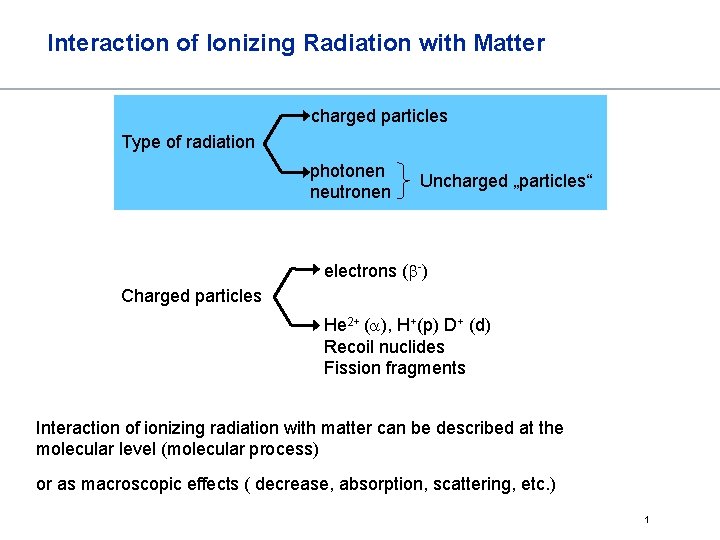
Interaction of Ionizing Radiation with Matter charged particles Type of radiation photonen neutronen Uncharged „particles“ electrons (b-) Charged particles He 2+ (a), H+(p) D+ (d) Recoil nuclides Fission fragments Interaction of ionizing radiation with matter can be described at the molecular level (molecular process) or as macroscopic effects ( decrease, absorption, scattering, etc. ) 1

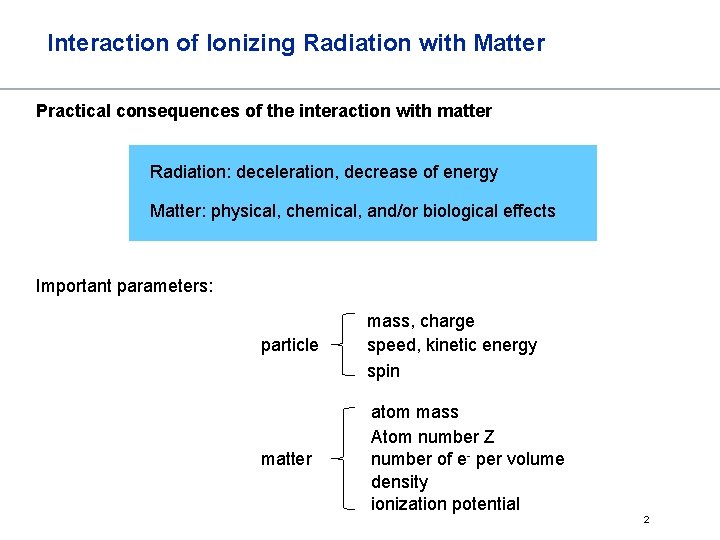
Interaction of Ionizing Radiation with Matter Practical consequences of the interaction with matter Radiation: deceleration, decrease of energy Matter: physical, chemical, and/or biological effects Important parameters: particle mass, charge speed, kinetic energy spin matter atom mass Atom number Z number of e- per volume density ionization potential 2

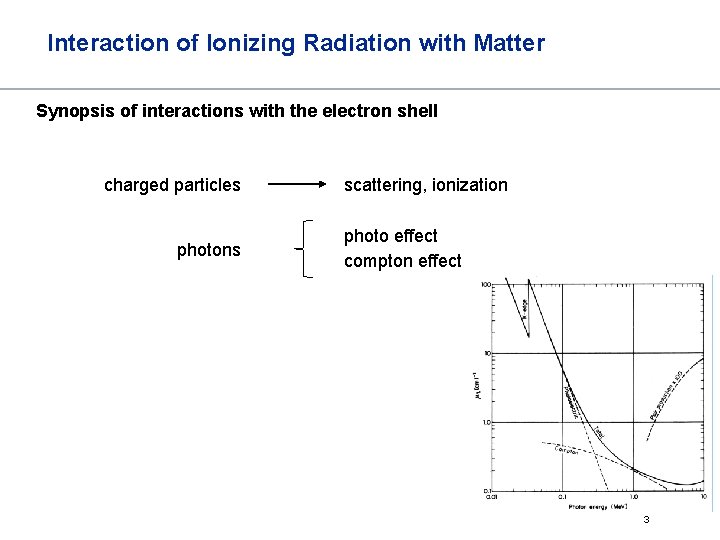
Interaction of Ionizing Radiation with Matter Synopsis of interactions with the electron shell charged particles photons scattering, ionization photo effect compton effect 3

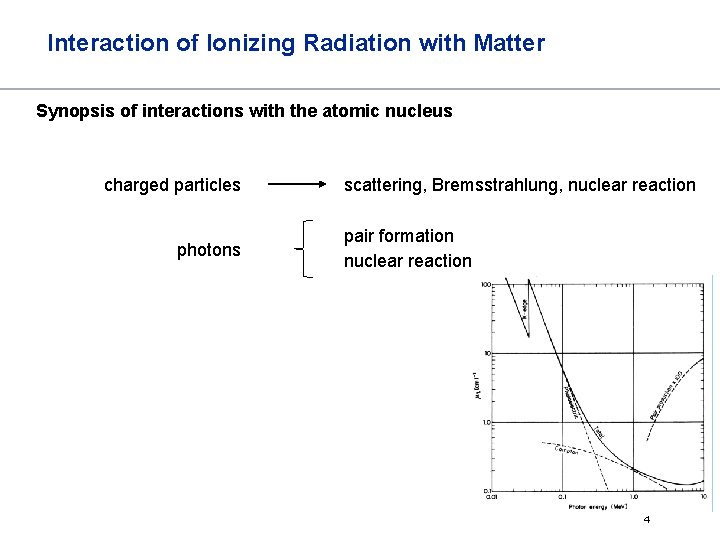
Interaction of Ionizing Radiation with Matter Synopsis of interactions with the atomic nucleus charged particles photons scattering, Bremsstrahlung, nuclear reaction pair formation nuclear reaction 4

Interaction of Ionizing Radiation with Matter Ionizing radiation Direct ionizing radiation: a, b-, b+, … Energy is high enough to ionize by collision Indirect ionizing radiation: n, Ionization as a consequents of nuclear reactions in the absorbing matter In the context of radiation absorption, two definitions are important: linear stopping power also important and linear energy transfer Without Bremsstrahlung SI and LI are equal, otherwise there will be a substantial difference 5

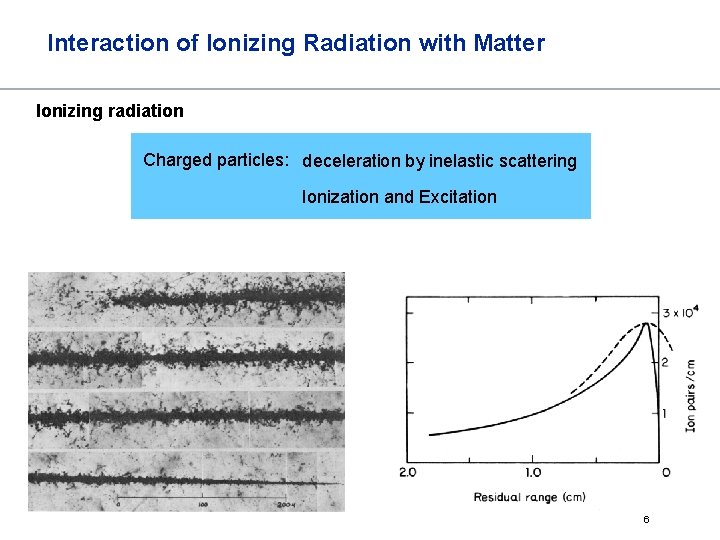
Interaction of Ionizing Radiation with Matter Ionizing radiation Charged particles: deceleration by inelastic scattering Ionization and Excitation 6

Interaction of Ionizing Radiation with Matter Ionizing radiation By collision with electrons, the incident particle ionizes matter The mean energy to remove an electron is called the W-factor for air is 33. 85 e. V/IP When the charged particle travels through matter, it makes an energy dependent number of ionization / length this is called specific ionization (SI) The mean energy loss per path length (LET) can determined by: LET = SI∙W LET = Linear Energy Transfer 7

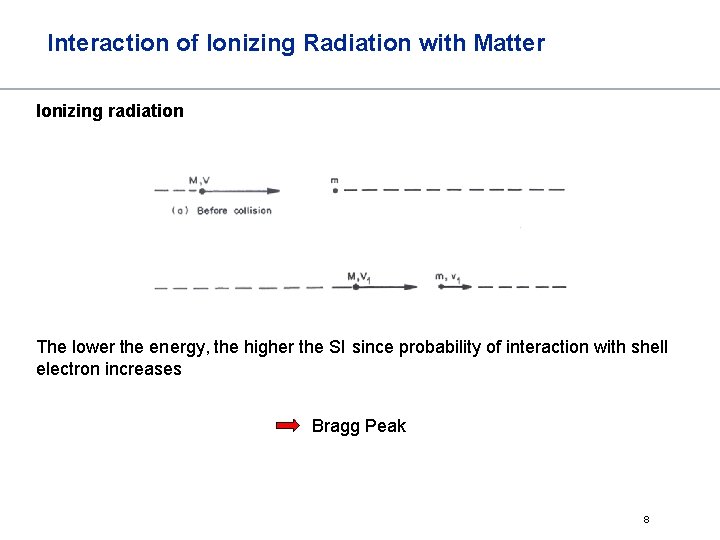
Interaction of Ionizing Radiation with Matter Ionizing radiation The lower the energy, the higher the SI since probability of interaction with shell electron increases Bragg Peak 8

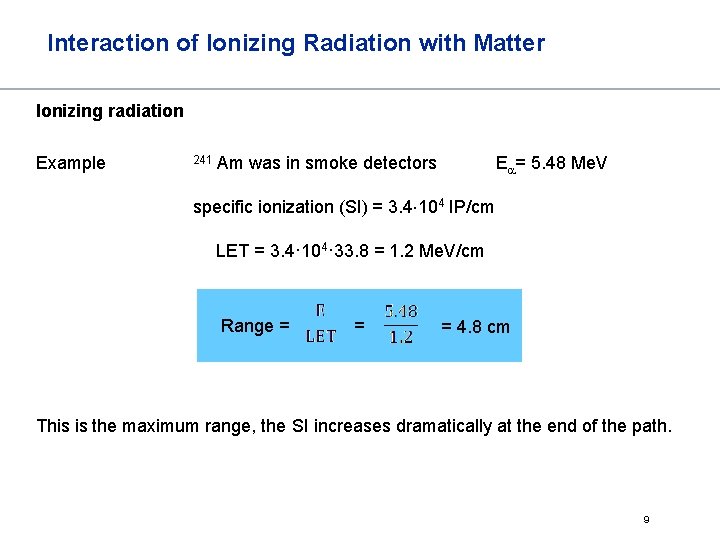
Interaction of Ionizing Radiation with Matter Ionizing radiation Example 241 Am was in smoke detectors Ea= 5. 48 Me. V specific ionization (SI) = 3. 4 104 IP/cm LET = 3. 4· 104· 33. 8 = 1. 2 Me. V/cm Range = = = 4. 8 cm This is the maximum range, the SI increases dramatically at the end of the path. 9

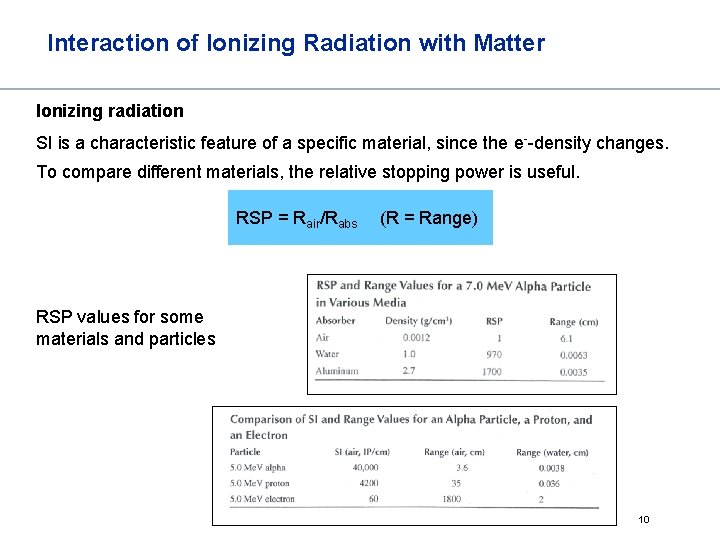
Interaction of Ionizing Radiation with Matter Ionizing radiation SI is a characteristic feature of a specific material, since the e--density changes. To compare different materials, the relative stopping power is useful. RSP = Rair/Rabs (R = Range) RSP values for some materials and particles 10

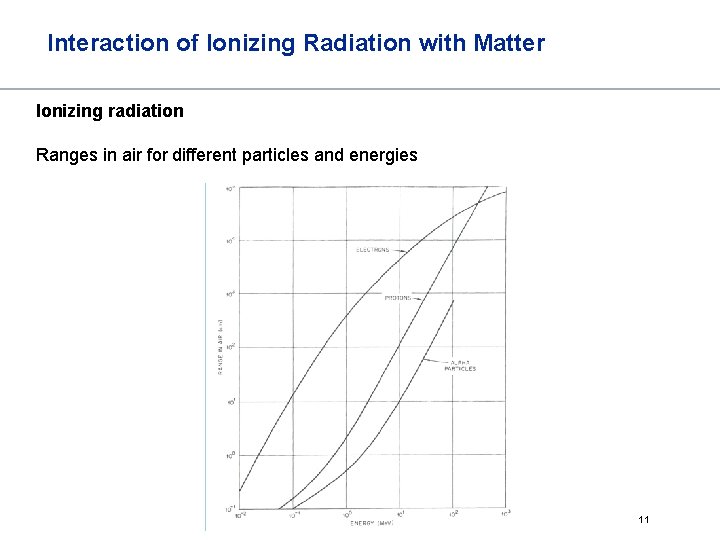
Interaction of Ionizing Radiation with Matter Ionizing radiation Ranges in air for different particles and energies 11

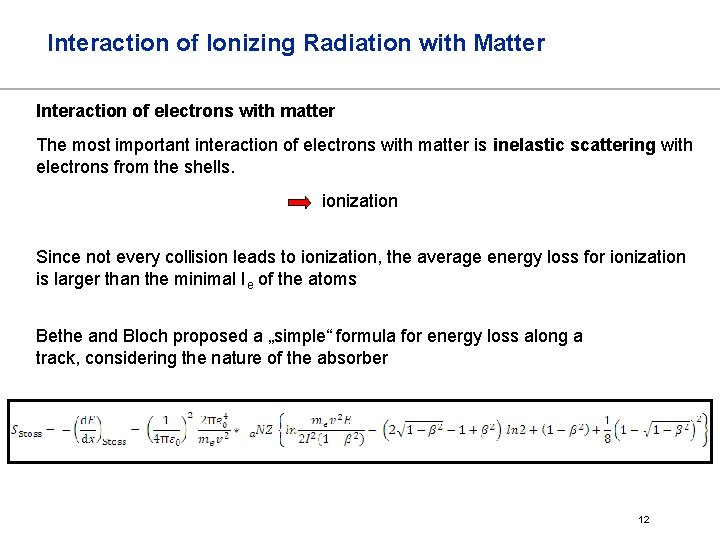
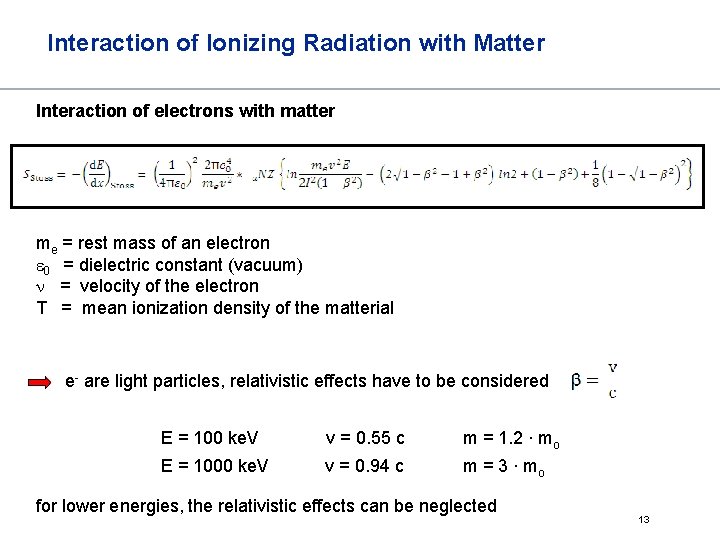
Interaction of Ionizing Radiation with Matter Interaction of electrons with matter The most important interaction of electrons with matter is inelastic scattering with electrons from the shells. ionization Since not every collision leads to ionization, the average energy loss for ionization is larger than the minimal Ie of the atoms Bethe and Bloch proposed a „simple“ formula for energy loss along a track, considering the nature of the absorber 12

Interaction of Ionizing Radiation with Matter Interaction of electrons with matter me = rest mass of an electron e 0 = dielectric constant (vacuum) n = velocity of the electron T = mean ionization density of the matterial e- are light particles, relativistic effects have to be considered E = 100 ke. V v = 0. 55 c m = 1. 2 ∙ mo E = 1000 ke. V v = 0. 94 c m = 3 ∙ mo for lower energies, the relativistic effects can be neglected 13

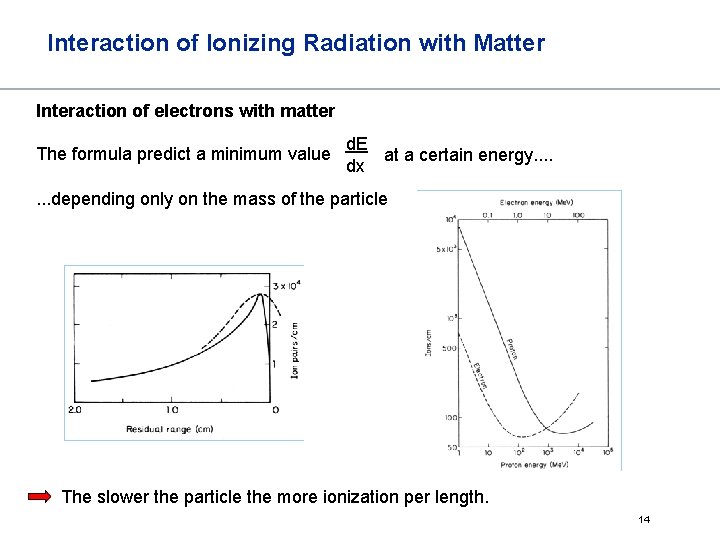
Interaction of Ionizing Radiation with Matter Interaction of electrons with matter The formula predict a minimum value d. E at a certain energy. . dx . . . depending only on the mass of the particle The slower the particle the more ionization per length. 14

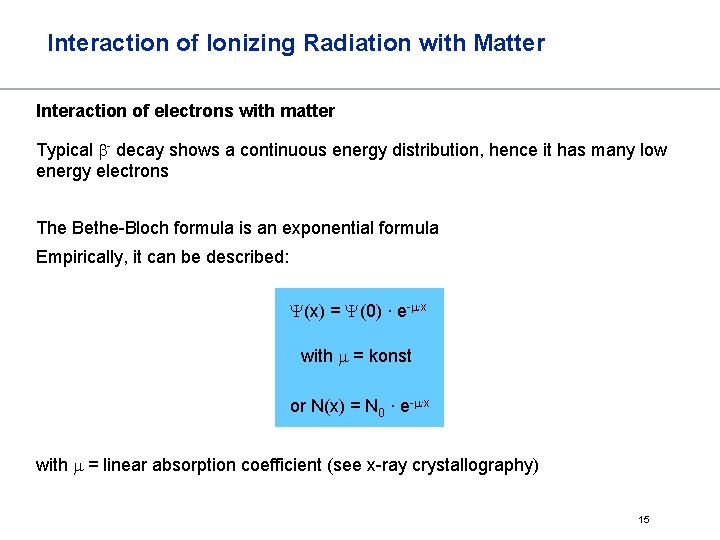
Interaction of Ionizing Radiation with Matter Interaction of electrons with matter Typical b- decay shows a continuous energy distribution, hence it has many low energy electrons The Bethe-Bloch formula is an exponential formula Empirically, it can be described: Y(x) = Y(0) ∙ e-m∙x with m = konst or N(x) = N 0 ∙ e-m∙x with m = linear absorption coefficient (see x-ray crystallography) 15

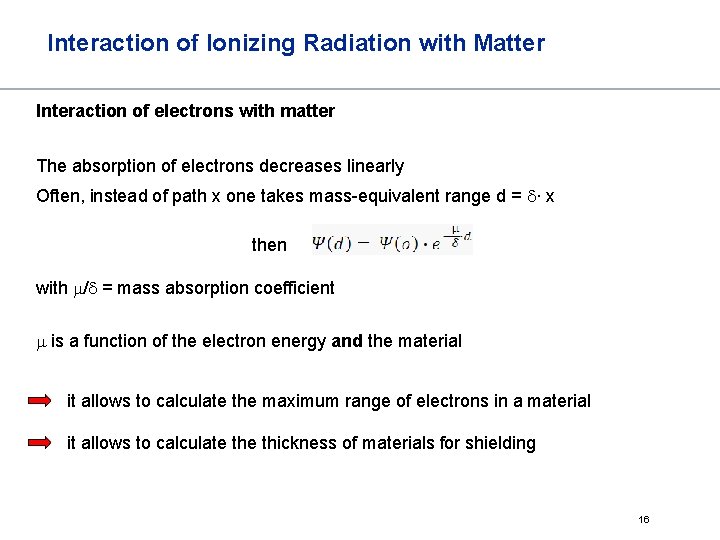
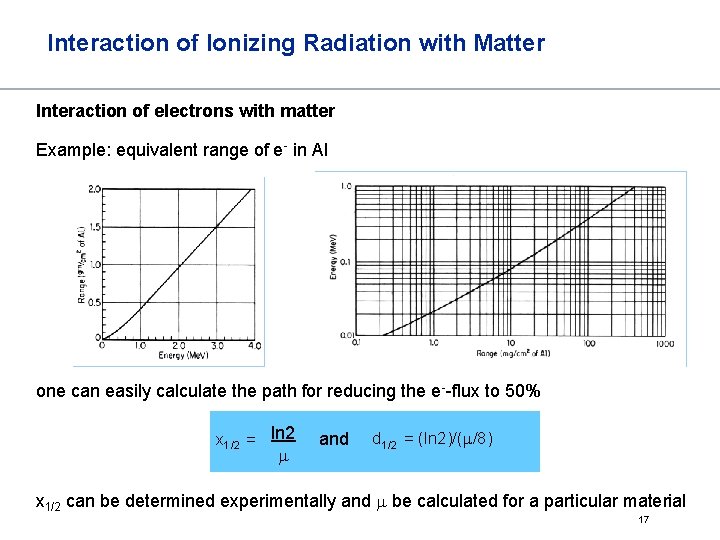
Interaction of Ionizing Radiation with Matter Interaction of electrons with matter The absorption of electrons decreases linearly Often, instead of path x one takes mass-equivalent range d = d∙ x then with m/d = mass absorption coefficient m is a function of the electron energy and the material it allows to calculate the maximum range of electrons in a material it allows to calculate thickness of materials for shielding 16

Interaction of Ionizing Radiation with Matter Interaction of electrons with matter Example: equivalent range of e- in Al one can easily calculate the path for reducing the e--flux to 50% x 1/2 = ln 2 m and d 1/2 = (ln 2)/(m/8) x 1/2 can be determined experimentally and m be calculated for a particular material 17

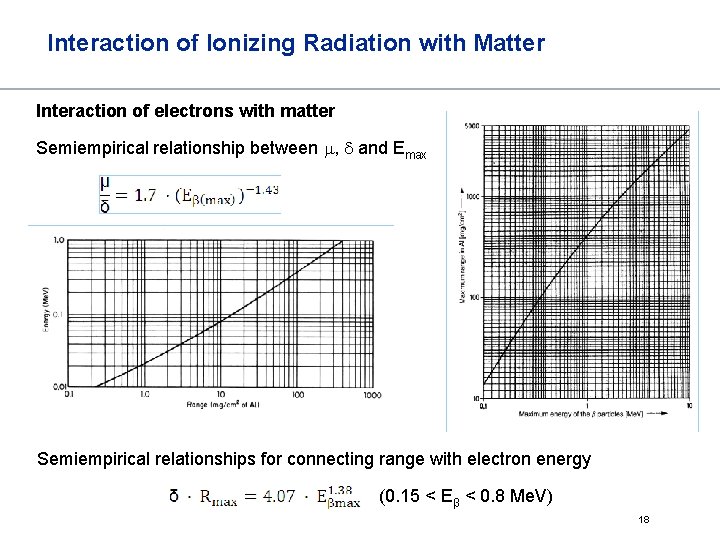
Interaction of Ionizing Radiation with Matter Interaction of electrons with matter Semiempirical relationship between m, d and Emax Semiempirical relationships for connecting range with electron energy (0. 15 < Eβ < 0. 8 Me. V) 18

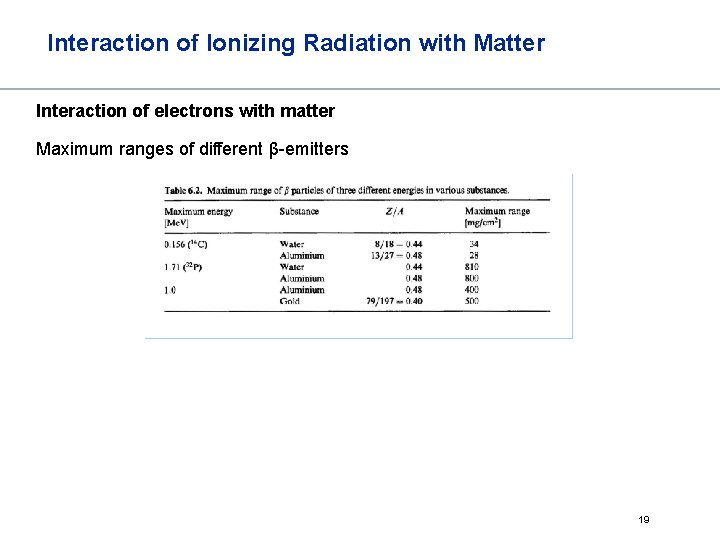
Interaction of Ionizing Radiation with Matter Interaction of electrons with matter Maximum ranges of different β-emitters 19

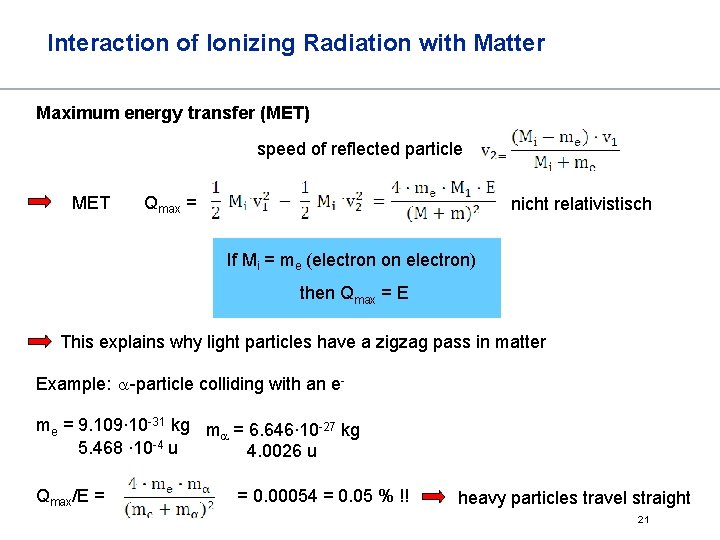
Interaction of Ionizing Radiation with Matter Interaction of charged particles with matter How much energy can be lost in a single collision? Of particular interest: collision with a shell electron Maximum energy transfer incoming particle : mass Mi, speed Vi 1 electron : mass me speed 0 after collision : Mi, v 2, me, ve Energy: ½ Mi·v 12 = ½ Mi·v 22 + ½ me· ve 2 momentum: Mi ·v 1 = Mi·v 2 + me·ve (non-relativistic) 20

Interaction of Ionizing Radiation with Matter Maximum energy transfer (MET) speed of reflected particle MET Qmax = nicht relativistisch If Mi = me (electron on electron) then Qmax = E This explains why light particles have a zigzag pass in matter Example: a-particle colliding with an eme = 9. 109∙ 10 -31 kg m = 6. 646∙ 10 -27 kg a 5. 468 ∙ 10 -4 u 4. 0026 u Qmax/E = = 0. 00054 = 0. 05 % !! heavy particles travel straight 21

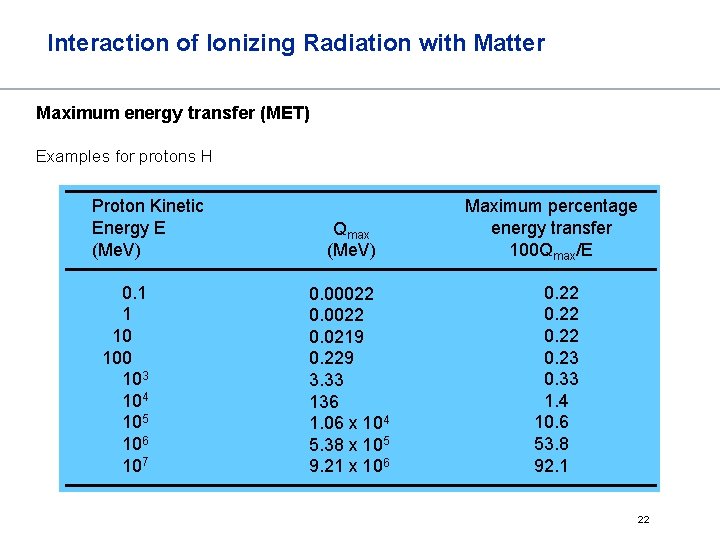
Interaction of Ionizing Radiation with Matter Maximum energy transfer (MET) Examples for protons H Proton Kinetic Energy E (Me. V) 0. 1 1 10 103 104 105 106 107 Qmax (Me. V) Maximum percentage energy transfer 100 Qmax/E 0. 00022 0. 0219 0. 229 3. 33 136 1. 06 x 104 5. 38 x 105 9. 21 x 106 0. 22 0. 23 0. 33 1. 4 10. 6 53. 8 92. 1 22

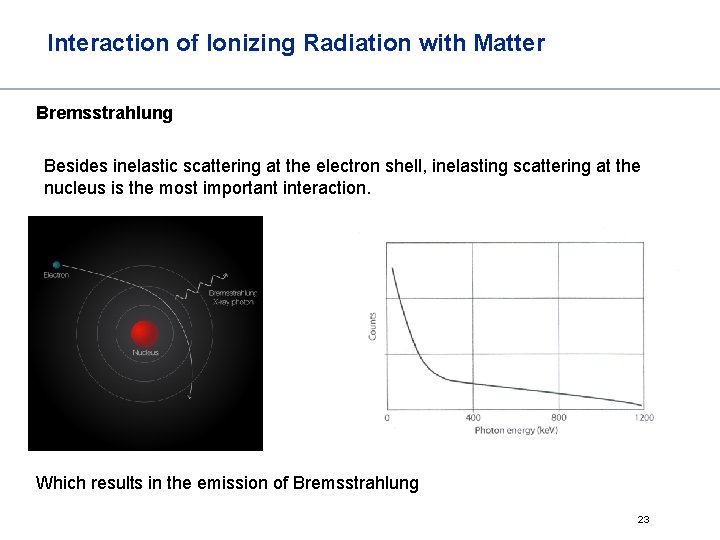
Interaction of Ionizing Radiation with Matter Bremsstrahlung Besides inelastic scattering at the electron shell, inelasting scattering at the nucleus is the most important interaction. Which results in the emission of Bremsstrahlung 23

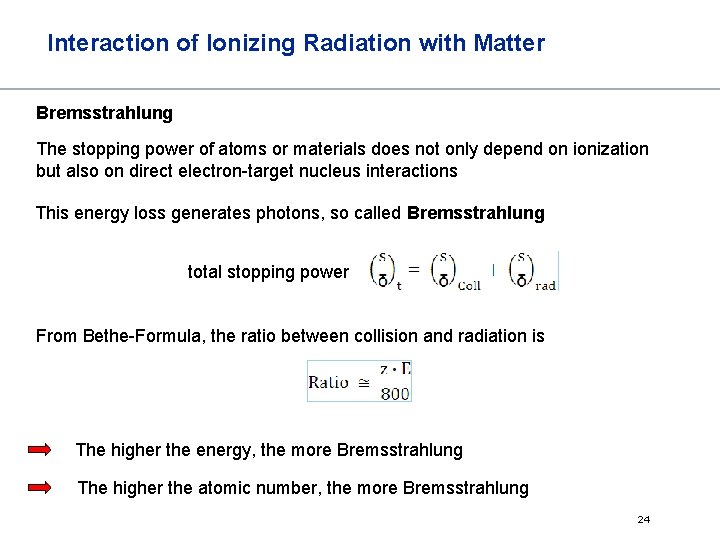
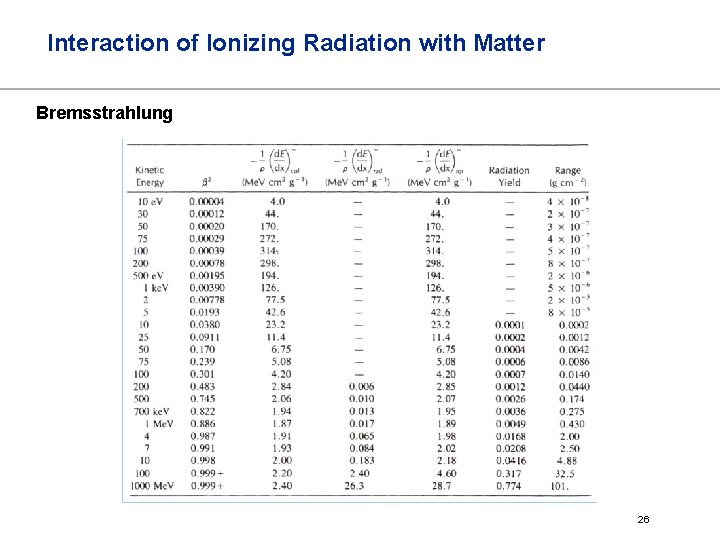
Interaction of Ionizing Radiation with Matter Bremsstrahlung The stopping power of atoms or materials does not only depend on ionization but also on direct electron-target nucleus interactions This energy loss generates photons, so called Bremsstrahlung total stopping power From Bethe-Formula, the ratio between collision and radiation is The higher the energy, the more Bremsstrahlung The higher the atomic number, the more Bremsstrahlung 24

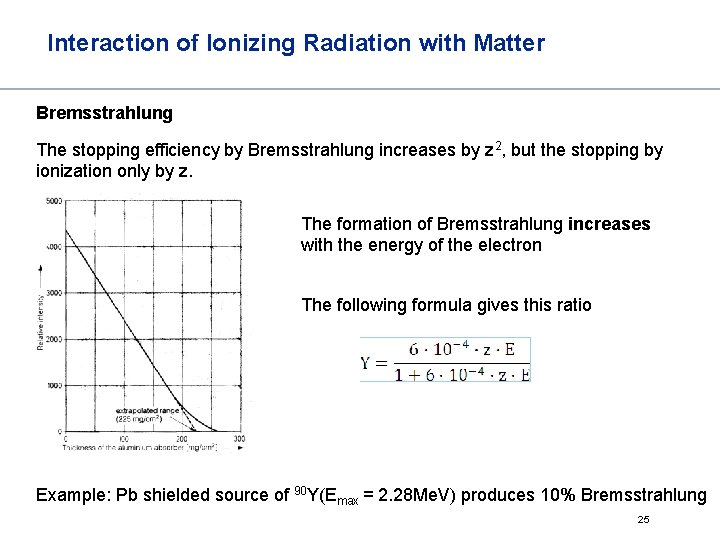
Interaction of Ionizing Radiation with Matter Bremsstrahlung The stopping efficiency by Bremsstrahlung increases by z 2, but the stopping by ionization only by z. The formation of Bremsstrahlung increases with the energy of the electron The following formula gives this ratio Example: Pb shielded source of 90 Y(Emax = 2. 28 Me. V) produces 10% Bremsstrahlung 25

Interaction of Ionizing Radiation with Matter Bremsstrahlung 26

Interaction of Ionizing Radiation with Matter Bremsstrahlung Example: Pb shielded source of 90 Y(Emax = 2. 28 Me. V) produces 10% Bremsstrahlung Don‘t shield b-emitters with lead !! 27

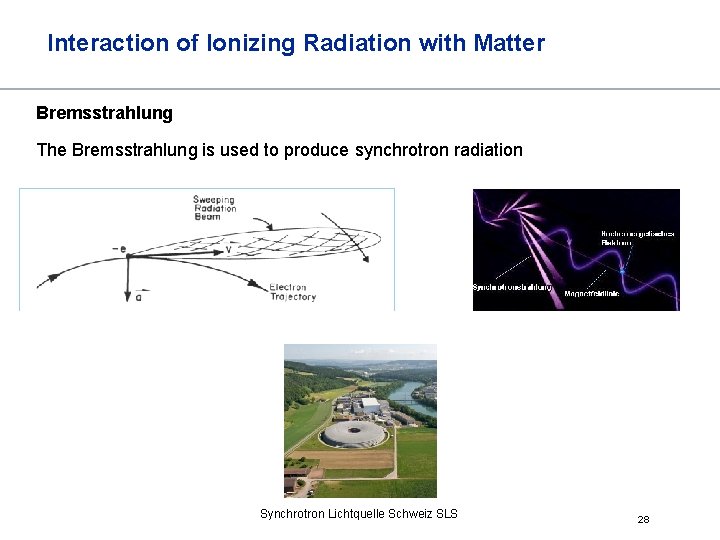
Interaction of Ionizing Radiation with Matter Bremsstrahlung The Bremsstrahlung is used to produce synchrotron radiation Synchrotron Lichtquelle Schweiz SLS 28

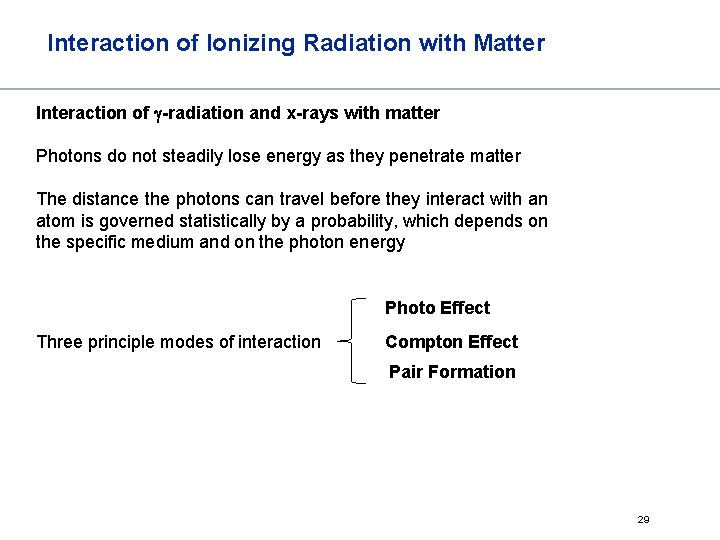
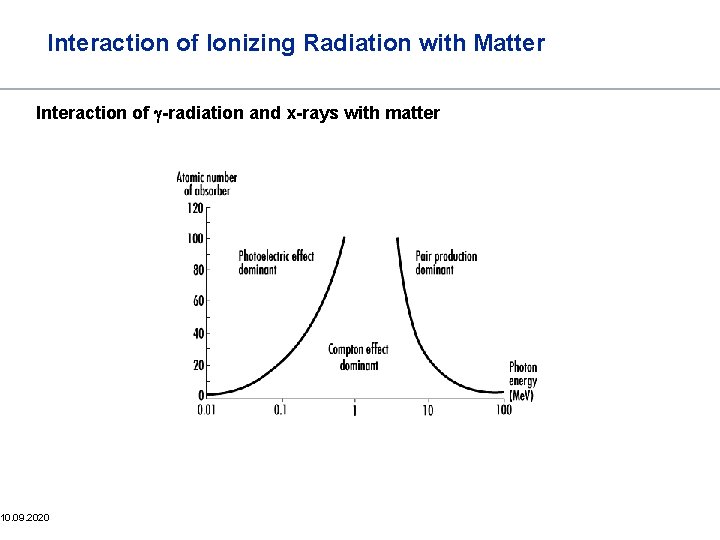
Interaction of Ionizing Radiation with Matter Interaction of g-radiation and x-rays with matter Photons do not steadily lose energy as they penetrate matter The distance the photons can travel before they interact with an atom is governed statistically by a probability, which depends on the specific medium and on the photon energy Photo Effect Three principle modes of interaction Compton Effect Pair Formation 29

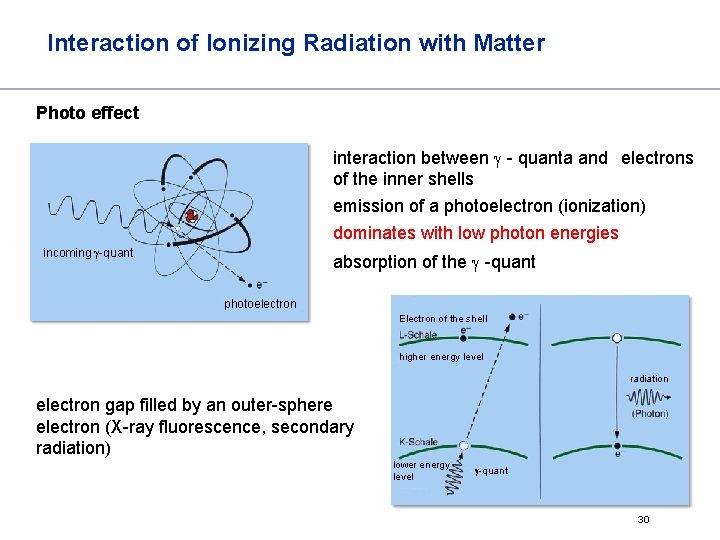
Interaction of Ionizing Radiation with Matter Photo effect interaction between - quanta and electrons of the inner shells emission of a photoelectron (ionization) dominates with low photon energies incoming -quant absorption of the -quant photoelectron Electron of the shell higher energy level radiation electron gap filled by an outer-sphere electron (X-ray fluorescence, secondary radiation) lower energy level -quant 30

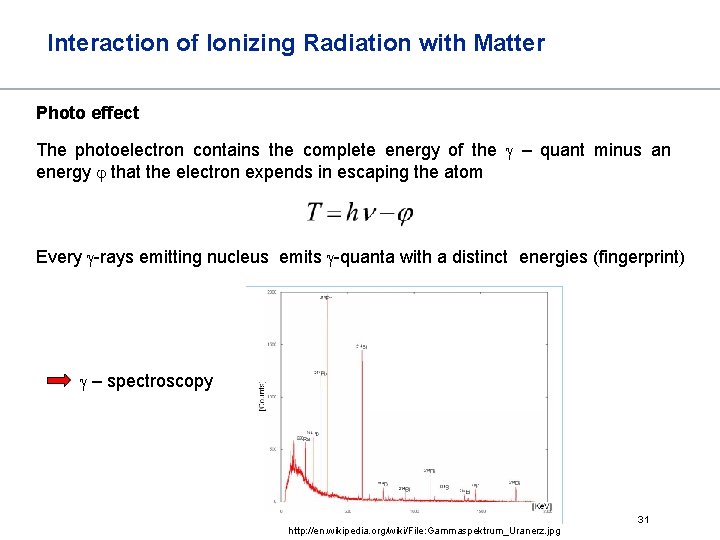
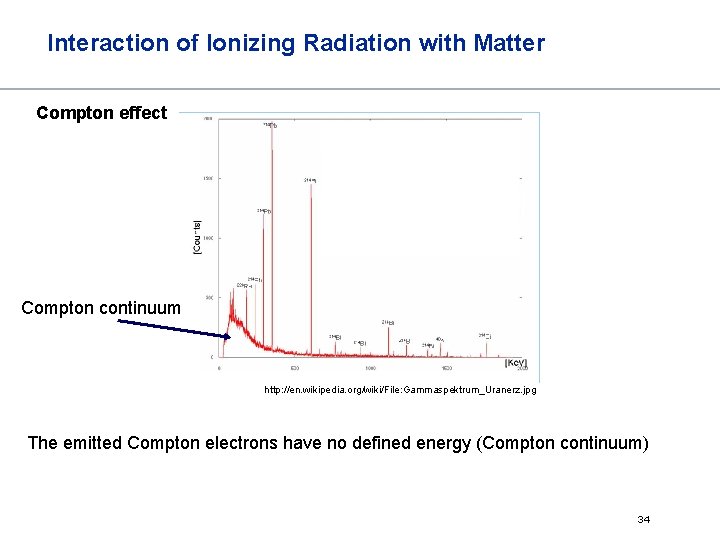
Interaction of Ionizing Radiation with Matter Photo effect The photoelectron contains the complete energy of the – quant minus an energy that the electron expends in escaping the atom Every -rays emitting nucleus emits -quanta with a distinct energies (fingerprint) – spectroscopy http: //en. wikipedia. org/wiki/File: Gammaspektrum_Uranerz. jpg 31

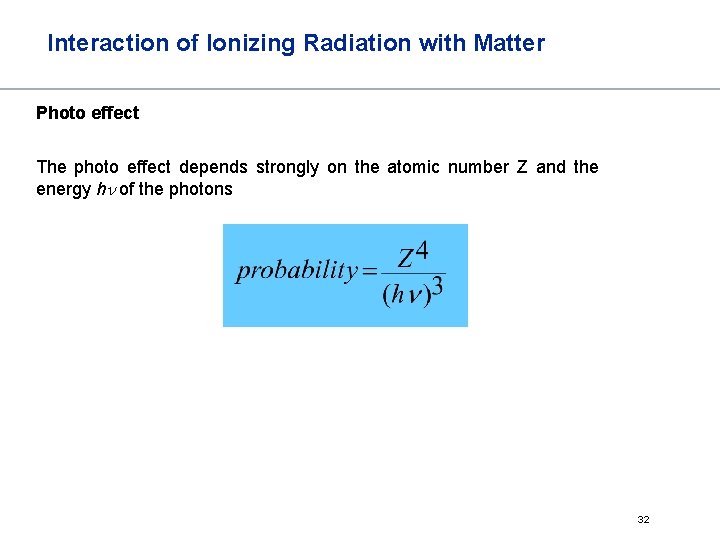
Interaction of Ionizing Radiation with Matter Photo effect The photo effect depends strongly on the atomic number Z and the energy hn of the photons 32

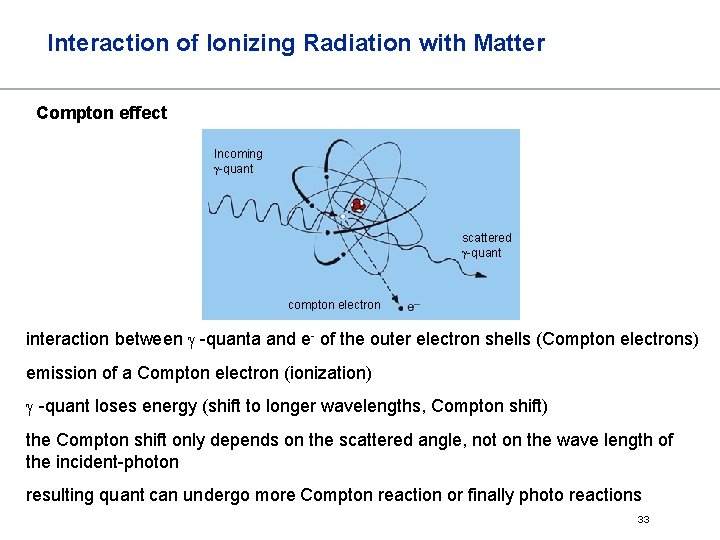
Interaction of Ionizing Radiation with Matter Compton effect Incoming -quant scattered -quant compton electron interaction between -quanta and e- of the outer electron shells (Compton electrons) emission of a Compton electron (ionization) -quant loses energy (shift to longer wavelengths, Compton shift) the Compton shift only depends on the scattered angle, not on the wave length of the incident-photon resulting quant can undergo more Compton reaction or finally photo reactions 33

Interaction of Ionizing Radiation with Matter Compton effect Compton continuum http: //en. wikipedia. org/wiki/File: Gammaspektrum_Uranerz. jpg The emitted Compton electrons have no defined energy (Compton continuum) 34

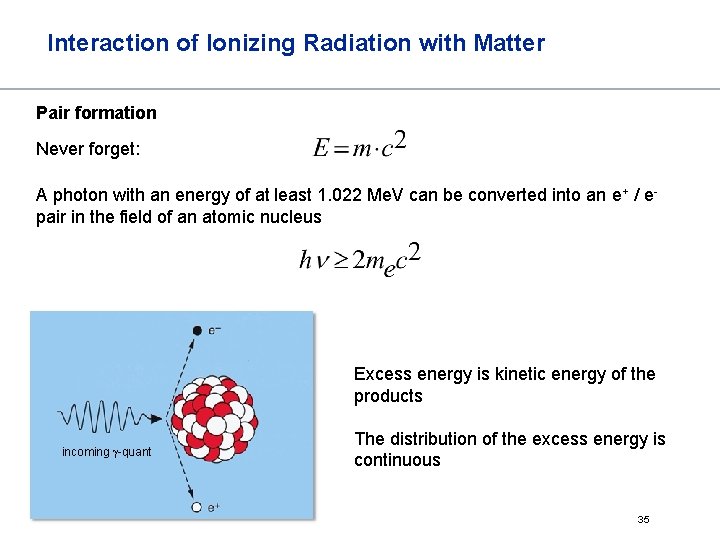
Interaction of Ionizing Radiation with Matter Pair formation Never forget: A photon with an energy of at least 1. 022 Me. V can be converted into an e+ / epair in the field of an atomic nucleus Excess energy is kinetic energy of the products incoming -quant The distribution of the excess energy is continuous 35

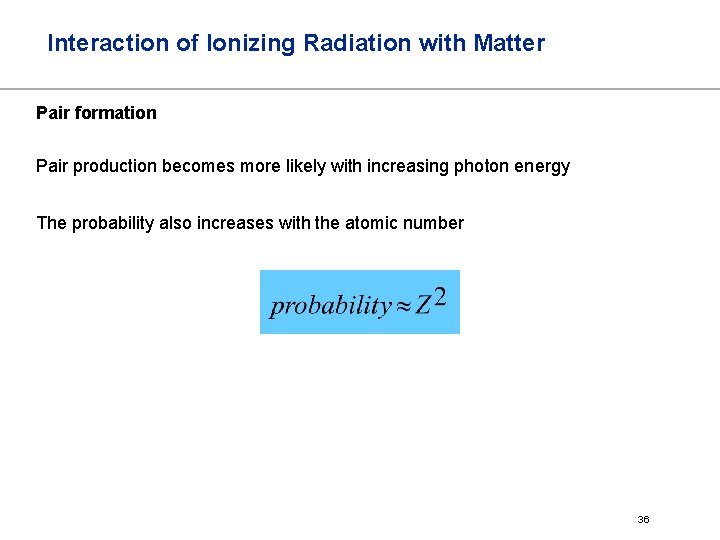
Interaction of Ionizing Radiation with Matter Pair formation Pair production becomes more likely with increasing photon energy The probability also increases with the atomic number 36

Interaction of Ionizing Radiation with Matter Annihilation of positrons The produced positron immediately reacts with an electron Since the total momentum before the decay is zero, two photons must be produced in order to conserve momentum The produced photons going off in opposite directions Due to the photon energy is 511 ke. V (1. 022 Me. V/2) 37

Interaction of Ionizing Radiation with Matter Pair formation Disadvantage: The presents of 511 ke. V annihilation photons around any positron source is always a potential radiation hazard Advantages: Pair Formation helps to convert high energy photons (> 1. 022 Me. V) into photons with less energy (511 ke. V) easier to shield Question: How would you shield a -emitter ? 38

Interaction of Ionizing Radiation with Matter Interaction of g-radiation and x-rays with matter 10. 09. 2020

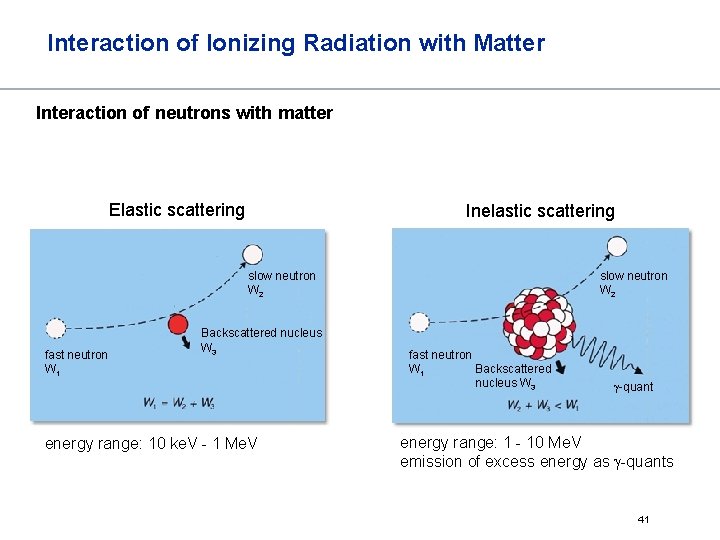
Interaction of Ionizing Radiation with Matter Interaction of neutrons with matter Neutrons have no charge and don‘t interact with the shell electron (no direct ionization) Interactions between neutrons and matter are interactions with nuclei (only secondary ionization processes) Classification of Neutrons Thermal Neutrons: Energy distribution according to the Maxwell – Boltzmann equation Energy ≈ 0. 025 e. V (most probable energy in the distribution at 20°C) Slow Neutrons: Also called “intermediate” of “resonance” neutrons. Energy ≤ 0. 1 Me. V Fast Neutrons: Energy ≤ 20 Me. V Relativistic Neutrons: Energy > 20 Me. V 40

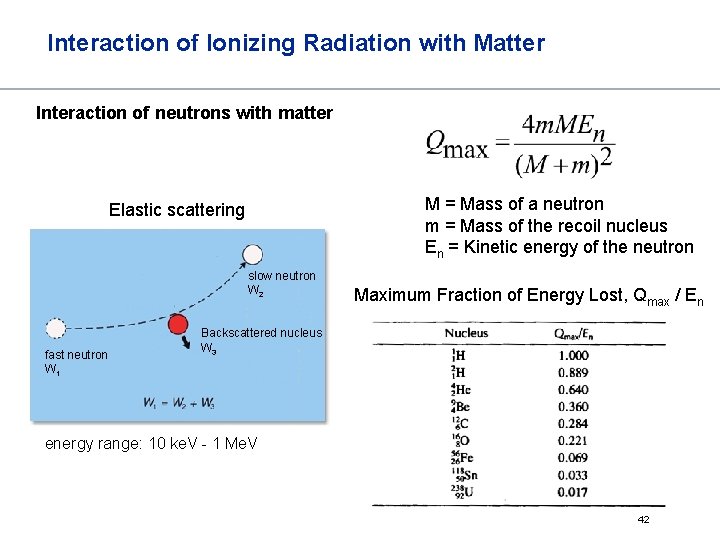
Interaction of Ionizing Radiation with Matter Interaction of neutrons with matter Elastic scattering Inelastic scattering slow neutron W 2 fast neutron W 1 Backscattered nucleus W 3 energy range: 10 ke. V - 1 Me. V slow neutron W 2 fast neutron Backscattered W 1 nucleus W 3 -quant energy range: 1 - 10 Me. V emission of excess energy as -quants 41

Interaction of Ionizing Radiation with Matter Interaction of neutrons with matter M = Mass of a neutron m = Mass of the recoil nucleus En = Kinetic energy of the neutron Elastic scattering slow neutron W 2 fast neutron W 1 Maximum Fraction of Energy Lost, Qmax / En Backscattered nucleus W 3 energy range: 10 ke. V - 1 Me. V 42

Interaction of Ionizing Radiation with Matter Interaction of neutrons with matter Slowing-down neutrons is called neutron moderation If a neutron reaches thermal energies, it will move about randomly by elastic scattering until absorbed by a nucleus Nuclear reaction: (n, p), (n, 2 n), (n, a), (n, ) Neutron Activation Analysis 43